Abstract
Adolescence is a phase of development during which many physiological and behavioral changes occur, including increased novelty seeking and risk taking. In humans, this is reflected in experimentation with drugs. Research demonstrates that drug use that begins during adolescence is more likely to lead to addiction than drug use that begins later in life. Despite this, relatively little is known of the effects of drugs in adolescence, and differences in response between adolescents and adults.
PCP and ketamine are popular club drugs, both possessing rewarding properties that could lead to escalating use. Drug sensitization (or reverse tolerance), which refers to an increase in an effect of a drug following repeated use, has been linked with the development of drug cravings that is a hallmark of addiction.
The current work investigated the acute response and the development of sensitization to PCP and ketamine in adolescent and adult rats. Periadolescent Sprague-Dawley rats (30 days or 38 days of age), and young adults (60 days of age) received PCP (6 mg/kg IP) or ketamine (20 mg/kg IP) once every three days, for a total of five drug injections. Adolescents and adults showed a stimulant response to the first injection of either drug, however the response was considerably greater in the youngest adolescents and lowest in the adults. With repeated administration, adults showed a robust escalation in activity that was indicative of the development of sensitization. Adolescents showed a flatter trajectory, with similar high levels of activity following an acute treatment and after five drug treatments. The results demonstrate important distinctions between adolescents and adults in the acute and repeated effects of PCP and ketamine.
Keywords: Phencyclidine, Ketamine, Locomotor, Addiction, Sensitization
1. Introduction
Recreational drug use often begins in adolescence and drugs of abuse can alter the brain and behavior during this vulnerable phase of life. Age of first exposure to an addictive substance is a large contributing factor to future dependence and abuse, with earlier onset translating to greater abuse potential later in life (Anthony and Petronis, 1995). Approximately 9% of individuals aged 12–17 are current users of some illicit substance (SAMHSA, 2015). The transition from drug use to addiction is not clearly understood; however, it is widely accepted that repeated administration of addictive drugs results in changes to the brain that increase motivation to seek drugs, ultimately contributing to compulsive use (Chambers et al., 2003; Dale et al., 2015; Robinson and Berridge, 1993, 2000, 2001, 2003; Trujillo and Akil, 1995; Trujillo et al., 2008; Wise and Bozarth, 1987; Zarate et al., 2006).
Two drugs of abuse that are often used by youth are Phencyclidine (PCP) and ketamine, especially in dance clubs and party settings (Dillon et al., 2003; Hopfer et al., 2006; Trujillo et al., 2011). These drugs are referred to as dissociatives because of their unique psychoactive effects, including alterations in visual perception and out-of-body experiences. Some of the desired effects of dissociatives are an increase in empathy, religious ecstasy, and transcendence of time and space; however, psychotomimetic effects, anxiety, panic, paranoia, and cognitive impairments can also ensue (Jansen, 2000; Kleinloog et al., 2015; White and Ryan, 1996).
Aside from their recreational use, PCP and ketamine have medical applications that include analgesia and anesthesia (Jouguelet-Lacoste, et al., 2015; Nalini et al., 2016; White and Ryan, 1996). Ketamine produces fewer dissociative effects than PCP and the effects are shorter lived, allowing for broader clinical use. Ketamine is better tolerated in children than adults. Children exhibit decreased psychotomimetic effects that are characterized by bad dreams and hallucinations (Cho et al., 2014; Green et al., 2009; Green et al., 2011). Aside from applications in pain relief, ketamine is also a fast-acting antidepressant that has been used with notable success in cases where traditional antidepressants are not effective. PCP and ketamine both produce schizophrenic-like symptoms that make them useful in the laboratory as pharmacological tools in animal models of schizophrenia (Elsworth et al., 2015; Jentsch et al., 2000; Jentsch and Roth, 1999; Laruelle et al., 2003; Morgan et al., 2006; Olney and Farber, 1995; White and Ryan, 1996; Xu et al., 2015).
PCP and ketamine share a mechanism of action as non-competitive antagonists of N-methyl-D-aspartate (NMDA) receptors. Ketamine has a lower affinity at this site and a shorter duration of action, thus exerting fewer undesirable effects than PCP (Corssen and Domino, 1966; Tricklebank et al., 1989; Wong et al., 1986). NMDA receptors increase during early development, peak in adolescence and then begin to decline, reaching a stable number in adulthood (Colwell et al., 1998; Henson et al., 2008; Insel et al., 1990; Luo et al., 1996). In humans, this phenomenon may help explain age-dependent disorders that involve glutamate and that often emerge during the teenage-years, such as schizophrenia, depression, and drug abuse. Additionally, because NMDA receptors are late to reach maturity, the drug response to drugs that act at NMDA receptors may differ between adolescents and adults.
One important behavioral endpoint in the neuroplasticity of drug abuse is sensitization (Castellani and Adams, 1981; Nabeshima et al., 1987; Robinson et al., 1998; Robinson and Becker, 1986; Robinson & Berridge, 1993; Trujillo, 2002; Trujillo and Akil, 1995; Trujillo et al., 2008; Uchihashi et al., 1993; White and Kalivas, 1998; Xu and Domino, 1999). Behavioral sensitization occurs when repeated administration of a drug leads to an increased behavioral response, such as increased locomotor activity. Sensitization is produced by neural changes that are also thought to be involved in the development of drug cravings. Drug cravings are considered to be a key component in the transition from casual drug use to compulsive abuse (Robinson and Berridge, 1993, 2000, 2001; 2003; Trujillo and Akil, 1995; Wise and Bozarth, 1987).
Similarly to other drugs of abuse, repeated administration of PCP and ketamine produces locomotor sensitization in animal models (Abekawa et al., 2002; Iwamoto, 1986; Phillips et al., 2001; Scalzo & Holson, 1992; Trujillo et al., 2008; Uchihashi et al., 1993; Xu & Domino; 1994a; 1994b).
Age differences in the development of sensitization have been examined for several drugs of abuse. Specifically, adolescents show reduced locomotor stimulation and less sensitization than adults to psychostimulants such as cocaine (Laviola et al., 1995; Izenwasser and French, 2002; Wiley et al., 2008; Zombeck et al., 2009) and methamphetamine (Zombeck et al., 2009). Age differences in the development of sensitization to dissociatives have not been well studied and are therefore less understood.
Given the popularity of club drugs in adolescents, the present work examined age-differences in the acute response and development of sensitization to PCP and ketamine. Because the acute response and the development of sensitization to psychomotor stimulants are reduced in adolescents, it was hypothesized that a similar pattern would occur in response to PCP and ketamine.
2. Methods
2.1. Animals
Twelve periadolescent (30 days old at the start of the experiment), 12 adolescent (38 days old at the start of the experiment), and 12 adult (60 days old at the start of the experiment) male Sprague-Dawley rats (Harlan Laboratories) were used in these studies. Ages were selected based on previous literature indicating that early adolescence appears at approximately 28–30 days of age and early adulthood by 60 days (Laviola et al., 1999; Spear and Brake, 1983; 2000; 2004). Animals were allowed to acclimate to the vivarium for at least one week prior to testing. A 12-hour light/dark cycle was maintained. Animals were group housed and had ad libitum access to food and water. The experimental protocol was approved by the California State University San Marcos Institutional Animal Care and Use Committee (IACUC) and is in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2 Drugs
2.2.1
Phencyclidine hydrochloride (6 mg/kg; National Institute on Drug Abuse Drug Supply Program) was dissolved in 0.9% saline and injected intraperitoneally (IP) at a volume of 1 ml/kg.
2.2.2
Ketamine hydrochloride (20 mg/kg; Sigma-Aldrich) was dissolved in 0.9% saline and injected intraperitoneally (IP) at a volume of 1 ml/kg.
2.2.3
No saline control groups were included; the primary purpose of this work was to compare the response in adults and adolescents. However, there were no baseline differences prior to drug administration for ambulations between ketamine-treated adults and adolescents (177±26.65 vs 131.8±29), t(10)=1.147, n.s.; nor between PCP-treated adults and adolescents (190.8±40.77 vs 134.5±61.77), t(10)=0.7611, n.s. There were also no baseline differences in fine movements between ketamine-treated adults and adolescents (344.7±45.6 vs 248.5±44.14), t(10)=1.515, n.s.; nor between PCP-treated adults and adolescents (337.2±76.6 vs 176.3±62.25), t(10)=1.628, n.s. Moreover, previous research from our laboratory and others (Collins and Izenwasser, 2004; Spear and Brake, 1983) has shown no differences in activity between adolescents and adults in response to an injection of saline.
