Abstract
Background
Objective of the study is to assess prevalence and survival among end stage renal disease patients with restless legs syndrome (RLS) within a national database (USRDS).
Methods
A case-control, retrospective analysis was performed. Differences in characteristics between the groups, RLS and those with no sleep disorder (NSD), were determined using χ2 tests. Cox proportional hazard regression was used to assess survival between those with RLS and propensity score matched controls.
Results
Cases of restless legs syndrome were defined as patients that had received an ICD-9 code of 333.94 at any point during their treatment (n = 372). RLS group demonstrated a significantly higher proportion of patients with major depressive disorder, dysthymic disorder, anxiety, depression, minor depressive disorder, and psychological disorder. The difference between the survival was not statistically significant in those without sleep disorder as compared to those with RLS (HR =1.16±0.14, p = 0.3).
Conclusions
True prevalence of RLS in dialysis patients can only be estimated if knowledge gap for care providers in diagnosis of RLS is addressed. RLS patients also have increased incidence of certain psychological disorders which needs to be addressed.
Electronic supplementary material
The online version of this article (doi:10.1186/s12882-017-0660-0) contains supplementary material, which is available to authorized users.
Keywords: Restless legs syndrome, Hemodialysis, End-stage renal disease, Mortality, Diagnosis, USRDS
Background
Individuals undergoing hemodialysis therapy due to End-Stage Renal Disease (ESRD) commonly report disturbances in their sleep [1–3]. A review of several studies suggested that the prevalence of sleep symptoms could be as high as 44% [1]. In this population, sleep disorders most often manifest as obstructive sleep apnea, excessive sleepiness, and restless legs syndrome (RLS)/Willis-Ekbom disease [4–6]. The effects of sleep disorders have been well documented, however it is worth noting that disrupted sleep can significantly impact quality of life, resulting in depression, psychological consequences, and even reduced socioeconomic status, particularly in the ESRD population [7–10]. Additionally, recurrent sleep disruption and sleep disorders have been associated with increased risk of cardiovascular disease, coronary artery disease and hypertension, and may also increase mortality [11–17].
Restless legs syndrome is cited as one of the most common movement disorders in the general population, with reported prevalence of 1.2% to 15% [18, 19]. In the end-stage renal disease population however, specifically those on hemodialysis, the prevalence of RLS ranges from 6.6% to 62% [9, 20–23]. This wide variability in reported prevalence may be driven by a number of factors, such as the heterogeneity of study populations, a previous lack of standardized diagnosis criteria, and a number of other confounders and comorbidities. The ESRD population tends to experience a high incidence of paresthesia, itching, cramps and peripheral neuropathy, which have likely contributed to such a high reported prevalence [24, 25]. To address the issues surrounding diagnosis of restless legs syndrome/Willis-Ekbom disease, the International Restless Legs Syndrome Study Group (IRLSSG) issued improved diagnostic criteria in 2012, after reaching an international and interdisciplinary consensus [24].
Despite the improvements to diagnosis criteria, sleep disorders continue to be under-recognized by renal healthcare providers [26]. Given the implications of frequent sleep disruption due to sleep disorders, specifically RLS, and the overall inconsistent estimates of prevalence in the ESRD population, we conducted a case-control analysis of the United States Renal Data System (USRDS). Our aim was to clarify RLS diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, evaluating significant comorbidities, and mortality rates among ESRD patients with and without RLS, with emphasis on those undergoing hemodialysis therapy.
Methods
De-identified USRDS Standard Analysis Files were used as the source of data in this analysis. Upon data retrieval, there were 2,138,876 patients in the database, however only 1,456,114 patients had a completed medical evidence report on file, with 62.8% of those on hemodialysis therapy. For the study population, however, we only considered ESRD patients greater than 18 years of age, who had initiated hemodialysis therapy between January 1, 2006 and December 31, 2008, and had a post-initiation survival of ≥3 months (n = 279,956). This survival criterion was introduced in order to reduce the odds that mortality was due to other acute causes. Patient survival was calculated as the time (in months) from dialysis initiation until transplant, death, or end of study data. Additionally, patients that had been diagnosed with Parkinson’s disease or secondary parkinsonism were excluded to reduce the likelihood of misdiagnosis of RLS due to pre-existing movement disorders [27]. The comorbidities of interest were identified using ICD-9-CM codes (Additional file 1: Table S1). Therefore, cases of restless legs syndrome were defined as patients that had received an ICD-9-CM code of 333.94 at any point during their treatment (n = 372), based on the 2012 classification. As a basis for comparison, patients were only considered for selection as a control if they had never been diagnosed with any sleep disorders (NSD) during the study period. After matching, the total number of cases and controls included in the survival analysis was 1092 (RLS, n = 273; NSD, n = 819). The analysis was approved by the SUNY Downstate Medical Center Institutional Review Board and the USRDS via data use agreements.
