Abstract
Cell fragments devoid of the nucleus and major organelles are found in physiology and pathology, for example platelets derived from megakaryocytes, and cell fragments from white blood cells and glioma cells. Platelets exhibit active chemotaxis. Fragments from white blood cells display chemotaxis, phagocytosis, and bactericidal functions. Signaling mechanisms underlying migration of cell fragments are poorly understood. Here we used fish keratocyte fragments and demonstrated striking differences in signal transduction in migration of cell fragments and parental cells in a weak electric field. cAMP or cGMP agonists completely abolished directional migration of fragments, but had no effect on parental cells. The inhibition effects were prevented by pre-incubating with cAMP and cGMP antagonists. Blocking cAMP and cGMP downstream signaling by inhibition of PKA and PKG also recovered fragment galvanotaxis. Both perturbations confirmed that the inhibitory effect was mediated by cAMP or cGMP signaling. Inhibition of cathode signaling with PI3K inhibitor LY294002 also prevented the effects of cAMP or cGMP agonists. Our results suggest that cAMP and cGMP are essential for galvanotaxis of cell fragments, in contrast to the signaling mechanisms in parental cells.
Signaling networks govern cell migration (Ridley et al., 2003). However, cell fragments devoid of the nuclear and major organelles, in which signaling is likely very different from that of the mother cells, are also able to manifest robust motility and directional migration. Blood platelets play an essential role in coagulation, and are specialized type of cells which were believed to be static and immobile once they adhere to a matrix (Valone et al., 1974). Recent experiments provide convincing evidence demonstrating that platelets are mobile, able to migrate over a surface, and transmigrate through a basement membrane and endothelium toward a chemoattractant source (Kraemer et al., 2010; Schmidt et al., 2011). Cell fragments from white blood cells (also called cytokineplasts or cytoplasts) retain chemotactic, phagocytic, and microbicidal function in vitro and in vivo (Malawista et al., 1989; Malawista et al., 1992; Malawista et al., 2006). Migration of fragments from glioma cells correlate with malignancy (Yount et al., 2007). Cells can also shed smaller fragments like exosomes. Cell fragments thus may play important roles in physiology and pathology through active participation in homeostasis, phagocytosis, and cell-cell communication (Mannel and Grau, 1997; Bang and Thum, 2012; Arnold and Kahwash, 2014). The mechanisms underlying migration of cell fragments, however, have not been well studied and remain poorly understood.
Fish epidermal keratocytes move rapidly with a smooth gliding motion, while maintaining a uniform shape and speed. Thus, following multiple studies (Rafelski and Theriot, 2004), we chose them as the experimental model for cell migration. Cell fragments from fish keratocytes exhibit robust motility like their parental cells and provide a good model for studying the mechanisms of cell motility (Verkhovsky et al., 1999).
We recently developed an experimental protocol to induce directional migration of fish keratocyte fragments (Sun et al., 2013). We used electric fields (EFs) as a directional cue to guide migration of cell fragments. EF-guided cell migration, termed galvanotaxis, has been reported for many cell types including corneal epithelial cells, keratinocytes, endothelial cells, lymphocytes, stem cells, and cancer cells (Mycielska and Djamgoz, 2004; Zhao et al., 2006; Brown and Dransfield, 2008; Lin et al., 2008; Feng et al., 2012; Yang et al., 2013; Cortese et al., 2014). Our previous study (Sun et al., 2013) showed that keratocyte fragments respond to a direct-current electric field (dcEF), by migrating toward the anode, unlike their mother cells, that migrate toward the cathode.
We set to determine the roles of cyclic mononucleotides, example cyclic AMP and cyclic GMP (cAMP and cGMP) in migration of cell fragments. cAMP and cGMP are among the most important second messengers in many biological processes. cAMP and cGMP often exert opposing effects on cellular responses to extracellular factors. The ratio of cAMP and cGMP, which regulate the cytosolic level of Ca2+, stimulates the bi-directional turning responses of nerve growth cones to netrin-1, which guides axons growth (Nishiyama et al., 2003). Involvement of cAMP and/or cGMP kinase in galvanotaxis-related pathways has been suggested for neural crest cells and skin keratinocytes (Nuccitelli et al., 1993; Pullar and Isseroff, 2005). In Dictyostelium cells, the preferential migration direction in EFs can be reversed by modulating multiple signaling pathways including cGMP (Sato et al., 2009). Here we found that cAMP and cGMP are essential for galvanotaxis of cell fragments, but not for of their parental cells, suggesting there are different signal transduction mechanisms in galvanotaxis of cells and cell fragments.
Materials and Methods
Primary isolation of keratocytes
Fish scales were removed from the flanks of black skirt tetra Gymnocorymbus ternetzi and seeded on a cell culture dish as previously described (Sun et al., 2013). Briefly, individual scales were spread out on the bottom of a culture dish and covered by a 22 mm glass coverslip with a stainless steel nuts on top to press down fish scales for attachment to the petri dish, and cultured in Leibovitz’s L-15 culture media, supplemented with 14.2 mM HEPES pH7.4, 10% Fetal Bovine Serum, and 1% Antibiotic-Antimycotic (Invitrogen, Carlsbad, CA) at room temperature. Keratocyte sheets were observed in 2 days and used for experiments.
Cell fragment induction
After Fish scales were removed from culture plates, attached keratocytes were washed twice with phosphate buffered saline (PBS), then treated briefly with 0.05% Trypsin/0.02% EDTA (Invitrogen) for dissociation. Isolated cells were reseeded in a 1 cm × 2 cm electrotaxis chamber and incubated for 1–2 h at room temperature to allow sufficient attachment. Attached cells were treated with staurosporin at 50 nM (Sigma–Aldrich, St. Louis, MO) in culture media for 20–30 min at 37°C to induce cytoplasmic fragmentation. Treated cells were then washed with culture medium again before migration experiments.
Cell migration assay
The membrane permeable cAMP analogue Sp-cAMP (Merck Millipore, Billerica, MA) and Rp-cAMP (Sigma–Aldrich), cGMP analogue Sp-cGMP (Sigma–Aldrich) and Rp-cGMP (Santa Cruz Biotechnology, Santa Cruz, CA) were used in this study. After induction of keratocyte fragmentation with staurosporin, cells and fragments were incubated with different concentrations of agonists and/or antagonists in each electrotaxis chamber for 30 min at room temperature. After incubation, cells were stimulated with EFs for 0.5–1 h and time lapse images were recorded. Pharmacological inhibitors were used at previously determined optimal concentrations of 10 μM KT5720, 10 μM KT5823, 50 μM LY294002, or 50 μM Blebbistatin (Sigma–Aldrich).