Doses were selected based on previous work in the laboratory demonstrating a moderate stimulant response at the respective doses (Garcia & Trujillo, 2007a; Sullivan & Trujillo, 2007a; Trujillo et al., 2006; Trujillo & Warmoth, 2005).
2.3. Apparatus
A Kinder Scientific Motor Monitor System was used to assess locomotor activity. This system consists of eight plexiglass enclosures (16″ X ″16 X ″15). Each enclosure has an array of photocells (sixteen in each dimension) placed 5 cm above the floor. The enclosures are interfaced with a personal computer for data collection. The computer collects photocell beam breaks (activity counts) and calculates different types of activity, including ambulations (interruptions of successive photocell beams leading to displacement of the animal horizontally, typically associated with forward movement) and fine movements (repeated interruptions of the same photocell beams, associated with short repetitive movements, as seen with stereotypic activity such as head-bobbing).
2.4. Procedures
Rats (n=6/group) were handled once daily for three days prior to the study. On test days, they were habituated to the testing room for 30 minutes followed by habituation in the locomotor chambers for an additional 30 minutes. Animals were then injected with either PCP or ketamine and placed once again into the chambers and activity was assessed for 120 minutes post-injection. Drug administration and locomotor testing occurred on an intermittent schedule, once every three days (note that the PND 38 group was tested only following the first injection).
2.5. Data Analysis
Ambulation counts (interruption of successive photocell beams representing horizontal movements throughout enclosure) and fine movement counts (repeated interruption of the same photocell beams representing repetitive stereotypic movements) were the dependent measures used to examine locomotor activity of the animals.
Given differences in the duration of action between the drugs, the analyses for PCP focused on 120 minutes post injection, while for ketamine, the analyses focused on 10 minutes post injection. Acute (Day 1) differences between animals at postnatal day (PND) 30 and PND 38, and adults at PND 60 were compared using a 2-way mixed-model ANOVA (Group by Time) followed by Fisher’s LSD post-hoc analyses. Responses to the drugs after the first (Day 1) and the fifth drug administration (Day 5) were compared using a 2-way Repeated Measures ANOVA to examine sensitization (Day by Time).
Behavioral observations of the animals suggested that the adolescents showed more horizontal activity and less stereotypy in response to PCP and ketamine than adults. The Kinder Scientific system offers a measure of horizontal locomotion (ambulations) and a measure of stereotypy (fine movements), thereby offering an opportunity to compare the relative contributions of the two types of activity. When divided, these measures offer potential differences in the pattern of locomotion (ambulations/fine movements = locomotor index). For the locomotor index, a bias toward horizontal locomotion would be reflected in a numerical value greater than 1, while a bias toward stereotypy would be reflected in a value less than 1. The locomotor index for adolescents and adults in response to the drugs was compared by a 1-way ANOVA followed by Fisher’s LSD post-hoc analysis.
3. Results
3.1. STUDY 1: PCP 6.0 mg/kg (Adult vs. Periadolescent)
3.1.1. Day 1
3.1.1.1. Ambulations
Adult (PND 60) animals showed a modest increase in ambulations in response to administration of PCP (6 mg/kg) on Day 1 of treatment. By comparison, periadolescents at postnatal day (PND) 30 and PND 38 showed a much stronger locomotor response to the acute administration of PCP. PND 30 animals exhibited the highest locomotor activity and remained high throughout the session. PND 38 animals reached an initial peak similar to the PND 30 group and gradually declined to adult levels. A 2-way mixed-model ANOVA of ambulations showed significant effects of Group and Time, as well as an Interaction [Group: F(2, 15)=11.18, p <.01; Time: F(11, 165)=4.376 p< .0001; Group × Time: F(22, 165)=4.536, p< .0001]. Post-hoc analyses showed differences in ambulations between the PND 30 and PND 38 groups at 60–120 minutes; between PND 38 and PND 60 groups at 20–50 minutes and between PND 30 and PND 60 groups at 30–120 minutes (p< .05). There was no difference between PND 30 and PND 38 groups (p> .05) (Fig 1A). A 1-way ANOVA comparing total ambulations post-injection showed a difference among the groups [F(2,15)=11.18, p< .01] that was significant between PND 60 and either PND 30 or PND 38 groups, but was not different between PND 30 and PND 38 groups (Fig 1B).
Figure 1.
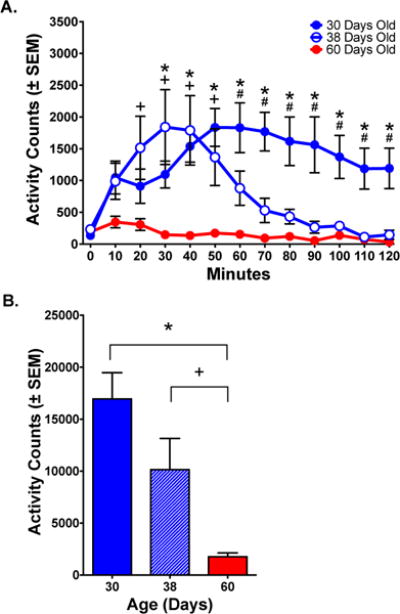
Ambulations (horizontal locomotion) in response to PCP (6.0 mg/kg). (A) Time course of response. Activity was highest for PND 30 animals and lowest for PND 60 animals. PND 38 animals showed an initial peak similar to PND 30 animals that gradually declined to adult levels. (B) Total ambulations over the 120-minute session. There were significant differences between the PND 60 group and either PND 30 or PND 38 groups, but no difference between PND 30 and PND 38 groups.
Significant differences between PND 30 and PND 60 animals (*, p< .05); PND 38 and PND 60 animals (+, p< .05); PND 30 and PND 38 animals (#, p< .05); Fisher’s test.
3.1.2.2. Fine Movements
Fine movements, a measure of stereotypy, showed a pattern similar to ambulations. After an acute injection of PCP, fine movements in PND 30 animals were the highest, and remained high throughout the session, but in PND 38 animals declined after an initial peak. Activity was lowest in the PND 60 group. A 2-way mixed-model ANOVA showed significant effects for Group, Time and Interaction [Group: F(2, 15)=11.79, p< .001; Time: F(11, 165)=14.77 p< .0001; Group × Time: F(22, 165)=4.368, p< .0001]. Post-hoc analyses showed differences in fine movements between PND 30 and PND 38 groups at 60–120 minutes; between PND 38 and PND 60 groups at 20–50 minutes and between PND 30 and PND 60 groups at 30–120 minutes, p< .01. There was no difference between PND 30 and PND 38 groups (p> .05) (Fig 2A). Total fine movements for the session were highest for PND 30, followed by PND 38 animals, and lowest for adults [F(2,15)=11.79, p< .001] (Fig 2B).
Figure 2.
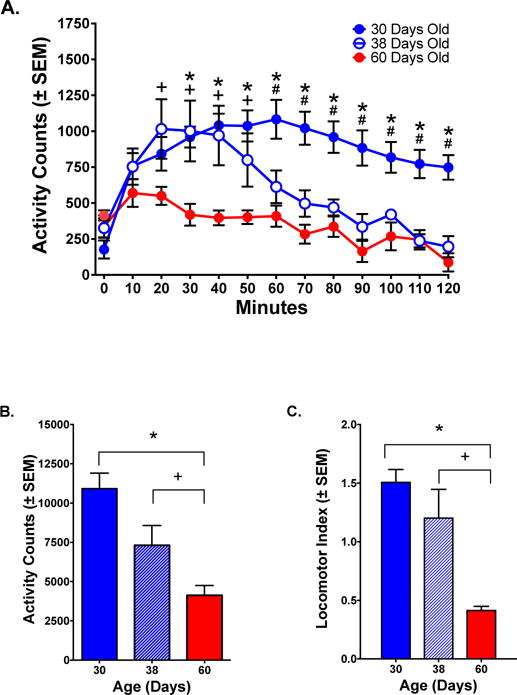
Fine movements (stereotypy) in response to PCP (6.0 mg/kg). (A) Time course of response. Fine movements were highest for PND 30 animals, lowest for PND 60 animals, and PND 38 animals showed an initial peak similar to PND 30 animals that gradually declined to adult levels. (B) Total fine movements over the 120-minute time course. There were significant differences between the PND 60 group and either PND 30 or PND 38 groups, but no difference between PND 30 and PND 38 groups. (C) Locomotor Index (ambulations/fine movements) determined the relative types of movement induced by PCP. PND 30 and PND 38 animals showed a bias toward horizontal locomotion reflected in a numerical value greater than 1.0, while PND 60 animals showed a bias toward stereotypy reflected in a value less than 1.0.