Statistical analysis
Differences in characteristics between the groups (RLS, NSD) were determined using χ2 tests for associations of categorical variables (%), and two-sided two-sample t-tests for differences in means (±SD). We then performed propensity score matching between RLS cases and controls by deriving the propensity score from logistic regression based on all variables in Table 1, and conducted greedy matching through a SAS macro for this purpose [28, 29]. Matching was done in a one-to-one stepwise manner, according to propensity score, where the best match was chosen first, followed by the next best until no further matches could be made. The result was a 1:3, cases to controls, matched population. Propensity scores were used as a means to control for any bias due to heterogeneity and imbalance.
Table 1.
Characteristics of study population and between-group comparison; USRDS 2006–2008
| Variable | Total n = 279,956 | RLS n = 372 | NSD n = 279,584 | P |
|---|---|---|---|---|
| Incidence age, years | 62.05 ± 15.39 | 64.22 ± 15.64 | 62.04 ± 15.39 | 0.007 |
| Gender | 0.2 | |||
| Female | 43.72 (122,400) | 47.3 (176) | 43.7 (122,224) | |
| Male | 56.28 (157,556) | 52.7 (196) | 56.3 (157,360) | |
| Race | <.0001 | |||
| White | 64.38 (180,239) | 86.29 (321) | 64.35 (179,918) | |
| Black | 29.93 (83,782) | 11.56 (43) | 29.95 (83,739) | |
| Asian | 3.32 (9282) | 1.34 (5) | 3.32 (9277) | |
| Native American | 1.08 (3026) | 0.27 (1) | 1.08 (3025) | |
| Other/Unknown | 1.3 (3627) | 0.54 (2) | 1.3 (3625) | |
| Ethnicity | 0.002 | |||
| Non-Hispanic/Latino | 85.68 (239,880) | 91.4 (340) | 85.68 (239,540) | |
| Hispanic/Latino | 14.32 (40,076) | 8.6 (32) | 14.32 (40,044) | |
| Vascular access type | 0.0002 | |||
| AV Fistula | 14.61 (40,902) | 23.12 (86) | 14.6 (40,816) | |
| Graft | 3.95 (11,058) | 4.3 (16) | 3.95 (11,042) | |
| Catheter | 80.28 (224,739) | 71.77 (267) | 80.29 (224,472) | |
| Other/Unknown | 1.16 (3257) | 0.81 (3) | 1.16 (3254) | |
| Body mass index (kg/m2)f | 28.74 ± 7.71 | 28.98 ± 7.57 | 28.74 ± 7.71 | 0.5 |
| Serum creatinine (mg/dl)f | 6.58 ± 3.46 | 6.64 ± 3.74 | 6.58 ± 3.46 | 0.8 |
| Serum albumin (g/dl)f | 3.11 ± 0.71 | 3.32 ± 0.64 | 3.11 ± 0.71 | <.0001 |
| Anemiaa | 8.37 (23,432) | 11.83 (44) | 8.37 (23,388) | 0.02 |
| Cardiovascular | ||||
| Coronary artery diseaseb | 21.09 (59,054) | 17.2 (64) | 21.1 (58,990) | 0.07 |
| Congestive heart failure | 32.18 (90,088) | 26.34 (98) | 32.19 (89,990) | 0.02 |
| Cerebrovascular disease | 9.4 (26,329) | 8.06 (30) | 9.41 (26,299) | 0.4 |
| Peripheral vascular disease | 14.04 (39,301) | 10.22 (38) | 14.04 (39,263) | 0.03 |
| Hypertension | 84.92 (237,737) | 83.6 (311) | 84.92 (237,426) | 0.5 |
| Other | ||||
| Diabetes | 52.78 (147,771) | 38.98 (145) | 52.8 (147,626) | <.0001 |
| Cancer | 6.81 (19,065) | 5.91 (22) | 6.81 (19,043) | 0.5 |
| Sleep disorders | ||||
| Any sleep disorder | 0.13 (372) | 100.0 (372) | 0 (0) | <.0001 |
| Restless legs syndrome | 0.13 (372) | 100.0 (372) | 0 (0) | <.0001 |
| Obstructive sleep apnea | 0.01 (17) | 4.57 (17) | 0 (0) | <.0001 |
| Psychological disorders | ||||
| Major depressive disorder | 0.37 (1030) | 1.61 (6) | 0.37 (1024) | <.0001 |
| Dysthymic disorder | 0.26 (727) | 2.15 (8) | 0.26 (719) | <.0001 |
| Anxiety | 0.6 (1691) | 4.03 (15) | 0.6 (1676) | <.0001 |
| Adjustment disorder | 0.03 (81) | 0.27 (1) | 0.03 (80) | 0.1 |
| Depressionc | 0.65 (1829) | 4.03 (15) | 0.65 (1814) | <.0001 |
| Minor depressive disorderd | 0.29 (808) | 2.42 (9) | 0.29 (799) | <.0001 |
| Psychological disordere | 1.24 (3459) | 8.06 (30) | 1.23 (3429) | <.0001 |
| Tobacco dependence | 6.39 (17,887) | 8.87 (33) | 6.39 (17,854) | 0.05 |
| Alcohol dependence | 1.57 (4384) | 0.81 (3) | 1.57 (4381) | 0.2 |
| Drug dependence | 1.55 (4339) | 1.08 (4) | 1.55 (4335) | 0.5 |
Age and clinical measures are mean ± SD. Categorical data are percentages (counts). No sleep disorder diagnosed (NSD)
aDetermined by ICD-9-CM codes 280.0–280.9, 285.0, 285.2, 285.8, 285.9
bAtherosclerosis, myocardial infarction (MI), ischemic heart disease (IHD)