Electric field stimulation and time lapse image recording
Prior to EF stimulation, seeded keratocytes in chamber were filled with culture media. EFs were applied through silver/silver chloride electrodes immersed in Steinberg’s solution (pH 7.4) reservoirs that were connected to culture medium by two agar bridges (1.5% agar in Steinberg’s solution) as described previously (Song et al., 2007). Unless stated otherwise, a field strength of 400 mV/mm was used. Field strengths were measured directly at the beginning and the end of the experiment. Time lapse imaging was performed using an inverted microscope (Carl Zeiss Axiovert 40) with a CCD digital camera controlled by SimplePCI 5.3 imaging system. Images were taken at 1 min intervals at room temperature.
Quantification and statistics
Time-lapse images of cell migration were analyzed using ImageJ software from the National Institutes of Health (http://rsbweb.nih.gov/ij/) using the MTrackJ and Chemotaxis tools. We characterized cell migration by quantifying the following two parameters: (i) Directedness, a commonly used indicator for directionality of cellular migration, which is defined as the cosine of the angle between the electrical field vector and the net cellular translocation direction. If a cell migrated perfectly along the field vector towards the cathode, the directedness would be 1; if the cell migrated directly toward the anode would have a directedness of −1. For a group of cells, an average directedness (Σi cos θ/N) close to 0 represented random migration. (ii) Cell migration rate was quantified as trajectory speed which presented as the accumulated migration distance per minute.
All experiments were repeated, and data are presented as mean ± standard error of the mean (s.e.m.). Statistical analysis was performed using SPSS software with unpaired, two-tailed Student’s t-test, P was set at 0.05 for rejecting null hypotheses.
Results
cAMP or cGMP agonists (Sp-cAMP or Sp-cGMP) abolished galvanotaxis of cell fragments
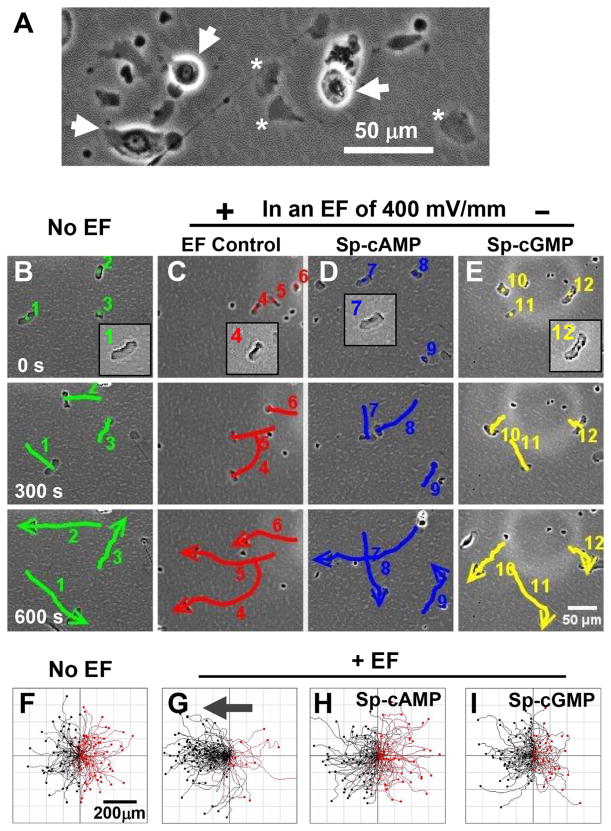
Fish keratocyte fragments can be observed clearly devoid of nuclei (Fig. 1A) and stained negative for endoplasmic reticulum and Golgi apparatus (Sun et al., 2013). Fragments exhibited random motility with trajectory speed of 8.52 ± 0.21 μm/min in the absence of an EF (Fig. 1B, F). Application of an EF guided fragments to migrate directionally towards the anode (Fig. 1C, G). The directedness values increased significantly in an EF with the same or similar migration speed (Cos θ = −0.68 ± 0.06, n = 100, P <0.001 compared to the no EF control of Cos θ = −0.005 ± 0.069, n = 100) (Fig. S1B and Movie S1).
Fig. 1.
cAMP and cGMP agonists abolished directional migration of cell fragments. (A) Generation of cell fragments (*). Cells are indicated with arrows for comparison. (B–E) Time-lapse images showing migration of fragments. Numbers indicate individual fragments. Lines show the trajectories, and arrows indicate the direction. (F–I) Migration trajectories of cell fragments. Cell fragments migrated directionally to the anode (the left) in an electric field of 400 mV/mm. Incubation with cell membrane permeable cAMP agonist (Sp-cAMP, 10 μM) or cGMP agonist (Sp-cGMP, 10 μM) abolished the anode directed migration. Polarity and the strength of the EF are as shown. Incubation time with Sp-cAMP and Sp-cGMP was 30 min. Cell trajectories are shown with the first position of all cells normalized to the center. Black and red lines indicate trajectories of fragments migrating toward anode and cathode, respectively.
To test the role of cyclic mononucleotides in directional migration of cell fragments, membrane permeable cAMP agonist Sp-cAMP, or cGMP agonist Sp-cGMP were added in culture media (10 μM) and incubated with keratocyte fragments for 30 min, then stimulated with an EF. Sp-cAMP is a potent activator of cAMP-dependent protein kinase I and II that mimics the effects of cAMP as a second messenger while being resistant to cyclic nucleotide phosphodiesterases. Sp-cGMP is a potent activator of cGMP-dependent protein kinases that mimics the effects of cGMP as a second messenger. Sp-cAMP or Sp-cGMP abolished directional migration of cell fragments with directedness value dropping to the no EF control value (Fig. 1D, E, H, I; Fig. S1B, and Movie S1). The migration speed remained unchanged after cAMP or cGMP treatment.
