Abstract
We report the size-controlled self-assembly of polymersomes through the cooperative self-assembly of nanoparticles and amphiphilic polymers. Polymersomes densely packed with magnetic nanoparticles in the polymersome membrane (magneto-polymersome) were fabricated with a series of different sized iron oxide nanoparticles. The distribution of nanoparticles in a polymersome membrane was size-dependent; while small nanoparticles were dispersed in a polymer bilayer, large particles formed well-ordered superstructure at the interface between the inner and outer layer of a bilayer membrane. The yield of magneto-polymersomes increased with increasing the diameter of incorporated nanoparticles. Moreover, the size of polymersomes was effectively controlled by varying the size of incorporated nanoparticles. This size-dependent self-assembly was attributed to the polymer chain entropy effect and the size-dependent localization of nanoparticles in polymersome bilayers. The transverse relaxation rates (r2) of magneto-polymersomes increased with increasing the nanoparticle diameter and decreasing the size of polymersomes, reaching 555 ± 24 s−1mM−1 for 241 ± 16 nm polymersomes, which is the highest value reported to date for superparamagnetic iron oxide nanoparticles.
Keywords: Block copolymer, nanoparticle, polymersome, vesicle, amphiphilic, superparamagnetic
TOC image
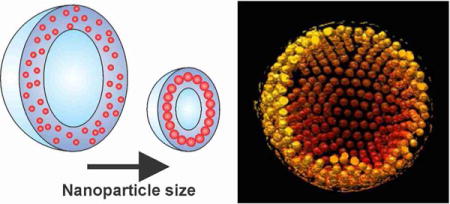
Vesicles made of amphiphilic polymers (polymersomes) have received significant attention in recent years for a number of applications such as in vivo imaging and drug delivery due to their structure and properties mimicking biological membranes1–3 as well as their chemical versatility.4–7 For successful implementation of polymersomes in many of the applications, it is critical to fabricate size-controlled polymersomes loaded with desired functional encapsulates.2, 8, 9 In nature, biological vesicles can adopt a broad range of membrane curvatures and sizes through the intricate and dynamic interactions between phospholipid bilayers and nanometer-scale cellular components (e.g., membrane proteins),10 which is important in many biological processes.11 Here, we show that a similar structural behavior occurs in the binary self-assembly of amphiphilic polymers and artificial nanoparticles, and demonstrate that size-controlled polymersomes can be formed by the incorporation of nanoparticles in the polymersome membrane.
It is indeed a challenging task to prepare uniform sized polymersomes with a desirable diameter. Commonly used fabrication techniques typically produce polydisperse assemblies with broad size distributions12, 13 because vesicle formation depends much on nonequilibrium aspects of the assembly process.14 For example, polymersomes formed by the film hydration method possess a size distribution ranging from ~ 100 nm to ~ 10 μm. Therefore, post-assembly processes such as membrane extrusion, sonication, freeze-thaw cycles are typically used to narrow the size distribution.15–18 Recently, a few elegant approaches have been developed to fabricate uniform sized polymer vesicles.19, 20 For example, Ryan et al. reported that micrometer-sized polymersomes with low polydispersity can be fabricated by the hydration of lithographically patterned block-copolymer films.19 Weitz and coworkers showed that highly monodisperse polymersomes can be fabricated using a microfluidic device.20 For smaller submicrometer-sized polymersomes, which are much needed for in vivo applications,9 inkjet,21 microfluidics,22 and nanoprecipitation23–25 methods have been successively utilized to make polymersomes with narrow size distribution.