cmajor depressive disorder, dysthymic disorder, adjustment disorder
dDysthymic disorder, adjustment disorder
eDepression, anxiety
fMissing data points
To assess survival, we initially produced an unadjusted Kaplan-Meier (KM) survival plot between the cases and controls using time to death, and employed right censoring. Between-group differences were tested by the log-rank test. We then ran an adjusted analysis with Cox proportional hazard regression, with adjustment for incidence age, sex, race, ethnicity, vascular access type, body mass index, serum albumin level, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, hypertension, diabetes, cancer, major depressive disorder, dysthymic disorder, anxiety, tobacco dependence, and restless legs syndrome. From this, we derived an adjusted survival plot. The survival analysis model was based on significant variables found in the univariable analyses (major depression, anxiety, incidence age, race, ethnicity, etc.) and several factors relevant to and associated with mortality (cardiovascular disease [30, 31], diabetes [32], cancer, BMI, etc).
Results
Characteristics of the study population are described in Table 1. Of the 279,956 patients examined, there was a mean incidence age of 62 ± 15.4 years. Within this cohort, 64.4% were White, 29.9% Black, 3.3% Asian, 1.1% Native American, and approximately 1.3% other or unknown races. Most patients were non-Hispanic/Latino (85.7%), and over half were male (56.3%). As previously described, individuals with ESRD often suffer from a multitude of metabolic, cardiovascular, and psychological comorbidities. We found a prevalence of diabetes (52.8%), coronary artery disease (21.1%), congestive heart failure (32.2%) and hypertension (84.9%). Psychological disorders were less common, with the greatest proportion having been diagnosed with anxiety (0.6%) and depression (0.7%). Prevalence of RLS was also estimated in this cohort. Three hundred and seventy-two documented cases of diagnosed restless legs syndrome were found which represents 0.1% of the study population.
Between-group characteristics
Our primary goal, however, was to examine the differences between RLS cases (n = 372) and non-sleep disorder (NSD) controls (n = 279,584). Table 1 also describes the between-group associations. When stratified by RLS status, significant differences in age and race were found. The cases and controls initiated dialysis therapy at an average of 64.22 ± 15.64 and 62.04 ± 15.39 years, respectively. There was also a larger proportion of Whites with RLS (86.3%) versus the control group (64.4%). Conversely, our data show only 11.6% of the RLS cases identified as Black/African-American, while this group accounted for nearly 30% of the controls. Serum albumin was statistically higher amongst the cases, while the NSD cohort demonstrated a higher proportion of patients with coronary artery disease, congestive heart failure, peripheral vascular disease and diabetes. Despite the relatively smaller number of patients with psychological conditions, the RLS group demonstrated a significantly higher proportion of patients with major depressive disorder, dysthymic disorder, anxiety, depression, minor depressive disorder, and psychological disorders.
Survival analysis
The Kaplan-Meier plot showed that survival in persons who have RLS was not different than those without RLS (Fig. 1a). The difference between the survival plots was not statistically significant at p = 0.2. Using Cox proportional hazards regression (Fig. 1b), the difference between the survival plots was not statistically significant with survival in those without sleep disorder as compared to those with RLS, HR = 1.16 ± 0.14, p = 0.3 (Table 2). In univariate analysis, age (p < .0001), race (p = 0.01), albumin (p = 0.0005), congestive heart failure (p = 0.004), hypertension (p = 0.003), and cancer (p = 0.03) were associated with an increase in mortality. However, when these covariates were adjusted for in the Cox models, there was no increased mortality in patients with RLS (Fig. 1).