Inhibition of galvanotaxis by cAMP and cGMP agonists was concentration dependent
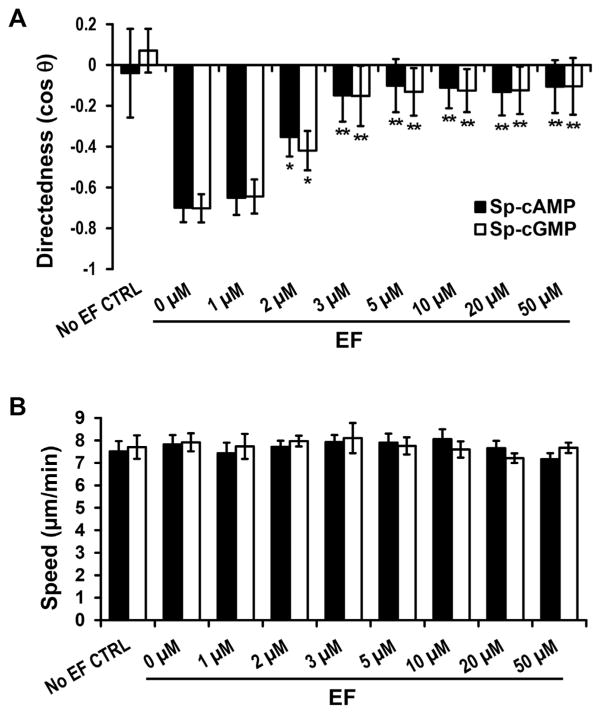
To determine the cAMP and cGMP dose dependence for galvanotaxis of cell fragments, Sp-cAMP or Sp-cGMP at different concentrations (from 1 to 50 μM) were used to treat the fragments (Fig. 2). Fragments in the presence of 1 μM Sp-cAMP or Sp-cGMP move with an average directedness of −0.65 ± 0.07 (Fig. 2A; n = 46) and −0.64 ± 0.05 (Fig. 2A; n = 47), respectively, similar to that of the control (0 μM) 0.70 ± 0.03 (Fig. 2A, n = 91).
Fig. 2.
cAMP and cGMP agonists inhibited the anode directionality, but not the speed. (A) cAMP and cGMP agonists inhibited directional migration of cell fragments in a dose-dependent manner. (B) cAMP and cGMP agonists did not affect migration speed of cell fragments. Data are shown as mean ± s.e.m. from 30–100 fragments; the results were consistent in three independent experiments. EF = 400 mV/mm; duration, 30 min. *P <0.05; **P <0.01 compared with their 0 μM controls).
Increasing the concentration to 2 μM, Sp-cAMP or Sp-cGMP significantly reduced the directional migration of fragment towards the anode. The directedness values halved, with an average directedness of −0.35 ± 0.09 (Sp-cAMP) and −0.42 ± 0.09 (Sp-cGMP), (P = 0.023 and P = 0.025, respectively compared to the control value) (Fig. 2A). Sp-cAMP or Sp-cGMP of 3 μM and above completely abolished galvanotaxis response of the fragments (Fig. 2). The dose-response curve shows a gradual decrease in the directional migration of the fragments, but no change in migration speed (Fig. 2B), over the concentration range, which suggest that cAMP or cGMP agonists are able to modulate cytoskeletal fragment directional response in EF, but do not affect their general motility.
cAMP or cGMP antagonists prevented the agonist-mediated attenuation of fragment galvanotaxis
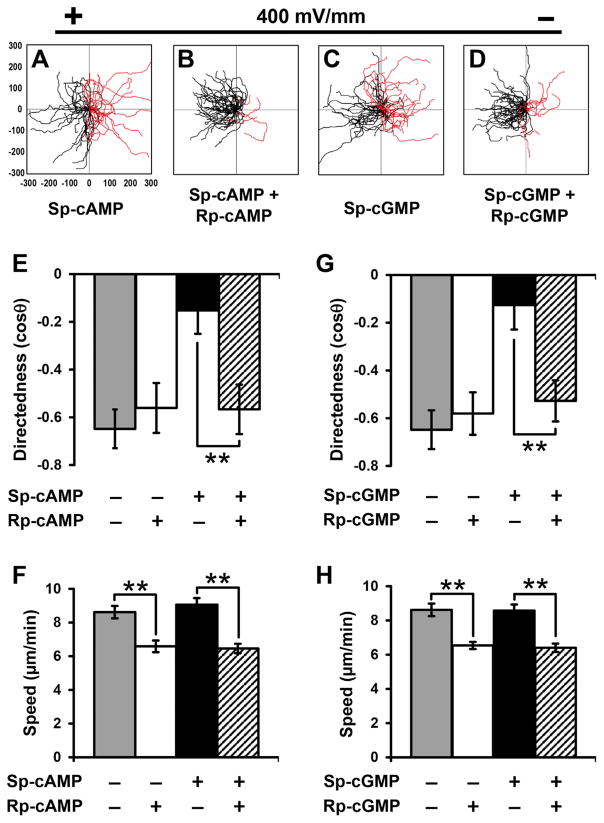
To confirm specific effects of cAMP or cGMP agonists on galvanotaxis of cell fragments, we incubated cultures with cAMP or cGMP antagonist (Rp-cAMP or Rp-cGMP), which blocked cAMP and cGMP downstream signaling. The cultures were stimulated with an EF in the presence or absence of Sp-cAMP and Sp-cGMP, respectively. Treatment of cell fragments with antagonists recovered galvanotaxis and cell fragments migrated directionally to the anode (Fig. 3B, D, compare to Fig. 3A,C, respectively). Quantitative analyses of galvanotaxis migration showed complete recovery of the directedness values after treatment of antagonists (Fig. 3E, G). Addition of Rp-cAMP or Rp-cGMP alone (10 μM) did not affect fragment directedness in an EF. Antagonist treatment significantly reduced the migration speed (P <0.01, Fig. 3F).
Fig. 3.
cAMP and cGMP antagonists prevented agonist-mediated attenuation of fragment galvanotaxis. (A–D) Migration trajectories of cell fragments in an electric field. Pre-incubation with cell membrane permeable cAMP antagonist (Rp-cAMP) and cGMP antagonist (Rp-cGMP) prevented the agonist-mediated attenuation of fragment galvanotaxis. (E–H) Directedness (E, G) and trajectory speed (F, H) of fragments in the presence and absence of cAMP or cGMP antagonists. Data are shown as mean ± s.e.m. from 50 to 80 fragments; the results were confirmed in three independent experiments. EF = 400 mV/mm; duration, 30 min. **P <0.01.
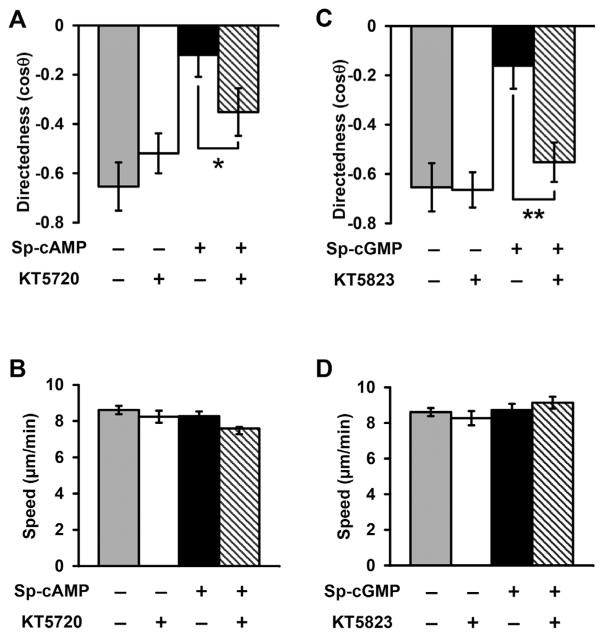
PKA and PKG inhibitors restored galvanotaxis in Sp-cAMP and Sp-cGMP treated fragments
Intracellular levels of cAMP and cGMP regulate PKA and PKG activities, respectively. To test whether PKA and PKG are involved in directional migration of cell fragments, we examined the effects of blocking PKA and PKG signaling on fragments galvanotaxis. Addition of a PKA inhibitor KT5720 (10 μM) to cell fragments in the presence of Sp-cAMP significantly restored directional migration, without significant effect on migration speed (Fig. 4A, B). Addition of a PKG inhibitor KT5823 (10 μM) also significantly restored galvanotaxis that was abolished by pretreatment of Sp-cGMP (Fig. 4C, D). These results suggest that intracellular cAMP and cGMP modulate fragment galvanotaxis through regulation of PKA and PKG activities.