Herein, we report a novel approach to fabricate size-controlled sub-micrometer polymersomes based on the cooperative self-assembly of inorganic nanoparticles and amphiphilic polymers and show how the size of nanoparticles influences the binary self-assembly. We show that the incorporated nanoparticles in polymersome membranes control the membrane curvature and the size of polymersomes through the size-dependent localization of nanoparticles in the polymersome membrane. Based on the approach, we fabricated uniform submicrometer sized superparamagnetic polymersomes loaded with iron oxide nanoparticles (magneto-polymersomes) in controllable diameters, and achieved exceptionally high transverse relaxation rates.26
RESULTS AND DISCUSSION
In typical experiments, magneto-polymersomes were formed by inducing the self-assembly of amphiphilic polymers in the presence of oleic acid-stabilized iron oxide nanoparticles by the slow water addition method, following our previously reported procedure (Figure 1a).27 A series of different sized iron oxide nanoparticles (5.6 ± 0.5 nm, 5.8 ± 0.7 nm, 6.4 ± 0.5 nm, 9.9 ± 0.8 nm, 10.8 ± 0.7 nm, 15.5 ± 0.8 nm, 16.3 ± 1.1 nm, and 19.9 ± 1.3) were synthesized by a modified literature procedure28, 29 to study the nanoparticle size effect on the self-assembly of magneto-polymersomes. The as-synthesized nanoparticles were assembled with an amphiphilic polymer of poly(acrylic acid) and polystyrene (PAA-b-PS) without any surface modification (Figure 1a).27 Briefly, PAA38-b-PS73 in 1,4 dioxane (dioxane, 1500 μL) and nanoparticles in tetrahydrofurane (THF, 50 μL) were first mixed together at 25 nanoparticle weight percent (np wt%). Then, 600 μL of water was slowly added to the solution at the rate of 10 μL/30 sec for 30 min to induce self-assembly of amphiphilic polymers and nanoparticles. Resulting co-assemblies were dispersed in water by dialysis and centrifugation and characterized by dynamic light scattering (DLS) and transmission electron microscopy (TEM).
Figure 1.
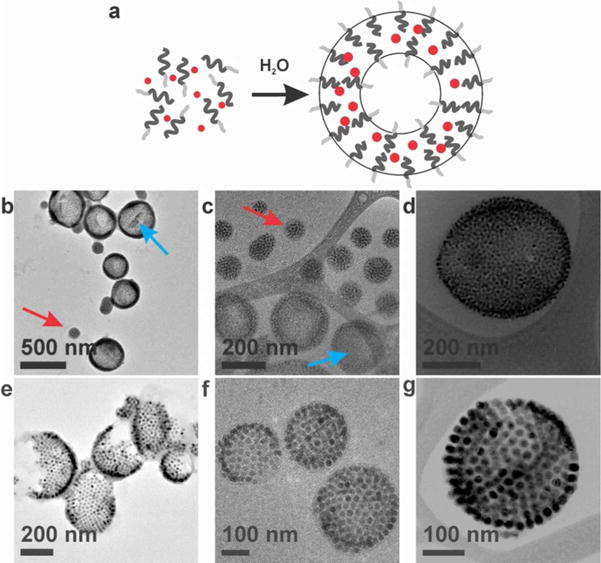
(a) Schematic representation of the self-assembly of magneto-polymersomes from iron oxide nanoparticles and amphiphilic block copolymers. Light gray lines, dark gray lines, and red dots represent PAA, PS, and iron oxide nanoparticles, respectively. (b–d) Conventional TEM (b) and cryo-TEM (c) and cryo-STEM (d) images of magneto-polymersomes assembled with 10.8 nm particles. Blue and red arrows indicate polymersomes and micelles, respectively. (e–g) Conventional TEM (e), cryo-TEM (f), and cryo-STEM (g) images of magneto-polymersomes assembled with 19.9 nm particles. Cryo-TEM images show intact magneto-polymersomes, indicating that the broken polymersomes observed in normal TEM (e) are due to the drying process.
The TEM images showed well-defined polymersomes densely loaded with nanoparticles in the polymersome membrane (Figure 1). Cryo-TEM images (Figure 1c–d and f–g) revealed that the broken polymersomes shown in normal TEM images (Figure 1e) are actually intact when dispersed in water (Figure 1f–g). They also show that large nanoparticles (19.9 nm, Figure 1f–g) form well-ordered curved two-dimensional superlattices30, 31 in the polymersome membrane (vide infra).