Fig. 1.
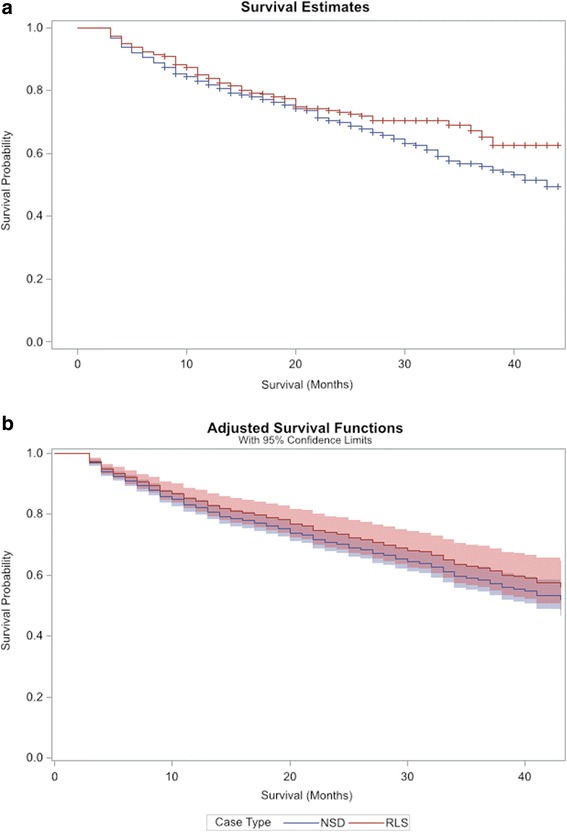
a Mortality in patients with ESRD by RLS status, unadjusted crude survival assessed by Kaplan-Meier analysis, Wilcoxon p = 0.2. b Mortality in patients with ESRD by RLS status, survival assessed by Cox regression analysis including 95% CI (shaded regions)
Table 2.
Survival analysis – propensity matched population (n = 1092)
| Variable | β | SE | HR | P |
|---|---|---|---|---|
| Incidence age, years | 0.03 | 0.005 | 1.03 | <.0001 |
| Female | −0.02 | 0.11 | 0.98 | 0.9 |
| Race | 0.22 | 0.09 | 1.25 | 0.01 |
| White | −0.53 | 0.72 | 0.59 | 0.5 |
| Black | −0.82 | 0.73 | 0.44 | 0.3 |
| Asian | −1.15 | 0.79 | 0.32 | 0.1 |
| Native American | −1.45 | 1.02 | 0.23 | 0.15 |
| Ethnicity | ||||
| Non-Hispanic/Latino | 0.26 | 0.19 | 1.3 | 0.2 |
| Vascular access type | ||||
| AV Fistula | −0.39 | 0.42 | 0.68 | 0.4 |
| Graft | −0.11 | 0.46 | 0.89 | 0.8 |
| Catheter | −0.07 | 0.39 | 0.93 | 0.9 |
| Body mass index (kg/m2) | −0.006 | 0.009 | 0.99 | 0.5 |
| Serum albumin (g/dl) | −0.27 | 0.08 | 0.77 | 0.0005 |
| Cardiovascular | ||||
| Coronary artery disease | 0.14 | 0.13 | 1.15 | 0.3 |
| Congestive heart failure | 0.34 | 0.12 | 1.41 | 0.004 |
| Cerebrovascular disease | 0.17 | 0.17 | 1.19 | 0.3 |
| Peripheral vascular disease | 0.24 | 0.15 | 1.27 | 0.1 |
| Hypertension | −0.43 | 0.15 | 0.65 | 0.003 |
| Other | ||||
| Diabetes | 0.1 | 0.12 | 1.1 | 0.4 |
| Cancer | 0.39 | 0.18 | 1.48 | 0.03 |
| Psychological disorders | ||||
| Major depressive disorder | 0.32 | 0.72 | 1.37 | 0.7 |
| Dysthymic disorder | 0.63 | 0.59 | 1.88 | 0.4 |
| Anxiety | 0.46 | 0.46 | 1.57 | 0.3 |
| Tobacco dependence | 0.14 | 0.22 | 1.16 | 0.5 |
| Case type | ||||
| No sleep disorders (NSD) | 0.15 | 0.14 | 1.16 | 0.3 |
Discussion
This study attempted to clarify the associations between RLS diagnosis, patient comorbidities, and mortality within the ESRD patient population, particularly those currently undergoing hemodialysis therapy. It is crucial to understand how sleep disorders like RLS may impact the health outcomes of this population. Results from our analyses reveal two interesting findings: 1) there are no significant differences in mortality between patients who are diagnosed with RLS and those with no sleep disorders, and 2) patients with RLS are more likely to have diagnoses of psychological conditions.
