ABSTRACT
Taxanes are mainstay treatment of triple negative breast cancer (TNBC) patients but resistance often develops. Using TNBC patient-derived orthoxenografts (PDX) we have recently discovered that a CD49f+ chemoresistant population with tumor-initiating ability is present in sensitive tumors and expands in tumors that have acquired resistance. Importantly, sensitivity to taxanes is recovered after long-term drug interruption. The characterization of this chemoresistant CD49f+ cells provides a unique opportunity to identify novel targets for the treatment of chemoresistant TNBC.
KEYWORDS: CD49f tumor-initiating cells, chemoresistance, docetaxel, PDX (patient-derived orthoxenografts), triple negative breast cancer (TNBC)
Triple negative breast cancer (TNBC) is the most heterogeneous and aggressive subtype of breast cancer. Mainstay treatment is chemotherapy, mostly anthracyclines and taxanes.1,2 The major cause of death is not the primary tumor but the metastases, which normally display resistance to treatment.3
The best way to study mechanisms of chemoresistance would be by direct comparison of paired biopsies collected before and after resistance appears, but these paired sensitive/resistant samples are often difficult to obtain. To overcome this limitation we took advantage of patient-derived orthoxenografts (PDXs) we have established in the laboratory. Our collection including TNBC, luminal, human epidermal growth factor receptor 2 (HER2) models and breast cancer 1/breast cancer 2 (BRCA1/BRCA2) mutated tumors represents the human breast cancer heterogeneity.4 An exhaustive characterization of these models confirmed that they mimic the main characteristics of the human tumors from which they derive, although differences are observed in some models. Moreover, luminal models were resistant to docetaxel whereas TNBC PDX models were initially sensitive, but they progressively acquired resistance by continued exposure to the drug mimicking the clinical scenario. These paired sensitive–resistant TNBC tumors constitute valuable tools for the study of resistance acquisition in TNBC. Interestingly, chemoresistant-derived TNBC tumors regained sensitivity after long-term maintenance in the absence of the drug. This phenomenon called “drug holidays” has been described for targeted therapies,5,6 but never for chemotherapy treatments. Using gene expression analyses from chemosensitive/resistant tumors we identified a predictive signature of residual disease after anthracycline/taxane based therapy in patients with basal-like disease, validating the clinical significance of our approach.
It has been shown that in a variety of neoplasias there are populations of cancer stem cells (CSCs) with tumor-initiating (TIC) ability, resistant to chemotherapy and therefore, responsible of recurrence and metastasis. No changes were observed in the most generally breast cancer stem cell markers, CD44+/CD24- population and aldehyde dehydrogenase (ALDH) + activity when sensitive and resistant-derived TNBC tumors were compared; however, an expansion of the CD49f+ population in both chemoresistant-derived TNBC models was observed. Moreover, CD49f-high expression was associated with non-pathological complete response or poor overall survival after chemotherapy in several clinical data sets.
We next wondered whether a chemoresistant CD49f+ population was initially present in our chemosensitive PDX tumors, or alternatively whether this population arose after long-term treatment with docetaxel. An increase in the frequency of CD49f+ cells was observed in residual disease after short-term treatments with docetaxel. Interestingly, the frequency of the CD49f+ population present in the “drug holidays” tumors was comparable to that in sensitive tumors of origin. Thus, a chemoresistant CD49f+ population is initially present in the sensitive tumors, survives to docetaxel treatment, is enriched in residual disease and expands in TNBC tumors with acquired resistance (Fig. 1).
Figure 1.
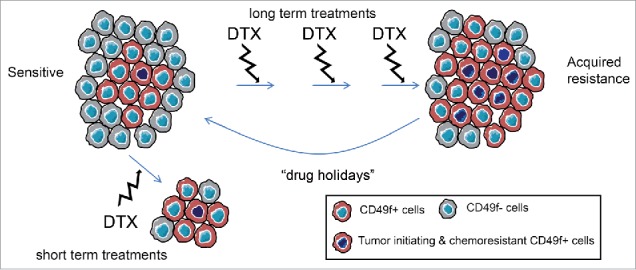
CD49f+ cell population dynamics upon docetaxel treatment in triple negative breast cancer. The acquisition of chemoresistance to docetaxel is associated to an expansion of a CD49f+ population. This population is present in the initially sensitive tumors, but after long-term treatment interruption sensitivity is recovered (drug holidays). In the short-term treatment, the surviving cells are the CD49f+ chemoresistant.