Significant differences between PND 30 and PND 60 animals (*, p< .05); PND 38 and PND 60 animals (+, p< .05); PND 30 and PND 38 animals (#, p< .05); Fisher’s test.
3.1.2.3. Locomotor Index
A locomotor index was assessed as ambulations/fine movements. In response to the first injection of PCP, the locomotor index was greater than 1.0 for PND 30 and PND 38 animals indicative of a bias toward horizontal activity. For PND 60 animals, the locomotor index was less than 1.0 indicative of a bias toward steretotypy. A 1-way ANOVA showed significant differences [F(2,15)=12.95, p= 0.0005] reflecting a bias toward horizontal activity relative to stereotypy in both groups of adolescents when compared to adults. Post-hoc analyses showed a significant difference between PND 60 and either PND 30 or PND 38 groups (p< .01), but no difference between PND 30 and PND 38 groups, (p> .05) (Fig 2C).
3.1.3. Day 5, PCP 6.0 mg/kg (Adult vs. Periadolescent)
3.1.3.1. Ambulations
After 5 injections, both the PND 30 group (now 42 days of age) and the PND 60 group (now 72 days of age) showed rapid increases in response following drug administration. Although the response in the adolescent group was greater than that in the adults, the difference was considerably less than that seen following the first injection (compare Figs 1A and 3A). A 2-way mixed-model ANOVA of ambulations on Day 5 showed a significant effect of Time [F(11, 110)=9.234 p< .0001], but no effect of Group or Interaction (p>.05). Post-hoc analyses showed differences in ambulations between PND 30 and PND 60 animals at 40–50 minutes (p< .05) (Fig 3A). Total ambulations post-administration did not significantly differ between the two groups, although the adolescent group appeared to show a greater response (p> .05) (Fig 3B).
Figure 3.
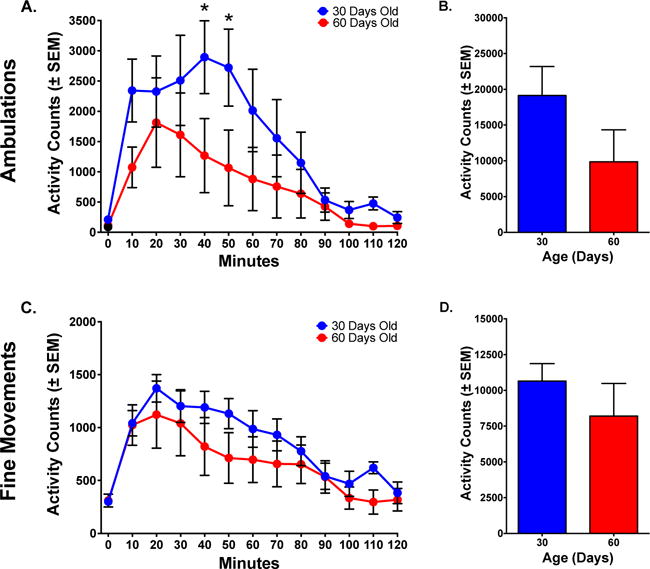
Locomotor response in adolescents and adults after five injections of PCP (6.0 mg/kg). (A) Time course of ambulations (horizontal activity). Ambulations in animals that began treatment at PND 30 were slightly higher than in animals that started at PND 60. (B) Total ambulations over the 120-minute session illustrate the similarities in activity between PND 30 and PND 60 animals. (C) Time course of fine movements (stereotypy). Fine Movements in animals that started at PND 30 were similar to animals that started at PND 60. (D) Total fine movements over the 120-minute time course illustrate the similarities in activity between PND 30 and PND 60 animals.
Asterisks reflect differences between PND 30 and PND 60 animals (p< .05).
3.1.3.2. Fine Movements
After 5 injections, the PND 30 group (now 42 days of age) and the PND 60 group (now 72 days of age) showed very similar responses following drug administration. A 2-way mixed-model ANOVA of fine movements showed a significant effect of Time [F(11,110)=18.67, p< .0001], but no Group effect or Interaction (p> .05) (Fig 3C). Analysis of the total response post-administration showed no significant difference between groups (p> .05) (Fig 3D).
3.1.4. Comparison of Day 1 and Day 5, PCP 6.0 mg/kg (Adult vs. Periadolescent)
3.1.4.1. Ambulations
A comparison of the first session (Day 1) to the fifth session (Day 5) showed an interesting pattern in adolescents. Day 1 ambulations remained moderately high throughout the session, while on Day 5 activity was significantly higher early in the session and showed reduced effects later in the session. A 2-way Repeated Measures ANOVA of PND 30 animals comparing Day 1 and Day 5 ambulations showed a significant effect of Time [F(11,55)=4.253 p<.0001] and an Interaction [F(11,55)=5.743 p< .0001], but no effect of Day (p> .05). Post-hoc analyses showed notable differences in the first 30 minutes post-injection (p< .05) (Fig 4A).
Figure 4.
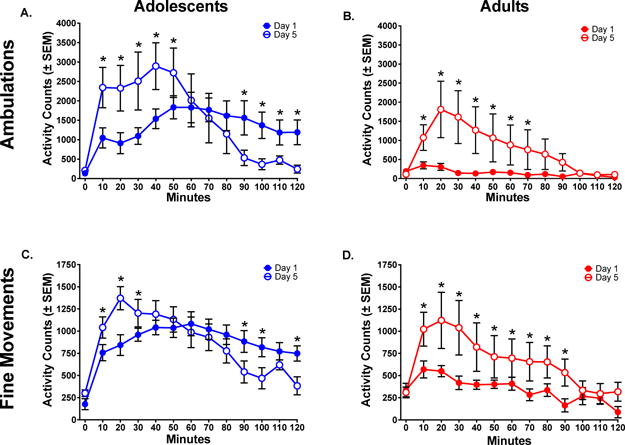
Changes in locomotor activity after five injections of PCP (6.0 mg/kg). (A) Time course of ambulations in adolescents. In animals that started at PND 30, PCP produced a shift in the time course of activity from Day 1 to Day 5. (B) Time course of ambulations in adults. In animals that started at PND 60, PCP produced increases in ambulations from Day 1 to Day 5, indicative of sensitization. (C) Time course of fine movements in adolescents. In animals that started at PND 30, PCP produced a shift in the time course of activity from Day 1 to Day 5. (D) Time course of fine movements in adults. In animals that started at PND 60, PCP produced increases in fine movements from Day 1 to Day 5, indicative of sensitization.
Asterisks reflect differences between PND 30 and PND 60 animals (p< .05).
In the adult group, ambulations showed a steep increase from the first session to the fifth session. A 2-way Repeated Measures ANOVA comparing Day 1 and Day 5 showed a significant effect of Time [F(11,55)=4.724 p< .0001] and an Interaction [F(11,55)=2.587 p< .0001], but no effect of Day. While there was not a main effect of Day, there were differences in simple effects. Post hoc analyses showed that Day 5 was consistently higher than Day 1 for the first 70 minutes post-injection (p< .05) (Fig 4B).
3.1.4.2. Fine Movements
Comparing Day 1 to Day 5 for fine movements in the adolescents showed modest differences across days. A 2-way Repeated Measures ANOVA showed a significant effect of Time [F(11,55)=10.78 p< .0001] and an Interaction [F(11, 55)=7.447 p< .0001], but no effect of Day (p> .05) (Fig 4C).