Fig. 4.
Protein kinase A (PKA) and protein kinase G (PKG) inhibitors recovered directional migration of cell fragments treated with Sp-cAMP and Sp-cGMP. (A, B) Directedness and trajectory speed of fragments treated with PKA inhibitor (KT5720) and/or cAMP agonist separately or simultaneously. KT5720 recovered directional migration in Sp-cAMP treated cell fragments. (C, D) Directedness and trajectory speed of fragments treated with PKG inhibitor (KT5823) and cGMP agonist separately and simultaneously. KT5823 recovered directional migration in Sp-cGMP treated fragments. Data are shown as mean ± s.e.m. from 50 to 100 fragments; the results were confirmed in three independent experiments. EF = 400 mV/mm; duration, 30 min. *P <0.05; **P <0.01. Fragments were treated with inhibitors and/or agonists for 30 min before experiments.
Inhibition of PI3Ks recovered galvanotaxis in the presence of cAMP or cGMP agonists
We then tested the effects of inhibition of PI3K or myosin II on attenuation of galvanotaxis of cAMP and cGMP agonists. In our previous study, we found that PI3K- and myosin-dependent pathways play competing roles in cell fragments and parental cells (Sun et al., 2013). Myosin II is required for anodal galvanotaxis of cell fragments, while PI3Ks are required for cathodal galvanotaxis of the parental cells. Inhibition of PI3K by LY294002 (50 μM) completely recovered directional migration of cell fragments treated with cAMP or cGMP agonists (Fig. 5A). Inhibition of myosin II with blebbistatin (50 μM) had no effect. Inhibition of PI3Ks and myosin II did not significantly affect the migration speed of cell fragments (P >0.05, Fig. 5B).
Fig. 5.
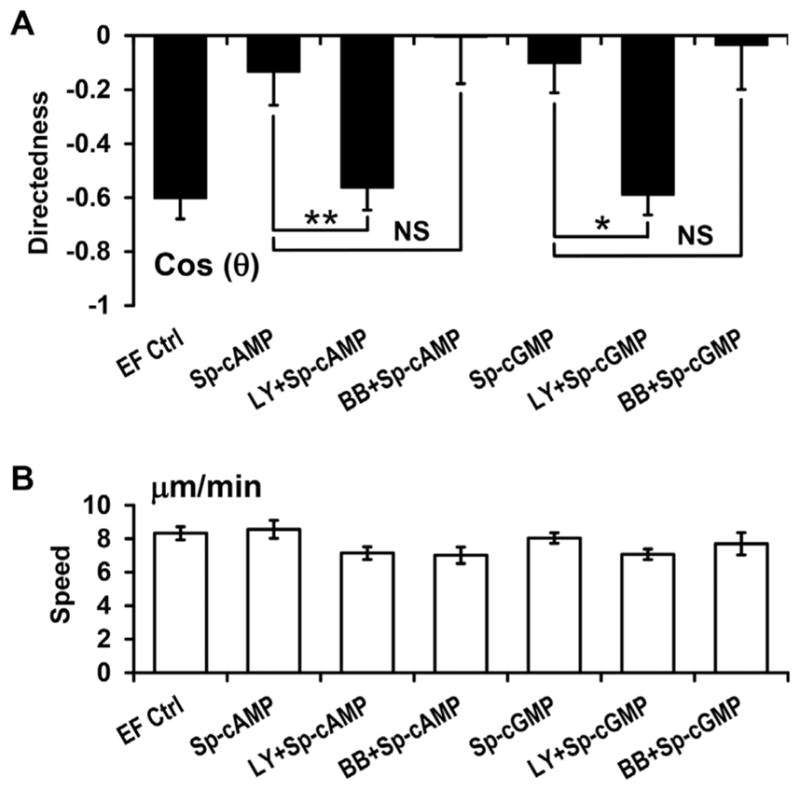
Inhibition of PI3Ks prevented the inhibitory effects of cAMP and cGMP agonists on directional migration of cell fragments. (A) Inhibition of PI3Ks with LY294002 (50 μM) completely recovered directional migration of cell fragments treated with cAMP and cGMP agonists. Inhibition of Myosin II with blebbistatin (BB; 50 μM) had no effect. (B) Inhibition of PI3Ks and myosin II did not affect the migration speed of cell fragments. Data are shown as mean ± s.e.m. from 30 to 80 fragments; the results were confirmed in three independent experiments. EF = 400 mV/mm; duration, 30 min. Fragments were treated with inhibitors and/or agonists for 30 min before experiments. *P <0.05; **P <0.01. NS: no significant difference.