We found a significantly higher proportion of patients with RLS had major depressive disorder, dysthymic disorder, anxiety, depression or minor depressive disorder, as compared to those with no sleep disorder. This may have been related to changes in quality of life in those with RLS. Further, our results did not show increased mortality in patients with RLS as compared to those with no sleep disorders. These findings are similar to Stefanidis et al. who reported no difference in 3-year mortality in 579 hemodialysis patients with and without RLS [33] but are in contrast to other studies which have reported higher mortality in patients with RLS [12, 34]. ESRD studies claiming greater likelihood of mortality for patients with sleep disorders appear to contain relatively homogeneous populations in their analyses. Our study, however, leverages the USRDS which has collected data on ESRD patients undergoing dialysis from across the United States and boasts a very diverse population. In addition, we have used diagnostic codes to assess RLS prevalence and comorbidities, along with cause-of-death reports to determine survival status. The greater heterogeneity in our patient population most likely drives important differences in results between the studies.
In this study, we used ICD-9-CM codes to identify cases of RLS, defined as 1) an urge to move the legs usually but not always accompanied by unpleasant sensations in the legs; 2) the urge to move the legs and unpleasant sensations worsen during periods of rest or inactivity; 3) symptoms are relieved by movement; 4) the patient demonstrates a circadian pattern with peak symptoms occurring at night or during the evening; and 5) the symptoms are distinct from other medical and behavioral conditions. We found a lower prevalence of RLS in the ESRD population than what has been reported in previous studies [12, 35]. One study conducted in India on patients with chronic renal failure also found a very low prevalence (1.5%) [36], however another study conducted by the same group found RLS to be prevalent in 6.6% of patients on hemodialysis [37]. Most previous studies did not use the most recent diagnostic criteria for RLS which could have resulted in improper diagnosis of RLS in those with legs cramps, peripheral neuropathy, positional discomfort, legs swelling, venous stasis, or arthritis. Another distinct difference is that previous studies have been mainly patient-based, conducted in small centers using survey data or diagnostic codes for restless legs syndrome that were lacking [20, 38]. Low reliability of questionnaires as a screening tool for RLS in a population of chronically dialyzed patients seems to be caused by the presence of other legs symptoms in these patients [39].
Lower prevalence of RLS using ICD-9-CM codes may also suggest a gap in knowledge [40] to recognize the disease or reduced priority in terms of documented comorbidities when reporting to Medicare/Medicaid. Kutner et al. found that RLS was considerably underdiagnosed among patients with kidney failure (0.9%) in the USRDS [41]. Unless a patient is proactive or the symptoms are so severe as to be difficult to miss, it is likely that many more cases of RLS are going unrecognized in this population. In fact, the Restless Legs Syndrome Foundation was established to raise awareness of RLS, improve treatments, and through research, find a cure [42, 43]. Although it was formed in 1992, the foundation recognizes that RLS is a common condition which most people do not know about. To address this, the RLS Foundation has established a network of 11 (9 US; 2 Europe) certified Quality Care Centers (QCC) that are staffed by specialists who provide expert care and tailored disease management of RLS. While there is a growing understanding that RLS is more common than previously believed, the QCC network was formed with the idea that as the number of participating hospitals increases, so too will the knowledge of RLS for both patients and physicians. Additionally, it is possible that physicians ascertain RLS to be a symptom of the overall condition of ESRD patients on hemodialysis, rather than a diagnosable condition, and thus fail to register it using ICD-9 coding [44].
Raising awareness and identifying true cases of RLS has greater implications for the health status of ESRD patients on dialysis. Failure to identify and treat this seemingly common condition can add to the stress experienced by ESRD patients and potentially contribute to additional morbidity [41]. Despite a small case population in this study, the proportion of psychological conditions present in the RLS patient group reveals concerning consequences of sleep disorders. This phenomenon, compounded with potential comorbidities found in the population, indicates a need in the medical community to undertake more careful monitoring of mental health status. Of particular concern is that diagnoses of psychological conditions may be under-reported or biased by self-reporting within the population, indicating a greater need for mental health surveillance [45–47]. Whether depression and/or anxiety are contributing to the development of RLS, and vice versa, remains to be determined. However, given the relative treatability of RLS–including iron replacement, drug treatments (dopamine agonists, alpha-2-delta calcium channel ligands, benzodiazepines, etc.), and non-drug therapies (massages, stretching, exercise, applying a cold compress, among others)–it is reasonable to assume that additional screening may improve the health status in this population.