In the adult group, fine movements showed an increase from the first session (Day 1) to the fifth session (Day 5). A 2-way Repeated Measures ANOVA showed a significant effect of Time [F(11, 55)=13.25, p< .0001] and an Interaction [F(11,55)=2.130, p< .05], but no effect of Day (p> .05). While there was no main effect of Day, post-hoc analyses showed that Day 5 was consistently higher than Day 1 for 90 minutes post-injection (p< .05) (Fig 4D).
3.2. STUDY 2: Ketamine 20.0 mg/kg (Adult vs. Periadolescent)
3.2.1. Day 1
4.1.2.1. Ambulations
After the first injection of ketamine (20 mg/kg), PND 30 periadolescents showed the strongest locomotor response, with considerably less activity in the PND 38 and PND 60 animals. Ambulations in the PND 30 group peaked slightly later than both PND 38 and PND 60 groups, but at a much higher level. A 2-way mixed-model ANOVA showed significant effects for Group, Time and Interaction [Group: F(2, 15)=11.77, p<.001; Time: F(14, 210)=17.06, p< .0001; Group × Time: F(28, 210)=2.877, p< .0001]. Post-hoc analyses showed differences in ambulations between PND 30 and PND 60 groups at 4–14 minutes and between PND 30 and PND 38 groups at 4–12 minutes post-administration, p< .05. Ambulations were not significantly different between PND 38 and PND 60 groups, p> .05 (Fig 5A). Since the ketamine response was so short-lived further analyses focused on the first 10 minutes post-administration. During this timeframe, PND 30 animals showed a response that was more than double that of PND 38 and PND 60 groups F(2,15)=10.63, p< .005; PND 38 animals were similar to PND 60 animals (p> .05) (Fig 5B).
Figure 5.
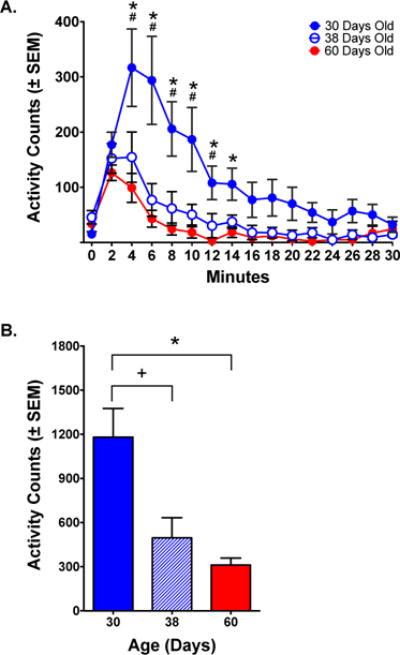
Ambulations (horizontal locomotion) in response to ketamine (20.0 mg/kg). (A) Time course of response. Activity was highest for PND 30 animals and lowest for PND 60 animals. PND 38 activity approximated that of PND 60 animals. (B) Total ambulations during the first 10 minutes post-administration. There were significant differences between the PND 30 group and either PND 38 or PND 60 groups, but no differences between PND 38 and PND 60 groups.
Symbols reflect differences between PND 30 and PND 60 animals (*, p< .05); PND 38 and PND 60 animals (+, p< .05); PND 30 and PND 38 animals (#, p< .05); Fisher’s test.
4.1.2.2 Fine Movements
The pattern for fine movements was similar to that seen for ambulations. After the first injection of ketamine, activity was highest for PND 30 periadolescents and showed a similar low response in PND 38 and PND 60 animals. The PND 30 group peaked later than both PND 38 and PND 60 groups, but at a higher level. A 2-way mixed-model ANOVA showed significant effects for Group, Time and Interaction [Group: F(2, 15)=4.710, p< .05; Time: F(14, 210)=36.87, p<.0001; Group × Time: F(28, 210)=3.227, p< .0001]. Post-hoc analyses showed differences between PND 30 and PND 60 groups between 2–12 minutes and between PND 30 and PND 38 groups between 2–12 minutes (p< .05); PND 38 and PND 60 groups did not differ (p> .05) (Fig 6A). Analysis of the total response during the first 10 minutes post-administration showed that PND 30 animals were highest and significantly greater than the other groups, F(2, 15)=3.119, p<.05; activity in PND 38 and PND 60 groups did not differ (p> .05) (Fig 6B).
Figure 6.
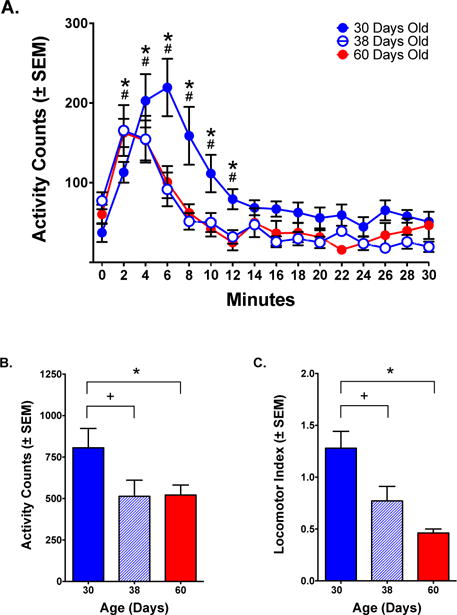
Fine movements (stereotypy) in response to ketamine (20.0 mg/kg). (A) Time course of response. Activity was highest for PND 30 animals and lowest for PND 38 and PND 60 animals. PND 38 and PND 60 animals closely followed the same pattern of behavior. (B) Total fine movements during the first 10 minutes post-administration. There were significant differences between the PND 30 group and either PND 38 or PND 60 groups, but no difference between the PND 38 and PND 60 groups. (C) Locomotor index (ambulations/fine movements) determined the relative types of movement induced by ketamine. PND 30 animals showed a bias toward horizontal locomotion reflected in a numerical value greater than 1.0, while PND 38, and to a greater extent, PND 60 animals showed a bias toward stereotypy reflected in a value less than 1.0.
Significant differences between PND 30 and PND 60 animals (*, p< .05); PND 38 and PND 60 animals (+, p< .05); PND 30 and PND 38 animals (#, p< .05); Fisher’s test.
4.1.2.3. Locomotor Index
Only PND 30 animals showed a locomotor index (ambulations/fine movements) greater than 1.0, indicative of more horizontal activity relative to stereotypy. The PND 38 and PND 60 groups were considerably less than 1.0. PND 60 animals showed the greatest bias toward stereotypy. A 1-way ANOVA showed differences in the locomotor index across groups [F(2, 15)=10.68, p= 0.0013]. Post-hoc analyses showed a significant difference between PND 30 animals and either PND 38 or PND 60 (p< .05), but no differences between PND 38 and PND 60, p > .05 (Fig 6C).
4.1.3. Day 5, Ketamine 20.0 mg/kg (Adult vs. Periadolescent)
4.1.3.1. Ambulations
On Day 5, adolescents (now 42 days of age) and adults (now 72 days of age) showed a rapid increase in ambulations that peaked at 4 minutes post-administration. A 2-way mixed-model ANOVA showed a significant effect of Time [Time: F(14, 140)=24.40, p< .0001], but no effect of Group or Interaction, p< .05. Post-hoc analyses showed greater ambulations in PND 30 animals than PND 60 animals at 4–6 minutes post-administration (p< .01) (Fig 7A). In comparing adolescents and adults during the first 10 minutes post-administration, it appears that the adolescent group was more active than the adult group, however the difference did not achieve statistical significance (p> .05), (Fig 7B).
Figure 7.
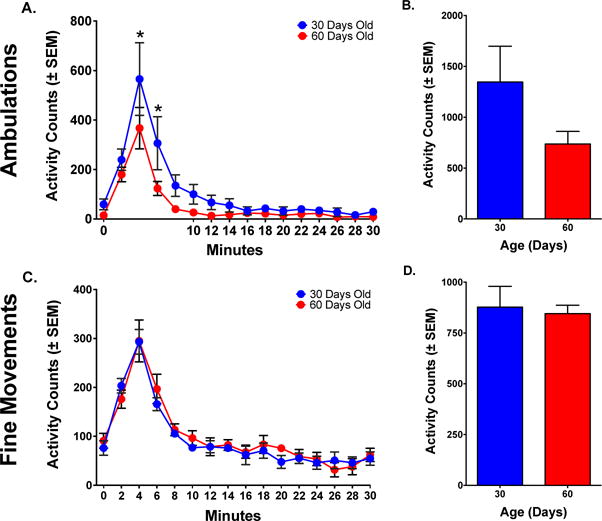
Locomotor response in adolescents and adults after five injections of ketamine (20.0 mg/kg). (A) Time course of ambulations (horizontal activity). Ambulations in animals that started at PND 30 were higher than in animals that started at PND 60. (B) Total ambulations over the first 10 minutes post-administration illustrate differences in activity between PND 30 and PND 60 animals that do not reach statistical significance. (C) Time course of fine movements (stereotypy). Fine movements in animals that started at PND 30 were similar to animals that started at PND 60. (D) Total fine movements during the first 10 minutes post-administration illustrate the similarities in activity between PND 30 and PND 60 animals.