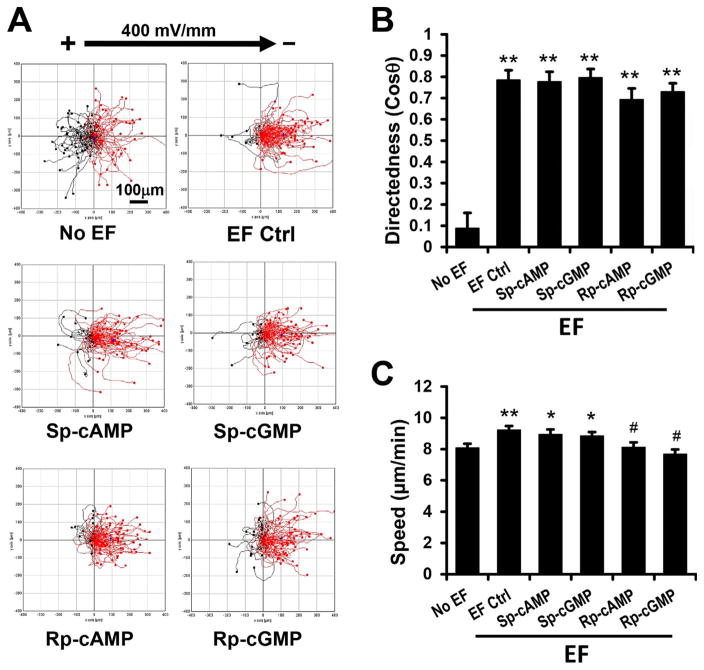
cAMP and cGMP did not affect parental keratocytes galvanotaxis
Finally, we determined the effects of cAMP and cGMP agonists on directional migration of the parental keratocytes. Without an EF, cells migrated in random directions (cosine θ = 0.09 ± 0.07). An EF induced robust cathode-directed migration of the cells (cosine θ = 0.78 ± 0.05, 400 mV/mm)(Fig. 6A). The trajectory speed was significantly increased in an EF compared to no EF control (Fig. 6C). Sp-cAMP or Sp-cGMP (10 μM) had no significant effects on migration directedness or migration speed (Fig. 6B). Cell treated with antagonistsRp-cAMP or Rp-cGMP (10 μM) had a slightly lower (not statistically significant) directedness value.
Fig. 6.
cAMP and cGMP did not affect parental keratocyte galvanotaxis. (A) Migration trajectories of parental keratocytes. Cells migrated directionally to the cathode (the right) in an electric field of 400 mV/mm. Incubation with cell membrane permeable agonist (Sp-cAMP, Sp-cGMP) or antagonist (Rp-cAMP, Rp-cGMP) did not affect the cathode directed migration of keratocytes. (B, C) Directedness (B) and trajectory speed (C) of keratocytes in the presence and absence of agonists or antagonists. Polarity and the strength of the EF are as shown. Incubation time with drugs was 30 min. Data are shown as mean ± s.e.m. from 50 to 100 cells, the results were confirmed in three independent experiments. EF = 400 mV/mm; duration, 30 min. *P <0.05; **P <0.01 compared with no EF. #P <0.01 compared with EF control.
Discussion
Our data suggest, for the first time, contrasting and unique roles for second messengers cAMP and cGMP in regulation of migration of cell fragments compared with whole cells (Fig. 7). Cell permeable agonists of cAMP or cGMP (Sp-cAMP and Sp-cGMP), completely abolished directional migration of cell fragments but did not affect the parental keratocyte behavior. The inhibitory effects were confirmed by cAMP or cGMP antagonists and PKA or PKG inhibitors. PI3K inhibition restored the fragments directional migration after treatment with cAMP and cGMP agonists.
Fig. 7.
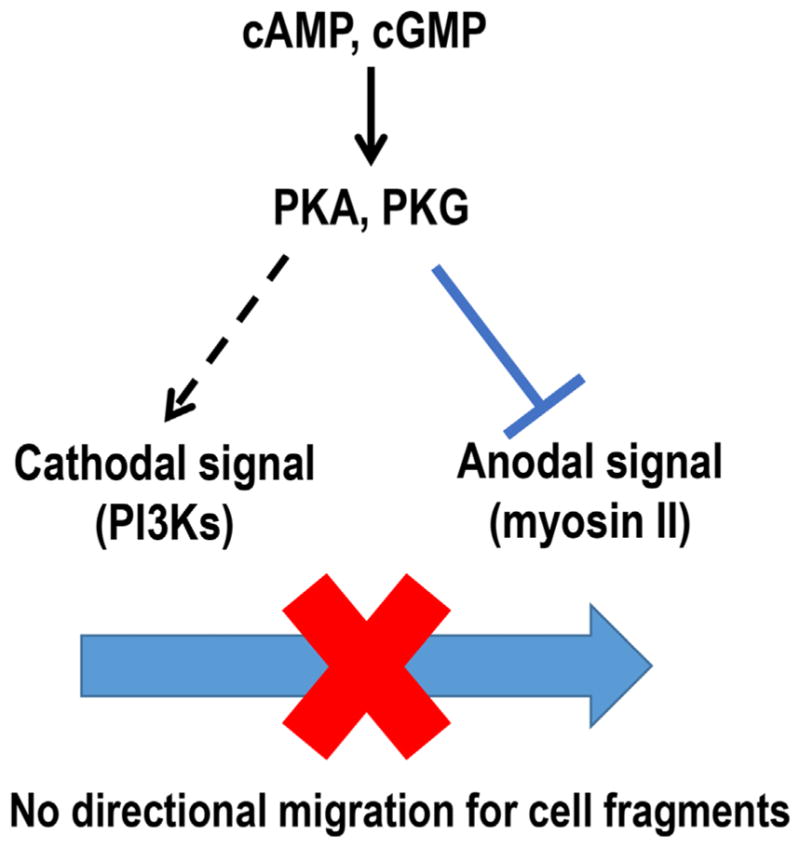
Schematic graph shows hypothetical mechanisms for cAMP and cGMP in regulation of anodal migration of cell fragments. Two biased signals determine the migration direction in an EF. Myosin-dependent pathway dominates in cell fragments and dictates the migration direction to the anode. cAMP and cGMP could regulate the directional signaling balance which controls galvanotaxis of cell fragments by inhibiting anodal signal and activating cathodal signal simultaneously.
Migration of cell fragments
Cell fragments are devoid of a nucleus and major organelles (Golgi apparatus and endoplasmic reticulum, for e.g.). The physiological roles played by cell fragments remain unclear, except for the essential role of platelets in hemostasis. Platelets, as a very specialized type of cell fragment, were long believed to be static and immobile when adhered to the extracellular matrix. Recent experiments have demonstrated active migration of platelets (Kraemer et al., 2010; Kraemer et al., 2011). Cell fragments from leukocytes are able to respond to chemotactic signals in the same direction as the mother leukocytes and can migrate to inflammatory lesions in vivo (Malawista and de, 1991; Malawista et al., 2006). Glioma cells in vitro spontaneously shed anucleate fragments which are independently motile. The motility was indistinguishable from those of their parent cells, and correlated well with cell invasiveness and might be related to the cell-invasive phenotype (Yount et al., 2007). In this study, we used fish keratocyte fragments as a robust model to study directional control during fragment migration. Fish keratocytes are thought to play important roles in epidermal wound healing. Similar to human keratinocytes, they response to endogenous electric fields and migrate directionally to the cathode. Cell fragments released from keratocytes reach a size up to 35 μm in diameter, which are slightly larger than mammalian cell fragments. These fragments could migrate autonomously much like intact cells. They polarized and migrated directionally in an applied EF, in the opposite direction to that of their mother cells (Sun et al., 2013). The parental cells polarized to the cathode, whereas the fragments polarized and migrated to the anode. Studying migration of cell fragments is significant for two reasons. The first is to try to understand the physiological and pathological roles for cell fragments in vivo. The second is to use cell fragments as a model system to understand the mechanisms underlying directional sensing and cell motility control.