Limitations
This study has several limitations. Principally, the structure of the USRDS dataset. It is limited by a lack of continuous validation of its methods, lack of complete comorbidity documentation at registration and throughout care, and lack of accuracy of cause-of-death reporting. Data is typically collected via the Centers for Medicare & Medicaid Services (CMS) 2728 form, which is a medical evidence report that is required for all newly diagnosed ESRD patients, regardless of Medicare status or treatment modality. The 2728 form provides critical information to aid caregivers and assure quality of care, however it is not as comprehensive as an electronic health record and is likely limited to the most important or relevant comorbid conditions. The form also describes patient comorbidities as having a current diagnosis or having had the diagnosis in the past ten years, making it difficult to draw strong conclusions about the impact of comorbidity on this patient population. Data is also collected from hospital encounters, however the diagnosis codes are restricted to inpatient visits. This means that physician encounters during dialysis sessions and outpatient visits are not captured, thus restricting the scope of data. The method in which data is collected leaves several variables with missing or incomplete data, and also excludes potentially important co-variables such as parathyroid hormone (PTH) levels, dialysis adequacy, and treatment regimens of RLS.
Therefore, it is reasonable to assume that cases of RLS were missed and might be found in the control group. Similarly, the overall prevalence of anxiety and depression in the population are lower than expected. It has been well documented that ESRD patients on dialysis have a high incidence of depression and anxiety [48–52], however this is not reflected in our dataset. We are unable to determine whether these conditions are typically under-reported by the nephrologists completing the medical evidence forms, by the attending physicians in the hospital, or if the patient population represented by our study do not suffer from mental health and/or sleep disorders at the same rate. Due to the de-identified nature of the USRDS dataset, we are unable to perform sensitivity/specificity analyses to confirm the presence or absence of comorbid conditions.
Finally, contrary to expectations, there was a lack of association between diabetes and mortality, as well as cardiovascular disease and mortality. Given these well-documented associations in previous literature, it is possible that the results suffer from misclassification of exposure biases as we rely solely on inpatient diagnosis data and the 2728 form–of which the limitations have been described.
Conclusion
This study examined the relationships between RLS diagnosis, comorbidities, and mortality in ESRD patients. Sleep disorders, like RLS, may impact health outcomes of this patient population, particularly those undergoing hemodialysis therapy. We used the USRDS database which has advantages given its size and almost complete inclusion of the ESRD population in the United States, but inherent limitations, such as completeness of data in the Medical Evidence Report at initiation of renal replacement therapy, have been well described.
We conclude that in a nationally representative sample, RLS diagnosis was not associated with mortality in hemodialysis patients. The high prevalence of other psychiatric diagnoses in patients with RLS calls for greater awareness in hemodialysis units but whether screening and management of these psychiatric diagnosis will improve quality of life will need to be tested in prospective studies.
Acknowledgements
The execution of the study could not have been achieved without the work of the United States Renal Data System in collecting health care data on U.S. patients diagnosed with end-stage renal disease.
Funding
Not applicable.
Availability of data and materials
Results from the study were drawn from the USRDS Standard Analysis Files, which can be obtained upon request at usrds.org. We cannot make the data available because it requires Data Use Agreements and IRB approval from an investigator’s home institution.
Abbreviations
- ESRD
End stage renal disease
- ICD-9-CM
International Classification of Diseases-Ninth Revision-Clinical Modification
- IRLSSG
International Restless legs Syndrome Study Group
- KM
Kaplan Meier
- NSD
No sleep disorder
- RLS
Restless Legs syndrome
- USRDS
United States Renal Data System
Additional file
Classification of sleep disorders and associated conditions. (DOCX 17 kb)
Authors’ contributions
JJD, AB, and MOS designed the study. JJD and UG conducted statistical analyses, with JJD generating the tables and UG creating the survival curve figures. KGL help to conceptualize and write the discussion and conclusion. DC contributed mental health consultation in the study design and results interpretation. MOS facilitated acquisition of the data set from the USRDS. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable, this study did not involve human or animal data or tissue. Use of the data was approved by the SUNY Downstate Medical Center Institutional Review Board and the USRDS via data use agreements.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12882-017-0660-0) contains supplementary material, which is available to authorized users.
Contributor Information
Joseph J. DeFerio, Email: jod2033@med.cornell.edu
Usha Govindarajulu, Email: usha.govindarajulu@downstate.edu.
Amarpali Brar, Email: amarpali.brar@downstate.edu.
Daniel Cukor, Email: daniel.cukor@downstate.edu.