Asterisks reflect differences between PND 30 and PND 60 animals (p< .05).
4.1.3.2. Fine Movements
After 5 injections, the PND 30 group (now 42 days of age) and the PND 60 group (now 72 days of age) showed virtually identical responses following drug administration. A 2-way mixed-model ANOVA showed a main effect of Time [F(14, 140)=48.04, p< .0001], but no effect of Group or an Interaction, (p> .05), and no differences as determined by post-hoc analyses (p> .05) (Fig 7C). Comparison of fine movements for the first 10 minutes post-administration further showed no significant difference between adolescents and adults (p> .05), (Fig 7D).
4.1.4. Comparison of Day 1 and Day 5, Ketamine 20.0 mg/kg (Adult vs. Periadolescent)
4.1.4.1. Ambulations
Comparing Day 1 to Day 5 in the adolescents, there appeared to be little difference following repeated administration. A 2-way Repeated Measures ANOVA of the first 10 minutes post-injection showed a significant effect of Time [F(14, 70)=56.04, p< .0001], but no Interaction or Day effect (p> .05). Post-hoc analyses showed greater ambulations on Day 5 than on Day 1 at 4 minutes post-injection (p< .01) (Fig 8A).
Figure 8.
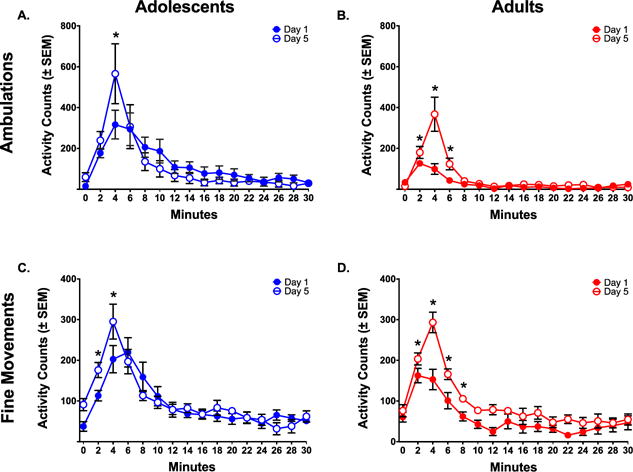
Changes in locomotor activity following five administrations of ketamine (20.0 mg/kg) (A) Time course of ambulations in adolescents. In animals that started at PND 30, ketamine produced little change in ambulations from Day 1 to Day 5. (B) Time course of ambulations in adults. In animals that started at PND 60, ketamine produced increases in ambulations from Day 1 to Day 5, indicative of sensitization. (C) Time course of fine movements in adolescents. In animals that started at PND 30, ketamine produced little change in fine movements from Day 1 to Day 5. (D) Time course of fine movements in adults. In animals that started at PND 60, ketamine produced increases in fine movements from Day 1 to Day 5, indicative of sensitization. Activity was consistently higher at more time points for PND 60 animals compared to PND 30 animals, indicative of more potent sensitization.
Asterisks reflect differences between PND 30 and PND 60 animals (p< .05).
In the adult group, there was a potent increase in ambulations from Day 1 to Day 5. A 2-way Repeated Measures ANOVA of the first 10 minutes post-injection showed a significant effect of Time [F(14,70)=19.43, p< .0001], of Day [F(1,5)=23.74, p< .005], and an Interaction [F(14,70)=9.263, p< .0001]. Post-hoc analyses showed higher ambulations on Day 5 than on Day 1 from 2–6 minutes post-injection (p< .05) (Fig 8B).
4.1.4.2. Fine Movements
Comparing Day 1 to Day 5 in the adolescents, there appeared to be little difference following repeated administration. A 2-way Repeated Measures ANOVA of the first 10 minutes post-injection showed a significant effect of Time [F(14, 70)=45.13 p< .0001] and an Interaction [F(14, 70)=2.429, p<.01], but no effect of Day (p> .05). Post-hoc analyses showed higher fine movements on Day 5 than on Day 1 between 2–4 minutes post-injection (p< .01) (Fig 8C).
In the adult group, there was a potent increase in fine movements from Day 1 to Day 5. A 2-way Repeated Measures ANOVA of the first 10 minutes post injection showed a significant effect of Time [F(14,70)=31.45, p< .0001], a significant effect of Day [F(1,5)=43.26, p< .05], and an Interaction [F(14,70)=3.231, p< .001]. Post-hoc analyses showed greater fine movements on Day 5 than on Day 1 between 2–8 minutes post-injection (p< .05) (Fig 8D).
5. Discussion
The present studies compared the stimulant effects of phencyclidine or ketamine, and sensitization to these drugs, in adolescents and adults. Since previous work demonstrated reduced locomotor stimulation in adolescents to psychostimulants such as cocaine (Laviola et al., 1995; Izenwasser and French, 2002; Wiley et al, 2008; Zombeck et al., 2009) and methamphetamine (Zombeck et al., 2009), it was hypothesized that periadolescent animals would exhibit reduced stimulant effects to PCP or ketamine when compared to adults. Surprisingly, young periadolescent animals (PND 30) exhibited a greater stimulant response to an acute administration of either PCP or ketamine when compared to older adolescents (PND 38) or adults (PND 60). PCP- or ketamine-induced activity in the older adolescent (PND 38) animals approximated the activity observed in adults, indicating maturation in response to these drugs between PND 30 and 38 of adolescence. Previous research has demonstrated a reduced response to psychomotor stimulants in adolescents, when compared to adults (Laviola et al., 1999; Spear, 2000). Because PCP and ketamine at the doses used in the present experiments produce psychomotor stimulation, we expected that differences between adolescents and adults would be similar to those obtained with amphetamine, cocaine and related drugs.
The finding that animals at 38 days of age begin to approximate behavior of adults at 60 days of age narrows the range at which adolescents transition to an adult-like response. Following an acute injection of PCP, PND 38 adolescents initially peaked at levels similar to PND 30 animals, but then decreased and approximated lower patterns of activity observed in adults halfway through the testing session. Similarly, following an acute injection of ketamine, an adult-like response emerged by 38 days of age. Adolescent PND 38 and adult PND 60 animals paralleled each other in overall response and behavioral patterns.
Dissociative drugs are not typically used daily, but instead are used on occasion by people at raves and party settings. The present study used intermittent administration to model an intermittent pattern of human use (Trujillo et al., 2008). It is possible that repeated daily administration would produce different results. Of relevance to this point, Venniro et al. (2015) demonstrated that resistance to extinction of ketamine self-administration is greater with intermittent sessions than with daily sessions. It is unclear if adolescents and adults would differ in sensitization following daily versus intermittent administration.
A comparison of locomotor activity after an acute (Day 1) injection and after 5 intermittent (Day 5) injections reflects an increase in behavior, indicative of sensitization in adults, but no increase in activity in adolescents. At first glance the results suggest that adolescents are not as susceptible to sensitization as adults. However, an alternative interpretation is that sensitization in adolescents is masked by maturational processes that diminish the response to PCP and ketamine. By the fifth injection, the periadolescent group was 42 days of age, older than the PND 38 animals tested on Day 1, and yet shows a response similar to PND 30 animals. In the absence of sensitization, at 42 days of age their activity in response to an acute dose of PCP or ketamine should have been similar to PND 38 and adult animals. The fact that the activity remained very high, equivalent to their response as young adolescents and similar to sensitized adults, reflects a sensitized response relative to the response expected of animals at 42 days of age. This leads us to conclude that the young adolescents were indeed sensitized by repeated administration of the dissociatives.