cAMP and cGMP in cell migration and galvanotaxis
As second messengers, cAMP and cGMP regulate many cell functions. Particularly, cAMP and cGMP play central roles in regulating platelet behavior (Smolenski, 2012). A number of studies have shown that cAMP and cGMP have both negative (i.e., inhibitory) and positive (i.e., enhancing) effects on cytoskeletal organization and cell migration (Whittard and Akiyama, 2001; Jacob et al., 2002; Chen et al., 2008; Tegenge et al., 2011). In several other systems, elevation of cAMP has been shown to be required for efficient cell migration. However, for endothelial cells, agents that activate intracellular PKA, such as forskolin or dibutyryl cAMP, suppress cell migration (Kim et al., 2000; Howe, 2004). Galvanotaxis in human keratinocytes is thought to be mediated by cAMP and cAMP-dependent signaling pathways (Pullar et al., 2001). Our results showed that cAMP and cGMP are essential for EF-guided anode migration of cell fragments, but not for the cathode migrating parental cells (Fig. 6). The mechanisms underlying different galvanotaxis between human keratinocytes and fish keratocytes, and that between cell fragments and parental cells, remain unclear.
In addition, antagonists Rp-cAMP and Rp-cGMP inhibited basal motility of cell fragments without affecting galvanotaxis directionality (Fig. 3). We also tested H89, a more specific inhibitor of PKA, to confirm our results. As shown in Figure S2, after H89 treatment at 10 μM, keratocytes and fragments migrated directly to the cathode and anode, respectively. The inhibition of PKA by H89 significantly decreased the basal motility of fragments in the presence or absence of EF, which is consistent with our previous data. The mechanisms underlying this inhibition remain unknown and need further investigation. cAMP and cGMP could regulate cell adhesion and cytoskeleton (Elferink and VanUffelen, 1996; Howe, 2004). Moreover, both cAMP and cGMP are able to regulate intracellular calcium signaling, which is widely considered to be one of the most important modulators in cell migration. Being devoid of a nucleus and major organelles, cell fragments could not synthesize new proteins from Golgi nor release calcium from endoplasmic reticulum. So, cell fragments, compared to parental cells, may have different mechanisms to regulate intracellular signaling networks. Therefore, inhibiting cAMP or cGMP activity may affect cell fragment mobility.
cAMP and cGMP in determination of migration direction
Spatial regulation of PKA via anchoring is an important factor in cell movement (Tkachenko et al., 2011). Importantly, cAMP can induce switching in turning direction of nerve growth cones (Song et al., 1997). The ratio of cAMP to cGMP activities set the polarity of netrin-1-induced axon guidance: high ratios favor attraction, whereas low ratios favor repulsion (Song et al., 1997). This switching mechanism between attraction and repulsion to a guidance cue may be species- and cell-type dependent. Embryonic rat spinal commissural neurons were always attracted to netrin-1 and never repelled, suggesting that the mechanisms underlying cyclic nucleotide-regulated switching are different from the signal transduction mechanisms required for chemoattraction to netrin-1 (Moore and Kennedy, 2006).
Cell fragments to a certain extent resemble nerve growth cones. During formation of the cell fragments and before the fragments are completely separated from the mother cells, the fragments already assume galvanotaxis independent of and opposite to that of their parental cells (Sun et al., 2013). Increasing intracellular levels of cAMP and cGMP in cell fragments inhibited EF-directed anode migration. Fragments were blinded to the EF while maintaining normal migratory speeds even at the highest concentration of Sp-cAMP and Sp-cGMP tested (e.g., 20 μM and 50 μM) (Fig. 2). cAMP and cGMP agonists are therefore capable of modulating cell fragment directional migration without affecting motility. Both Rp-cAMP and Rp-cGMP are cell membrane permeable and resistant to cyclic nucleotide phosphodiesterases. These two antagonists competitively inhibit cAMP- and cGMP- dependent protein kinases, respectively. Pre-incubation with those antagonists prevented the agonist mediated attenuation of fragments galvanotaxis, confirming the negative regulation role of cAMP, and cGMP (Fig. 3).
Protein kinase A and protein kinase G are downstream of cAMP and cGMP, respectively, in galvanotaxis of cell fragments
Cyclic mononucleotides mediate many cellular responses, including cell migration, through their downstream protein kinase activities. PKA is present in most cells and functions as an effector of elevated cAMP following hormone and neurotransmitter stimulation. PKG is highly expressed in special cell types such as smooth muscle cells and platelets, as well as intestinal, kidney and brain cells. Inhibition of PKA and PKG recovered directional migration in Sp-cAMP and Sp-cGMP treated fragments, respectively (Fig. 4). It is worth noting that inhibition of PKA by KT5720 could only partially recover fragment galvanotaxis in the presence of Sp-cAMP while the inhibitor alone did not significantly affect migration directedness. PKG may also be involved in cAMP mediated fragments galvanotaxis, because cAMP could activate PKG (Lin et al., 2002). These results suggest that cAMP and cGMP agonists attenuate fragments galvanotaxis at least partially through PKA and PKG.
Anodal migration versus cathodal migration
As the smallest motile and EF-sensing unit, fragments contain both the EF sensor and the relevant signal transduction pathways. We proposed two competing pathways that determine migration direction in an EF: a PI3K-dependent pathway controlling migration to the cathode, and a myosin-dependent pathway controlling migration to the anode (Sun et al., 2013). In cell fragments, the myosin-dependent pathway, as an anodal biased signal, dominates, and directs fragments migrating to the anode. Cathodal biased signals do not play a major role in anodal migration since inhibition of cathodal signal, such as PI3K, do not affect fragment galvanotaxis, neither on the migration directions nor the migration speed. According to this model, weakening anodal signals and/or activate cathodal signals would disrupt the anode-cathode axis polarization and result in loss of migration direction. The key factors that regulate these two biased signals in cells and fragments remains unknown.
Here, we propose that second messengers cAMP and cGMP could be one of the factors that govern both anodal and cathodal signals in cell fragment galvanotaxis (Fig. 7). The cAMP/PKA pathway could inhibit RhoA and decrease myosin light chain kinase (MLCK) activity by modulating myosin light-chain kinase/phosphotase (MLCP) activity ratio (Howe, 2004; Aslam et al., 2010). Moreover, cAMP could affect PI3K activity through the activation of Ras signaling (Tsygankova et al., 2000). Similar to cAMP, cGMP could also up-regulate PI3K while down-regulating myosin phosphorylation. In contrast to their roles in cells, cAMP and cGMP are critical in regulation of directional signaling balance (Fig. 7). The diversified regulation of anodal signal (myosin II) and cathodal signal (PI3K) activities at the same time disorientated cell fragments in an electric field and abolished the galvanotaxis. Indeed, inhibition of cathode migration signaling with LY294002 recovered galvanotaxis of cell fragments after treatment with Sp-cAMP or Sp-cGMP (Fig. 5).