Analyses of residual disease after docetaxel treatment in multiple TNBC PDXs revealed an increase in CD49f (ITGA6) mRNA expression in most sensitive TNBC PDX, whereas no changes in CD49f expression were observed in resistant TNBC PDX. Moreover, higher CD49f expression levels were also observed in cells from TNBC cell lines that survive docetaxel treatment. Altogether, these results demonstrate that despite the heterogeneity of the TNBC disease, a chemoresistant CD49f+ population is present in most TNBC sensitive tumors and that modulation of this population associates with docetaxel resistance in this subtype (Fig. 1).
Finally, we functionally challenged the CD49f+ cells as breast CSC analyzing the two most common properties associated with CSC: tumor-initiating ability and chemoresistance. CD49f+ and CD49f- cells from our sensitive TNBC PDX were orthotopically injected in immunocompromised mice. The CD49f+ cells from TNBC showed an increased tumor-initiating ability than the negative ones, and CD49f+-derived tumors show higher levels of CD49f+ population and were more resistant to docetaxel compared to the initial sensitive ones or the CD49f–derived tumors.
Gene expression analyses of CD49f+ and CD49f- cells from sensitive and resistant tumors revealed downregulation of keratins, claudins and P-cadherin (P-CADH), and upregulation of proliferation related genes in the CD49f+ resistant population. This signature predicts residual disease following anthracycline/taxane based therapy in basal-like breast cancer.
PDXs have become popular models in the study of cancer and constitute a unique tool to investigate tumor heterogeneity, tumoral evolution and cancer resistance. They fill the gap between basic research knowledge and clinical research, with clear advantages over cancer cell lines as state-of-the-art translational models.7,8
The “drug holidays” effect showed in our resistance acquired TNBC PDXs has important implications for clinical decisions and drug scheduling, particularly in metastatic TNBC disease where no targeted therapies are available.
Our results evidence the intra-tumor heterogeneity of the TNBC disease where different tumoral populations, with different drug sensitivities can coexist in the same tumor. The existence of a chemoresistant CD49f+ population with TIC ability in most sensitive TNBC would have to be clinically validated in the neoadjuvant clinical setting, ideally in the residual disease after taxane-based therapy. The gene expression signature predictive of residual disease identified constitute a closer approach to personalized medicine. Further characterization of this chemoresistant CD49f+ cells can reveal novel therapeutic targets for the treatment of the metastatic TNBC disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants to Eva González Suárez by the Spanish Ministry of Economy and Competitivity MINECO and from the ISCIII (SAF2011-22893, SAF2014-55997), PIE13/00022, co-funded by FEDER funds/ European Regional Development Fund (ERDF) - a way to build Europe-), by a Career Catalyst Grant from the Susan G Komen Foundation CCR13262449 and by institutional funds provided by the Generalitat de Catalunya to the PEBC.
References
- 1.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, et al.. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol 2014; 25:1871-88; PMID:25234545; https://doi.org/ 10.1093/annonc/mdu385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F; ESMO Guidelines Committee . Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26(Suppl. 5):v8-30; PMID:26314782; https://doi.org/ 10.1093/annonc/mdv298 [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol 2012; 23(Suppl 6):vi46-51; PMID:23012302; https://doi.org/ 10.1093/annonc/mds195 [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Miragaya J, Palafox M, Paré L, Yoldi G, Ferrer I, Vila S, Galván P, Pellegrini P, Pérez-Montoyo H, Igea A, et al.. Resistance to taxanes in triple negative breast cancer associates with the dinamics of a CD49f+ tumor initiating population. Stem Cell Reports 2017; 8(5):1392-407; PMID:28457887; https://doi.org/ 10.1016/j.stemcr.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M, Stuart DD. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013; 494(7436):251-5; PMID:23302800; https://doi.org/ 10.1038/nature11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, Haanen J, Blank C, Wesseling J, Willems SM, et al.. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014; 508(7494):118-22; PMID:24670642; https://doi.org/ 10.1038/nature13121 [DOI] [PubMed] [Google Scholar]
- 7.Dobrolecki LE, Airhart SD, Alferez DG, Aparicio S, Behbod F, Bentires-Alj M, Brisken C, Bult CJ, Cai S, Clarke RB, et al.. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev 2016; 35(4):547-73; PMID:28025748; https://doi.org/ 10.1007/s10555-016-9653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, et al.. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 2017; 17(4):254-68; PMID:28104906; https://doi.org/ 10.1038/nrc.2016.140 [DOI] [PubMed] [Google Scholar]