In addition to measuring ambulations and fine movements, a ratio of these two measures of activity was calculated. The locomotor index served as an assessment of the relationship between horizontal activity (ambulations) and stereotypy (fine movements). A higher locomotor index indicates more horizontal locomotion in relation to stereotypy, while a lower locomotor index indicates more stereotypy in relation to horizontal activity. After an acute administration of PCP or ketamine, PND 30 animals showed the highest Locomotor Index and PND 60 animals showed the lowest Locomotor Index. Thus, consistent with our qualitative observations during the experiments, adolescents had more horizontal locomotion and less dissociative-induced stereotypy and ataxia than adults. Considering potential clinical implications, the greater stimulant response may reflect more reinforcing aspects of drug response, since the brain substrates of locomotor stimulation are closely tied to those of reward (Wise and Bozarth, 1987; Li and Markou, 2015; Trujillo et al., 2006; Watson et al., 1989). On the other hand, stereotypy and ataxia may be more indicative of negative side-effects of the dissociatives. If this is the case, then the subjective experience in adolescence may be more positive and less negative than in adults, a pattern that has been reported for nicotine (Adriani et al., 2003; Bjork et al., 2004; Chambers et al., 2003; Dahl, 2004; Dawes et al., 2000; Jamner et al., 2003; Laviola et al., 1999; O’Dell et al., 2006; Smith, 2003; Spear 2000, 2004; Witt, 1994; Yuan et al., 2015). This may help to explain why dissociatives are particularly attractive to adolescents.
Parise et al. (2013) obtained results consistent with the current finding that adolescents produce a greater locomotor response to ketamine (20 mg/kg) than adults. On the other hand, Wiley et al. (2008) reported reduced stimulant effects of ketamine in adolescents, and Wilson et al. (2007) reported negligible differences in ketamine-induced locomotor activity between adults and adolescents (both used a 10mg/kg dose). Consistent with our results and those of Parise et al. (2013), Pesic and coworkers (2010), found that an acute dose of MK-801 (a highly potent and selective NMDA receptor antagonist), induced a greater increase in locomotor behavior in adolescents compared to adults. Differences in experimental approaches, including dose, time course of testing, habituation, animal strain, specific ages of the animals and other variables may be responsible for the differing results. Thus, although there have been some differences reported, there is increasing evidence that dissociatives produce a greater locomotor response in adolescents at low to moderate doses (Garcia and Trujillo, 2007a; Garcia and Trujillo, 2007b; Hartfield and Trujillo, 2006; Heller and Trujillo, 2007; Sullivan and Trujillo, 2007a; Sullivan and Trujillo, 2007b; Trujillo et al., 2006; Trujillo and Warmoth, 2005).
Related to the current findings, there are other critical differences in response to dissociatives between adolescents and adults. When awakening from ketamine anesthesia, adult patients often experience an emergence reaction characterized by bad dreams and psychotomimetic effects, while this is less prominent in children or adolescents (Cho et al., 2014; Green et al., 2009; Green et al., 2011). Similarly, while adult laboratory rats show neurotoxic effects of high doses of dissociatives, this is not seen in young adolescent animals (Farber et al., 1995; Farber and Olney, 2003; Noguchi et al., 2005; Olney and Farber, 1995). Moreover, antidepressant effects of ketamine are seen in adult rats, but not in adolescents (Nosyreva et al., 2014). The differences in responses to PCP and ketamine at the different ages offers potential insight into the neurobiological systems that underlie the effects of these drugs, suggesting that glutamatergic NMDA receptors and/or downstream systems rapidly mature during adolescence. For example, NMDA receptors increase during early development, peak in adolescence and then begin to decline, reaching a stable number in adulthood (Colwell, et al., 1998; Insel et al., 1990; McDonald et al., 1990; Simeone et al., 2004; Spear, 2000; Henson et al., 2008; Luo et al., 1996). It is notable that changes in locomotor response to PCP and ketamine parallel changes in receptors. The greater concentration of NMDA receptors in adolescence may allow for more pronounced effects of the antagonists.
Other developmental differences in glutamatergic function during adolescence have been found that may contribute to the greater response in adolescents to the dissociatives. For example, electrophysiological parameters related to NMDA receptor/dopamine interactions in the forebrain are immature in early adolescence, and the adult pattern does not emerge until late adolescence (Flores-Barrera et al., 2014; O’Donnell, 2011; Tseng and O’Donnell, 2005; Tseng et al., 2007; Wahlstrom et al., 2010). In addition, differences in other neurotransmitter systems in adolescence have been identified that may also contribute (Andersen and Navalta, 2004; Monti et al., 2005; Smith 2003; Spear 2000, 2004, Yuan et al., 2015). Given the many systems that are changing during adolescence, it is not surprising that psychoactive drugs would produce different behavioral responses during this vulnerable developmental phase (Andersen and Navalta, 2004; Collins and Izenwasser, 2004; Laviola et al, 1999; Monti et al., 2005; Spear 2000, 2004, Yuan et al., 2015).
An alternative explanation that needs to be considered in the differences between adolescents and adults is pharmacokinetic factors (such as differences in drug metabolism). For example, the current findings could potentially be explained by reduced metabolism of the dissociatives in adolescents, resulting in higher brain concentrations of the drugs. However, evidence suggests that differences in response across age groups are not simply due to metabolism or to differences in brain concentrations of drug. Although relatively little work has been done exploring differences in pharmacokinetics of psychoactive drugs between adolescents and adult laboratory animals, there is some relevant work. Similar to research in humans showing more rapid elimination of a variety of drugs in adolescents when compared to adults (de Wildt et al., 1999; Geller, 1991; Kearns, 2000; Vitiello and Jensen, 1995), evidence shows lower plasma levels of cocaine (McCarthy et al., 2004), nicotine (O’Dell et al., 2006; Trauth et al., 2000) and ethanol (Little et al., 1996) following administration to adolescents in animals. Although similar work has not yet been completed for ketamine or PCP, lower levels of ketamine are seen in young adult rats, when compared to older adults (Veilleux-Lemieux et al., 2013). Thus, there is a general tendency for adolescents (both humans and laboratory animals) to have lower blood levels of psychoactive drugs than adults following equivalent doses. In this regard, research in humans has revealed lower plasma levels and greater dose requirements for ketamine in younger individuals (Grant et al., 1983; Lockhart and Nelson, 1974). If this holds for PCP, the greater effect in periadolescents occurs despite reduced levels of the compounds. Of importance to this discussion, other behavioral responses to ketamine are reduced in adolescents when compared to adults. For example, ketamine anesthesia emergence reactions (Cho et al., 2014; Green et al., 2009; Green et al., 2011), antidepressant effects of ketamine (Nosyreva et al., 2014) and ketamine-induced conditioned taste aversion (Gochez et al., 2012) are reduced in adolescence. A pharmacokinetic difference could not easily explain why some dissociative-induced responses are greater in adolescents than adults, while others are reduced. Further research will clarify the potential role of pharmacokinetics in the differences between adolescents and adults in response to these dissociatives.
Age differences in behavioral endpoints of drug administration are of interest in the scientific community as they offer potential strategies for prevention and treatment of drug abuse and addiction. Once drug use is initiated, it often continues and transitions to addiction in older populations. It remains unclear whether adolescents are more resilient or more vulnerable to the neurobiological impacts of psychoactive drugs. Neuroanatomically, during adolescence, the prefrontal cortex is not fully developed, the limbic system is increasing in volume, and the dopamine fiber density peaks and begins to decline to adult levels. Repeated use of psychoactive drugs is likely to alter these circuits in ways that can put the individual at risk for later drug dependence (Andersen and Sonntag, 2014; Crews et al., 2007; Volkow and Li, 2004; Yuan et al., 2015).
Because use of club drugs, including PCP and ketamine, is prevalent in teens and young adults (Bachman et al., 2011), it is critical to gain a better understanding of how use of these drugs during adolescence may differ from that in adults. The current results suggest that adolescents experience greater stimulant effects of PCP and ketamine, and that they are sensitized by repeated use.
Highlights.
Drug-induced locomotor stimulation and sensitization are closely tied to reward and addiction.
PCP or ket produce stronger stimulation in adolescent than adult rats after a single injection.
Following either repeated PCP or ket, adults sensitize more than adolescent rats.