Kathleen G. Lee, Email: kal2040@med.cornell.edu
Moro O. Salifu, Email: moro.salifu@downstate.edu
References
- 1.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Merlino G, Piani A, Dolso P, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21(1):184–190. doi: 10.1093/ndt/gfi144. [DOI] [PubMed] [Google Scholar]
- 3.Hanly P. Sleep disorders and end-stage renal disease. Curr Opin Pulm Med. 2008;14(6):543–550. doi: 10.1097/MCP.0b013e3283130f96. [DOI] [PubMed] [Google Scholar]
- 4.Stepanski E, Faber M, Zorick F, Basner R, Roth T. Sleep disorders in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1995;6(2):192–197. doi: 10.1681/ASN.V62192. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel PL, Miller G, Mendelson WB. Sleep apnea syndrome in chronic renal disease. Am J Med. 1989;86(3):308–314. doi: 10.1016/0002-9343(89)90301-X. [DOI] [PubMed] [Google Scholar]
- 6.Kosmadakis GC, Medcalf JF. Sleep disorders in dialysis patients. Int J Artif Organs. 2008;31(11):919–927. doi: 10.1177/039139880803101101. [DOI] [PubMed] [Google Scholar]
- 7.Molzahn AE, Northcott HC, Dossetor JB. Quality of life of individuals with end stage renal disease: perceptions of patients, nurses, and physicians. Anna j. 1997;24(3):325–333. [PubMed] [Google Scholar]
- 8.Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20(3):571–577. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- 9.Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996;28(3):372–378. doi: 10.1016/S0272-6386(96)90494-1. [DOI] [PubMed] [Google Scholar]
- 10.Unruh ML, Levey AS, D'Ambrosio C, Fink NE, Powe NR, Meyer KB. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43(5):900–909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Eeden SK, Albers KB, Davidson JE, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort Study from Kaiser Permanente northern California. Sleep. 2015;38(7):1009–1015. doi: 10.5665/sleep.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Manna G, Pizza F, Persici E, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26(6):1976–1983. doi: 10.1093/ndt/gfq681. [DOI] [PubMed] [Google Scholar]
- 13.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14(2):163–165. [PubMed] [Google Scholar]
- 14.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walters AS, Rye DB. Evidence continues to mount on the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep. 2010;33(3):287. doi: 10.1093/sleep/33.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz RL, Pressman MR, Hovick ET, Peterson DD. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35(6):1052–1060. doi: 10.1016/S0272-6386(00)70039-4. [DOI] [PubMed] [Google Scholar]
- 17.Molnar MZ, Szentkiralyi A, Lindner A, et al. Restless legs syndrome and mortality in kidney transplant recipients. Am J Kidney Dis. 2007;50(5):813–820. doi: 10.1053/j.ajkd.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kavanagh D, Siddiqui S, Geddes CC. Restless legs syndrome in patients on dialysis. Am J Kidney Dis. 2004;43(5):763–771. doi: 10.1053/j.ajkd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18(2):128–147. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995;26(5):751–756. doi: 10.1016/0272-6386(95)90438-7. [DOI] [PubMed] [Google Scholar]
- 21.Huiqi Q, Shan L, Mingcai Q. Restless legs syndrome (RLS) in uremic patients is related to the frequency of hemodialysis sessions. Nephron. 2000;86(4):540. doi: 10.1159/000045861. [DOI] [PubMed] [Google Scholar]
- 22.Takaki J, Nishi T, Nangaku M, et al. Clinical and psychological aspects of restless legs syndrome in uremic patients on hemodialysis. Am J Kidney Dis. 2003;41(4):833–839. doi: 10.1016/S0272-6386(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 23.Kawauchi A, Inoue Y, Hashimoto T, et al. Restless legs syndrome in hemodialysis patients: health-related quality of life and laboratory data analysis. Clin Nephrol. 2006;66(6):440–446. doi: 10.5414/CNP66440. [DOI] [PubMed] [Google Scholar]
- 24.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions ("mimics") Sleep Med. 2009;10(9):976–981. doi: 10.1016/j.sleep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960–967. doi: 10.2215/CJN.00990207. [DOI] [PubMed] [Google Scholar]
- 27.Bliwise DL, Zhang RH, Kutner NG. Medications associated with restless legs syndrome: a case-control study in the US renal data system (USRDS) Sleep Med. 2014;15(10):1241–1245. doi: 10.1016/j.sleep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons L. Reducing bias in a propensity score matched pair sample using greedy matching techniques. Paper presented at: the twenty sixth annual SAS users group international conference, 2001; Cary. NC.
- 29.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 30.Herzog CA. How to manage the renal patient with coronary heart disease: the agony and the ecstasy of opinion-based medicine. J Am Soc Nephrol. 2003;14(10):2556–2572. doi: 10.1097/01.ASN.0000087640.94746.47. [DOI] [PubMed] [Google Scholar]
- 31.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 32.United States Renal Data System. 2014 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases;2014.