In conclusion, our data demonstrated that elevation of intracellular cAMP or cGMP blinded cell fragments to extracellular electric fields. The loss of directional migration of cell fragments was mediated by PKA and PKG. Loss of directional migration can be recovered by inhibiting the competing PI3K signaling pathway or using cAMP and cGMP antagonists. Our results suggest a unique signaling mechanism for cAMP/PKA and cGMP/PKG in directional migration of cell fragments, contrary to their roles in cells. Strategies aiming to modulate migration of cell fragments should take into account the difference from the mechanisms in whole cells.
Supplementary Material
Fig. S1. cAMP and cGMP agonists abolished directional migration of cell fragments.
(A) Migration speed. Tt/T: the trajectory speed, Td/T: displacement speed. (B) Cell membrane permeable cAMP agonist (Sp-cAMP) and cGMP agonist (Sp-cGMP) abolished the anode directed migration. EF = 400 mV/mm. Incubation time with Sp-cAMP and Sp-cGMP was 30 min. Data are shown as mean ± s.e.m. from 100 fragments, the results were confirmed in 3 independent experiments.
Fig. S2. H89 inhibited the basal motility of cell fragments
(A) H89, a specific PKA inhibitor, did not affect the opposite directional migration of keratocytes and cell fragments. (B) H89 significantly inhibited the basal motility of fragments in the presence or absence of EF. Data are shown as mean ± s.e.m. from 30–50 fragments. EF = 400 mV/mm; duration, 30 min. ** P < 0.01.)
Time-lapse video corresponding to Fig. 1 shows that cell fragments migrate directionally towards the anode (to the left) in an EF. cAMP and cGMP agonists abolished directional migration of cell fragments. The recording time is 30 min with frame interval of 1 minute. EF = 400 mV/mm.
Acknowledgments
Contract grant sponsor: National Institutes of Health;
Contract grant numbers: 1R01EY019101, 1R01GM068952.
Contract grant sponsor: National Science Foundation;
Contract grant number: MCB-0951199.
This work was supported by NIH 1R01EY019101 and NIH 1R01GM068952, and in part by a grant from NSF [MCB-0951199]. This study was supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., UC Davis Ophthalmology. This work was also supported in part by UC Davis Dermatology and Ophthalmology Developmental funds, and UC Davis Bridging fund. Kan Zhu is supported by a fellowship from the China Scholarship Council. The authors thank Dr. Brian Reid for proofreading and editing and Liu Sui and Chenxi Ouyang for assistance in some initial experiments and data analysis.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- PKA
protein kinase A
- PKG
protein kinase G
- PI3K
phosphoinositide 3 kinase
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Authors’ Contribution
K.Z., Q.Z., A.M., and M.Z. conceived ideas and designed the experiments. K.Z., Y.H.S., A.M., M.Y., and B.L. performed the experiments and analysed the results. K.Z. and M.Z. wrote the article.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
Literature Cited
- Arnold MA, Kahwash SB. Phagocytized neutrophil fragments in the bone marrow: A phenomenon most commonly associated with hodgkin lymphoma. ISRN Hematol. 2014;2014:363854. doi: 10.1155/2014/363854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Hartel FV, Arshad M, Gunduz D, Abdallah Y, Sauer H, Piper HM, Noll T. CAMP/PKA antagonizes thrombin-induced inactivation of endothelial myosin light chain phosphatase: Role of CPI-17. Cardiovas Res. 2010;87:375–384. doi: 10.1093/cvr/cvq065. [DOI] [PubMed] [Google Scholar]
- Bang C, Thum T. Exosomes: New players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Brown SB, Dransfield I. Electric fields and inflammation: May the force be with you. Sci World J. 2008;8:1280–1294. doi: 10.1100/tsw.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang JJ, Huang XY. CAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J Biol Chem. 2008;283:13799–13805. doi: 10.1074/jbc.M800555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese B, Palama IE, D’Amone S, Gigli G. Influence of electrotaxis on cell behavior. Integr Biol. 2014;6:817–830. doi: 10.1039/c4ib00142g. [DOI] [PubMed] [Google Scholar]
- Elferink JG, VanUffelen BE. The role of cyclic nucleotides in neutrophil migration. Gen Pharmacol. 1996;27:387–393. doi: 10.1016/0306-3623(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30:349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Jacob A, Molkentin JD, Smolenski A, Lohmann SM, Begum N. Insulin inhibits PDGF-directed VSMC migration via NO/cGMP increase of MKP-1 and its inactivation of MAPKs. Am J Physiol Cell Physiol. 2002;283:C704–C713. doi: 10.1152/ajpcell.00110.2002. [DOI] [PubMed] [Google Scholar]
- Kim S, Harris M, Varner JA. Regulation of integrin alpha vbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J Biol Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- Kraemer BF, Borst O, Gehring EM, Schoenberger T, Urban B, Ninci E, Seizer P, Schmidt C, Bigalke B, Koch M, Martinovic I, Daub K, Merz T, Schwanitz L, Stellos K, Fiesel F, Schaller M, Lang F, Gawaz M, Lindemann S. PI3 kinase-dependent stimulation of platelet migration by stromal cell-derived factor 1 (SDF-1) J Mol Med. 2010;88:1277–1288. doi: 10.1007/s00109-010-0680-8. [DOI] [PubMed] [Google Scholar]
- Kraemer BF, Schmidt C, Urban B, Bigalke B, Schwanitz L, Koch M, Seizer P, Schaller M, Gawaz M, Lindemann S. High shear flow induces migration of adherent human platelets. Platelets. 2011;22:415–421. doi: 10.3109/09537104.2011.556277. [DOI] [PubMed] [Google Scholar]
- Lin CS, Liu X, Chow S, Lue TF. Cyclic AMP and cyclic GMP activate protein kinase G in cavernosal smooth muscle cells: Old age is a negative factor. BJU Int. 2002;89:576–582. doi: 10.1046/j.1464-410x.2002.02643.x. [DOI] [PubMed] [Google Scholar]
- Lin F, Baldessari F, Gyenge CC, Sato T, Chambers RD, Santiago JG, Butcher EC. Lymphocyte electrotaxis in vitro and in vivo. J Immunol. 2008;181:2465–2471. doi: 10.4049/jimmunol.181.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista SE, de Boisfleury-Chevance A. Cryopreserved cytoplasts from human polymorphonuclear leukocytes (cytokineplasts) are chemotactic at speeds comparable to those of fresh intact cells. J Leukoc Biol. 1991;50:313–315. doi: 10.1002/jlb.50.3.313. [DOI] [PubMed] [Google Scholar]