Highlights, PBB_2017_64.
Ketamine and phencyclidine are abused by young people
Behavioral effects of the drugs were compared in adolescent and adult rats
Young adolescents showed the greatest initial response to the drugs
Behavioral sensitization occurred and differed between adolescents and adults
The results have implications for drug abuse and addiction
Acknowledgments
Nigel Hart (Reginald Hartfield) lost his battle to cancer on June 2, 2012 while a doctoral student in the Department of Psychology at Texas A&M University. Nigel conducted the present studies in partial fulfillment of the requirements for his master’s degree in the Department of Psychology at California State University San Marcos. Nigel passed away before the preparation of this manuscript; however, his contributions are significant and include study design, data collection and interpretation, and earlier writings of this work. This research was supported by the National Institute of General Medical Sciences (GM 64783 and GM 81069), the National Science Foundation (HRD 1302873), and the Office for Training, Research, and Education in the Sciences. We would like to thank Colleen Heller for expert technical assistance.
Abbreviations
- ANOVA
analysis of variance
- IP
intraperitoneal
- ket
ketamine
- PCP
phencyclidine
- PND
postnatal day
- NMDA
N-Methyl-D-aspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Abekawa T, Honda M, Ito K, Inoue T, Koyama T. Effect of MS-153 on the development of behavioral sensitization to locomotion- and ataxia-inducing effects of phencyclidine. Psychopharmacology. 2002;160:122–131. doi: 10.1007/s00213-001-0958-1. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23(11):4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22(5–6):423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Sonntag KC. Juvenile methylphenidate reduces prefrontal cortex plasticity via D3 receptor and BDNF in adulthood. Front Synaptic Neurosci. 2014;6:1–8. doi: 10.3389/fnsyn.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40(1):9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Bachman JG, Staff J, O’Malley PM, Schulenberg JE, Freedman-Doan P. Twelfth-grade student work intensity linked to later educational attainment and substance use: new longitudinal evidence. Dev Psychol. 2011;47(2):344–363. doi: 10.1037/a0021027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DcW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani S, Adams PM. Acute and chronic phencyclidine effects on locomotor activity, stereotypy and ataxia in rats. Eur J of Pharmacol. 1981;73(2–3):143–54. doi: 10.1016/0014-2999(81)90086-8. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HK, Kim KW, Jeong YM, Lee HS, Lee YJ, Hwang SH. Efficacy of ketamine in improving pain after tonsillectomy in children: meta-analysis. PloS One. 2014;9(6):e101259. doi: 10.1371/journal.pone.0101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Cepeda C, Crawford C, Levine MS. Postnatal development of glutamate receptor-mediated responses in the neostriatum. Dev Neurosci. 1998;20(2–3):154–163. doi: 10.1159/000017310. [DOI] [PubMed] [Google Scholar]
- Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 1966;45(1):29–40. [PubMed] [Google Scholar]
- Crews G, He J, Hodge C. Adolescent cortical development: A critical period of vulnerabilty for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann NY Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dale E, Bang-Andersen B, Sánchez C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol. 2015;95(2):81–97. doi: 10.1016/j.bcp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Antelman SM, Vanyukov MM, Giancola P, Tarter RE, Susman EJ, Mezzich A, Clark DB. Developmental sources of variation in liability to adolescent substance use disorders. Drug Alcohol Depend. 2000;61(1):3–14. doi: 10.1016/s0376-8716(00)00120-4. [DOI] [PubMed] [Google Scholar]
- de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37(6):485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend. 2003;69:23–28. doi: 10.1016/s0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Groman SM, Jentsch JD, Leranth C, Redmond DE, Kim JD, Diano S, Roth RH. Primate phencyclidine model of schizophrenia: sex-specific effects on cognition, brain derived neurotrophic factor, spine synapses, and dopamine turnover in prefrontal cortex. Int J Neuropsychopharmacol. 2015;18(6):pyu048. doi: 10.1093/ijnp/pyu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, Olney JW. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38(12):788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Farber N, Olney JW. Drugs of abuse that cause developing neurons to commit suicide. Dev Brain Res. 2003;147(1–2):37–45. doi: 10.1016/j.devbrainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry. 2014;75(6):508–516. doi: 10.1016/j.biopsych.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Trujillo KA. Club drug interactions: Effects of ketamine and methamphetamine combinations on locomotor behavior in rats. Society Neurosci Abstracts. 2007a Abstract #813.15. [Google Scholar]
- Garcia C, Trujillo KA. The effects of ketamine and methamphetamine on locomotor behavior in rats. California State University Statewide Student Research Competition 2007b [Google Scholar]
- Geller B. Psychopharmacology of children and adolescents: pharmacokinetics and relationships of plasma/serum levels to response. Psychopharmacol Bull. 1991;27(4):401–409. [PubMed] [Google Scholar]
- Gochez A, Gonzalez CG, Rocha A, Trujillo KA. Adolescents and adults differ in ketamine-induced conditioned taste aversion. Society Neurosci Abstract. 2012 Abstract #258.04. [Google Scholar]
- Grant IS, Nimmo WS, McNicol LR, Clements JA. Ketamine disposition in children and adults. Br J Anaesth. 1983;55(11):1107–1111. doi: 10.1093/bja/55.11.1107. [DOI] [PubMed] [Google Scholar]
- Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57(5):449–461. doi: 10.1016/j.annemergmed.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Green SM, Roback MG, Krauss B, Brown L, McGlone RG, Agrawal D, McKee M, Weiss M, Pitetti RD, Hostetler MA, Wathen JE, Treston G, Garcia Pena BM, Gerber AC, Losek JD. Emergency Department Ketamine Meta-Analysis Study Group Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54(2):158–168e4. doi: 10.1016/j.annemergmed.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Hartfield N, Trujillo KA. Behavioral sensitization of PCP (“Angel dust”): Effects of interval between injection and environment. California State University Statewide Student Res Competition 2006 [Google Scholar]
- Heller CY, Trujillo KA. Sensitization to ketamine (“Special K”): Effects of injection interval and environmental conditioning. Society Neurosci Abstracts. 2007 Abstract #813.3. [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog FL, Philpot BD. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex. 2008;18(11):2560–2573. doi: 10.1093/cercor/bhn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer C, Mendelson B, Van Leeuwen JM, Kelly S, Hooks S. Club Drug Use among Youths in Treatment for Substance Abuse. Am J Addictions. 2006;15(1):94–99. doi: 10.1080/10550490500419144. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain—I.N-methyl-d-aspartate and quisqualate receptors. Neurosci. 1990;35(1):31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, French D. Tolerance and sensitization to the locomotor-activating effects of cocaine are mediated via independent mechanisms. Pharmacol Biochem Behav. 2002;73(4):877–882. doi: 10.1016/s0091-3057(02)00942-5. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Comparison of the pharmacologic effects of N-allylnormetazocine and phencyclidine: sensitization, cross-sensitization, and opioid antagonist activity. Psychopharmacology. 1986;89:221–229. doi: 10.1007/BF00310633. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Whalen CK, Loughlin SE, Mermelstein R, Audrain-McGovern J, Krishnan-Sarin S, Worden JK, Leslie FM. Tobacco use across the formative years: a road map to developmental vulnerabilities. Nicotine Tob Res. 2003;5(Suppl 1):S71–87. doi: 10.1080/14622200310001625573. [DOI] [PubMed] [Google Scholar]
- Jansen KL. A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs. 2000;32(4):419–433. doi: 10.1080/02791072.2000.10400244. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: Evidence for cognitive impulsivity. Biol Psychiatry. 2000;48:415–424. doi: 10.1016/s0006-3223(00)00926-4. [DOI] [PubMed] [Google Scholar]
- Jouguelet-Lacoste J, La Colla L, Schilling D, Chelly JE. The use of intravenouse infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med. 2015;16(2):383–403. doi: 10.1111/pme.12619. [DOI] [PubMed] [Google Scholar]
- Kearns GL. Impact of developmental pharmacology on pediatric study design: overcoming the challenges. J Allergy Clin Immunol. 2000;106(3 Suppl):S128–38. doi: 10.1067/mai.2000.109419. [DOI] [PubMed] [Google Scholar]
- Kleinloog D, Uit den Boogaard A, Dahan A, Mooren R, Klaassen E, Stevens J, Freijer J, vanGerven J. Optimizing the glutamatergic challenge model for psychosis, using S (+)-ketamine to induce psychotomimetic symptoms in healthy volunteers. J Psychopharmacol. 2015;29(4):401–413. doi: 10.1177/0269881115570082. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–58. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23(7):993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275(1):345–57. [PubMed] [Google Scholar]