- 33.Stefanidis I, Vainas A, Giannaki CD, et al. Restless legs syndrome does not affect 3-year mortality in hemodialysis patients. Sleep Med. 2015;16(9):1131–1138. doi: 10.1016/j.sleep.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Lin CH, Sy HN, Chang HW, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol. 2015;22(1):142–149. doi: 10.1111/ene.12545. [DOI] [PubMed] [Google Scholar]
- 35.Gigli GL, Adorati M, Dolso P, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5(3):309–315. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Bhowmik D, Bhatia M, Tiwari S, et al. Low prevalence of restless legs syndrome in patients with advanced chronic renal failure in the Indian population: a case controlled study. Ren Fail. 2004;26(1):69–72. doi: 10.1081/JDI-120028557. [DOI] [PubMed] [Google Scholar]
- 37.Bhowmik D, Bhatia M, Gupta S, Agarwal SK, Tiwari SC, Dash SC. Restless legs syndrome in hemodialysis patients in India: a case controlled study. Sleep Med. 2003;4(2):143–146. doi: 10.1016/S1389-9457(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 38.Hui DS, Wong TY, Ko FW, et al. Prevalence of sleep disturbances in chinese patients with end-stage renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;36(4):783–788. doi: 10.1053/ajkd.2000.17664. [DOI] [PubMed] [Google Scholar]
- 39.Cirignotta F, Mondini S, Santoro A, Ferrari G, Gerardi R, Buzzi G. Reliability of a questionnaire screening restless legs syndrome in patients on chronic dialysis. Am J Kidney Dis. 2002;40(2):302–306. doi: 10.1053/ajkd.2002.34508. [DOI] [PubMed] [Google Scholar]
- 40.Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO controversies conference on supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–459. doi: 10.1038/ki.2015.110. [DOI] [PubMed] [Google Scholar]
- 41.Kutner NG, Zhang R, Bliwise DL. Restless legs syndrome is underdiagnosed in the US renal data system. QJM. 2013;106(5):487. doi: 10.1093/qjmed/hct014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Restless Legs Syndrome Foundation Inc. About Us. 2016; https://www.rls.org/about-us. Accessed 05/04/2017.
- 43.Trenkwalder C. Restless legs syndrome: overdiagnosed or underdiagnosed? Nat Clin Pract Neurol. 2007;3(9):474–475. doi: 10.1038/ncpneuro0552. [DOI] [PubMed] [Google Scholar]
- 44.Calvino J, Cigarran S, Lopez LM, Martinez A, Sobrido MJ. Restless legs syndrome in non-dialysis renal patients: is it really that common? J Clin Sleep Med. 2015;11(1):57–60. doi: 10.5664/jcsm.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watnick S, Kirwin P, Mahnensmith R, Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41(1):105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 46.Shirazian S, Diep R, Jacobson AM, Grant CD, Mattana J, Calixte R. Awareness of chronic kidney disease and depressive symptoms: National Health and nutrition examination surveys 2005-2010. Am J Nephrol. 2016;44(1):1–10. doi: 10.1159/000446929. [DOI] [PubMed] [Google Scholar]
- 47.Cohen SD, Cukor D, Kimmel PL. Anxiety in patients treated with hemodialysis. Clin J Am Soc Nephrol. 2016; [DOI] [PMC free article] [PubMed]
- 48.Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042–3055. doi: 10.1681/ASN.2007030345. [DOI] [PubMed] [Google Scholar]
- 49.Cukor D, Coplan J, Brown C, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(3):484–490. doi: 10.2215/CJN.00040107. [DOI] [PubMed] [Google Scholar]
- 50.Cukor D, Coplan J, Brown C, et al. Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008;52(1):128–136. doi: 10.1053/j.ajkd.2008.02.300. [DOI] [PubMed] [Google Scholar]
- 51.Cukor D, Peterson RA, Cohen SD, Kimmel PL. Depression in end-stage renal disease hemodialysis patients. Nat Clin Pract Nephrol. 2006;2(12):678–687. doi: 10.1038/ncpneph0359. [DOI] [PubMed] [Google Scholar]
- 52.Yoong RK, Mooppil N, Khoo EY, et al. Prevalence and determinants of anxiety and depression in end stage renal disease (ESRD). A comparison between ESRD patients with and without coexisting diabetes mellitus. J Psychosom Res. 2017;94:68–72. doi: 10.1016/j.jpsychores.2017.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Results from the study were drawn from the USRDS Standard Analysis Files, which can be obtained upon request at usrds.org. We cannot make the data available because it requires Data Use Agreements and IRB approval from an investigator’s home institution.