- Malawista SE, Montgomery RR, van Blaricom G. Evidence for reactive nitrogen intermediates in killing of staphylococci by human neutrophil cytoplasts. A new microbicidal pathway for polymorphonuclear leukocytes. J Clin Invest. 1992;90:631–636. doi: 10.1172/JCI115903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista SE, Smith EO, Seibyl JP. Cryopreservable neutrophil surrogates: Granule-poor, motile cytoplasts from polymorphonuclear leukocytes home to inflammatory lesions in vivo. Cell Motil Cytoskeleton. 2006;63:254–257. doi: 10.1002/cm.20120. [DOI] [PubMed] [Google Scholar]
- Malawista SE, Van Blaricom G, Breitenstein MG. Cryopreservable neutrophil surrogates. Stored cytoplasts from human polymorphonuclear leukocytes retain chemotactic, phagocytic, and microbicidal function. J Clin Invest. 1989;83:728–732. doi: 10.1172/JCI113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannel DN, Grau GE. Role of platelet adhesion in homeostasis and immunopathology. Mol Pathol. 1997;50:175–185. doi: 10.1136/mp.50.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW, Kennedy TE. Protein kinase A regulates the sensitivity of spinal commissural axon turning to netrin-1 but does not switch between chemoattraction and chemorepulsion. J Neurosci. 2006;26:2419–2423. doi: 10.1523/JNEUROSCI.5419-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycielska ME, Djamgoz MB. Cellular mechanisms of direct-current electric field effects: Galvanotaxis and metastatic disease. J Cell Sci. 2004;117:1631–1639. doi: 10.1242/jcs.01125. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R, Smart T, Ferguson J. Protein kinases are required for embryonic neural crest cell galvanotaxis. Cell Motil Cytoskeleton. 1993;24:54–66. doi: 10.1002/cm.970240107. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J cell Sci. 2005;118:2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Isseroff RR, Nuccitelli R. Cyclic AMP-dependent protein kinase A plays a role in the directed migration of human keratinocytes in a DC electric field. Cell motil Cytoskeleton. 2001;50:207–217. doi: 10.1002/cm.10009. [DOI] [PubMed] [Google Scholar]
- Rafelski SM, Theriot JA. Crawling toward a unified model of cell mobility: Spatial and temporal regulation of actin dynamics. Ann Rev Biochem. 2004;73:209–239. doi: 10.1146/annurev.biochem.73.011303.073844. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Sato MJ, Kuwayama H, van Egmond WN, Takayama AL, Takagi H, van Haastert PJ, Yanagida T, Ueda M. Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc Natl Acad Sci USA. 2009;106:6667–6672. doi: 10.1073/pnas.0809974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EM, Munzer P, Borst O, Kraemer BF, Schmid E, Urban B, Lindemann S, Ruth P, Gawaz M, Lang F. Ion channels in the regulation of platelet migration. Biochem Biophys Res Commun. 2011;415:54–60. doi: 10.1016/j.bbrc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J Thromb Haemost. 2012;10:167–176. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- Song B, Gu Y, Pu J, Reid B, Zhao Z, Zhao M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nature Protoc. 2007;2:1479–1489. doi: 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. CAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Sun Y, Do H, Gao J, Zhao R, Zhao M, Mogilner A. Keratocyte fragments and cells utilize competing pathways to move in opposite directions in an electric field. Curr Biol. 2013;23:569–574. doi: 10.1016/j.cub.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegenge MA, Rockel TD, Fritsche E, Bicker G. Nitric oxide stimulates human neural progenitor cell migration via cGMP-mediated signal transduction. Cell Mol Life Sci. 2011;68:2089–2099. doi: 10.1007/s00018-010-0554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko E, Sabouri-Ghomi M, Pertz O, Kim C, Gutierrez E, Machacek M, Groisman A, Danuser G, Ginsberg MH. Protein kinase A governs a RhoA-RhoGDI protrusion-retraction pacemaker in migrating cells. Nat Cell Biol. 2011;13:660–667. doi: 10.1038/ncb2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankova OM, Kupperman E, Wen W, Meinkoth JL. Cyclic AMP activates Ras. Oncogene. 2000;19:3609–3615. doi: 10.1038/sj.onc.1203680. [DOI] [PubMed] [Google Scholar]
- Valone FH, Austen KF, Goetzl EJ. Modulation of the random migration of human platelets. J Clin Invest. 1974;54:1100–1106. doi: 10.1172/JCI107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- Whittard JD, Akiyama SK. Positive regulation of cell-cell and cell-substrate adhesion by protein kinase A. J Cell Sci. 2001;114:3265–3272. doi: 10.1242/jcs.114.18.3265. [DOI] [PubMed] [Google Scholar]
- Yang HY, Charles RP, Hummler E, Baines DL, Isseroff RR. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J Cell Sci. 2013;126:1942–1951. doi: 10.1242/jcs.113225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount G, Taft RJ, Luu T, Rachlin K, Moore D, Zhang W. Independent motile microplast formation correlates with glioma cell invasiveness. J Neurooncol. 2007;81:113–121. doi: 10.1007/s11060-006-9211-4. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. cAMP and cGMP agonists abolished directional migration of cell fragments.
(A) Migration speed. Tt/T: the trajectory speed, Td/T: displacement speed. (B) Cell membrane permeable cAMP agonist (Sp-cAMP) and cGMP agonist (Sp-cGMP) abolished the anode directed migration. EF = 400 mV/mm. Incubation time with Sp-cAMP and Sp-cGMP was 30 min. Data are shown as mean ± s.e.m. from 100 fragments, the results were confirmed in 3 independent experiments.
Fig. S2. H89 inhibited the basal motility of cell fragments
(A) H89, a specific PKA inhibitor, did not affect the opposite directional migration of keratocytes and cell fragments. (B) H89 significantly inhibited the basal motility of fragments in the presence or absence of EF. Data are shown as mean ± s.e.m. from 30–50 fragments. EF = 400 mV/mm; duration, 30 min. ** P < 0.01.)
Time-lapse video corresponding to Fig. 1 shows that cell fragments migrate directionally towards the anode (to the left) in an EF. cAMP and cGMP agonists abolished directional migration of cell fragments. The recording time is 30 min with frame interval of 1 minute. EF = 400 mV/mm.