- Li X, Markou A. Metabotropic glutamate receptor 7 (mGluR7) as a target for the treatment of psychostimulant dependence. CNS Neurol Disord Drug Targets. 2015;14(6):738–744. doi: 10.2174/1871527314666150529145332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20(8):1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Lockhart CH, Nelson WL. The relationship of ketamine requirement to age in pediatric patients. Anesthesiol. 1974;40(5):507–508. doi: 10.1097/00000542-197405000-00020. [DOI] [PubMed] [Google Scholar]
- Luo J, Bosy TZ, Wang Y, Yasuda RP, Wolfe BB. Ontogeny of NMDA R1 subunit protein expression in five regions of rat brain. Dev Brain Res. 1996;92(1):10–17. doi: 10.1016/0165-3806(95)00191-3. [DOI] [PubMed] [Google Scholar]
- McCarthy LE, Mannelli P, Niculescu M, Gingrich K, Unterwald EM, Ehrlich ME. The distribution of cocaine in mice differs by age and strain. Neurotoxicol Teratol. 2004;26(6):839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV, Young AB. Differential ontogenic development of three receptors comprising the NMDA receptor/channel complex in the rat hippocampus. Exp Neurol. 1990;110(3):237–247. doi: 10.1016/0014-4886(90)90035-q. [DOI] [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains and behavior. Alcohol Clin Exp Res. 2005;29(2):207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Perry EB, Cho HS, Krystal JH, D’Souza DC. Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology. 187(4):405–414. doi: 10.1007/s00213-006-0409-0. 2006. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Fukaya H, Yamaguchi K, Ishikawa K, Furukawa H, Kameyama T. Development of tolerance and supersensitivity to phencyclidine in rat safter repeated administration of phencyclidine. Eur J Pharmacol. 1987;135(1):23–33. doi: 10.1016/0014-2999(87)90753-9. [DOI] [PubMed] [Google Scholar]
- Nalini V, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298–306. doi: 10.4103/0970-9185.168149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi KK, Nemmers B, Farber NB. Age has a similar influence on the susceptibility to NMDA antagonist-induced neurodegeneration in most brain regions. Dev Brain Res. 2005;158(1–2):82–91. doi: 10.1016/j.devbrainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Autry AE, Kavalali ET, Monteggia LM. Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Front Mol Neurosci. 2014;7:94. doi: 10.3389/fnmol.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186(4):612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37(3):484–92. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology. 1995;13(4):335–345. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK, Kroeck KG, Iñiguez SD, Bolaños-Guzmán CA. Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol Psychiatry. 2013;74(10):750–759. doi: 10.1016/j.biopsych.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesić V, Popić J, Milanović D, Loncarević-Vasiljković N, Rakić L, Kanazir S, Ruzdijić S. The effect of MK-801 on motor activity and c-Fos protein expression in the brain of adolescent Wistar rats. Brain Res. 2010;1321:96–104. doi: 10.1016/j.brainres.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Phillips M, Wang C, Johnson KM. Pharmacological characterization of locomotor sensitization induced by chronic phencyclidine administration. J Pharmacol Exp Ther. 2001;296:905–913. [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction (Abingdon, England) 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Rev of Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22(2):347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- SAMHSA, Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. (HHS Publication No. SMA 15-4927, NSDUH Series H-50).Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 Retrieved from http://www.samhsa.gov/data/
- Scalzo FM, Holson RR. The ontogeny of behavioral sensitization to phencyclidine. Neurotoxicol Teratol. 1992;14:7–14. doi: 10.1016/0892-0362(92)90023-4. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol. 2004;19(5):343–360. doi: 10.1177/088307380401900507. discussion 361. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25(3):291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiology. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Sullivan B, Trujillo KA. Sensitization to ketamine in a ‘bump’ model: Robust effect following different patterns of administration. Society Neurosci Abstracts. 2007a Abstract #813.13. [Google Scholar]
- Sullivan B, Trujillo KA. Sensitization to PCP and ketamine following weekly injections. California State University Statewide Student Res Competition 2007b [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867(1–2):29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Singh L, Oles RJ, Preston C, Iversen SD. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989;167(1):127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. The neurobiology of opiate tolerance, dependence and sensitization: mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotox Res. 2002;4(4):373–391. doi: 10.1080/10298420290023954. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Excitatory amino acids and drugs of abuse: a role for N-methyl-D-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend. 1995;38(2):139–154. doi: 10.1016/0376-8716(95)01119-j. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Hartfield N, Heller C, Sullivan B, Warmoth KP. Sensitization to ketamine (“Special K”) following weekly administration: implications for “rave” use. Society for Neurosci Abstracts. 2006 Abstract #394.22. [Google Scholar]
- Trujillo KA, Smith ML, Sullivan B, Heller CY, Garcia C, Bates M. The neurobehavioral pharmacology of ketamine: implications for drug abuse, addiction, and psychiatric disorders. Institute for Laboratory Animal Research. 2011;52(3):366–378. doi: 10.1093/ilar.52.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Warmoth KP. Adolescent rats are more sensitive than adults to the acute and chronic behavioral effects of phencyclidine (PCP) Society for Neurosci Abstracts. 2005 Abstract #1031.11. [Google Scholar]
- Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63(2):178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62(7):730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15(1):49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Uchihashi Y, Kuribara H, Morita T, Fujita T. The repeated administration of ketamine induces an enhancement of its stimulant action in mice. Japanese J Pharmacol. 1993;61(2):149–151. doi: 10.1254/jjp.61.149. [DOI] [PubMed] [Google Scholar]
- Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2013;52(5):567–570. [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Mutti A, Chiamulera C. Pharmacological and non-pharmacological factors that regulate the acquisition of ketamine self-administration in rats. Psychopharmacology. 2015;232:4505–4515. doi: 10.1007/s00213-015-4077-9. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Jensen PS. Developmental perspectives in pediatric psychopharmacology. Psychopharmacol Bull. 1995;31(1):75–81. [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nature Rev Neurosci. 2004;5(12):963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cognition. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Trujillo KA, Herman JP, Akil H. Neuroanatomical and neurochemical substrates of drug-seeking behavior: overview and future directions. In: Goldstein A, editor. Mol Cellular Aspects Drug Addictions. Springer Verlag; New York: 1989. pp. 29–91. [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51(1–2):141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- White JM, Ryan CF. Pharmacological properties of ketamine. Drug Alcohol Rev. 1996;15(2):145–155. doi: 10.1080/09595239600185801. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. Age-dependent differences in sensitivity and sensitization to cannabinoids and ‘club drugs’ in male adolescent and adult rats. Addiction Biology. 2008;13(3–4):277–286. doi: 10.1111/j.1369-1600.2007.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A. Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav. 2007;91(2–3):202–7. doi: 10.1016/j.physbeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biology. 1994;62(3):168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Domino EF. Genetic differences in the locomotor response to single and daily doses of phencyclidine in inbred mouse strains. Behav Pharmacol. 1994a;5:623–629. doi: 10.1097/00008877-199410000-00008. [DOI] [PubMed] [Google Scholar]
- Xu X, Domino EF. Phencyclidine-induced behavioral sensitization. Pharmacol Biochem Behav. 1994b;47:603–608. doi: 10.1016/0091-3057(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Xu K, Krystal JH, Ning Y, Chen DC, He H, Wang D, Ke X, Zhang X, Ding Y, Liu Y, Gueorguieva R, Wang Z, Limoncelli D, Pietrzak RH, Petrakis IL, Zhang X, Fan N. Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatric Res. 2015;61:64–72. doi: 10.1016/j.jpsychires.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–3412. doi: 10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Domino EF. A Further Study on Asymmetric Cross-Sensitization Between MK-801 and Phencyclidine-Induced Ambulatory Activity. Pharmacol Biochem Behav. 1999;63(3):413–416. doi: 10.1016/s0091-3057(99)00004-0. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives General Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology. 2009;201(4):589–599. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]