To confirm the hollow structure of polymersomes, magneto-polymersomes assembled with 10.8 nm and 16.3 nm particles were imaged by TEM tomography (Figure 2, Movie 1, and Movie 2). At roughly the half-way slicing through the vesicle containing 10.8 nm particles (Figure 2a.v, Movie 1), clear polymer edges are seen on both sides of nanoparticles. The three-dimensional (3-D) surface rendering of the tomographic volume (Figure 2b) also confirms the hollow structure with nanoparticles located in the vesicle wall. The tomography data of magneto-polymersomes containing 16.3 nm particles (Figure 2c, Movie 2) shows well-ordered arrays of nanoparticles as well as the hollow structure.
Figure 2.
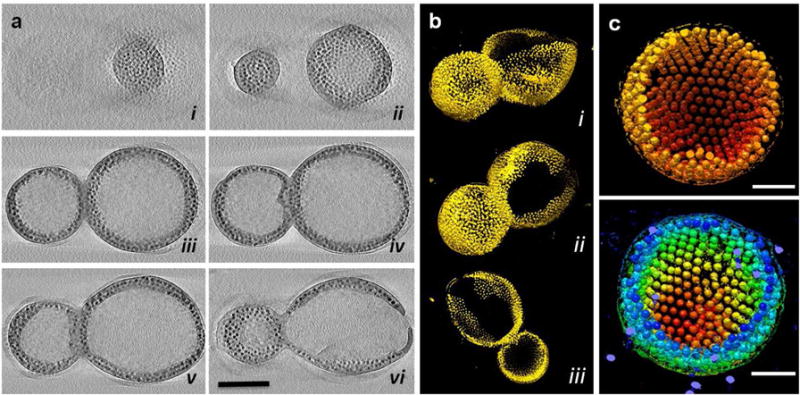
TEM tomography of magneto-polymersomes assembled with 10.8 and 16.3 nm iron oxide particles. (a) 0.23 nm thick X-Y computational slices (i–vi) of the 3-D tomographic volume containing magneto-polymersomes assembled with 10.8 nm iron oxide particles, shown in every 60 slices (13.8 nm) through the volume. The scale bar is for 200 nm. (b) 3-D surface rendering of the polymersomes shown in a, viewed from side (i), bottom (ii) and as a slab (iii). (c) 3-D surface rendering of a magneto-polymersome assembled with 16.3 nm iron oxide particles, showing nanoparticles form ordered arrays in the polymersome. The magneto-particles are colored red to yellow according to the radial position from the center (top) or red to blue according to the height (bottom). The scale bar is for 100 nm.
In order to investigate the nanoparticle size effect on the self-assembly of magneto-polymersomes, a range of different sized nanoparticles were self-assembled with a micelle-forming polymer with a relatively short PS, PAA38-b-PS73 at a constant nanoparticle weight percent (25 np wt%) (Figure 3). The incorporation of nanoparticles induced a micelle-to-vesicle morphology change, consistent with our previous result,27 yielding mixtures of magneto-polymersomes (Figure 3a–c, blue arrows) and magneto-micelles (Figure 3a–c, red arrows) in varying ratios. Interestingly, the population of polymersomes increased with the size of nanoparticles and reached 82% polymersome yield for 16.3 nm particles (Figure 3d), revealing that the diameter of incorporated nanoparticles is an important factor that affects the polymersome formation. We hypothesize that the size-dependent polymersome yield is due to the entropic cost of inserting large nanoparticles in a polymer domain. This factor should be especially important for amphiphilic polymers with a relatively short hydrophobic block used in this study. As mentioned above, cryo-TEM and TEM tomography data showed that large nanoparticles form ordered two-dimensional superlattices (Figure 2c, Figure 4c) presumably at the PS-PS interface between the inner and outer layer of the polymersome membrane (Figure 4d (right)). The formation of ordered superlattices is believed to be the result of the large entropic cost caused by inserting large nanoparticles within a PS domain. Small particles on the other hand can be dispersed in a polymer domain of polymersomes (Figure 4a, Figure 4d (left)) or micelles (Figure 1c) without causing too much entropic penalty. Similar behavior has been observed in thin film self-assembly studies of A-B block copolymers and A-grafted nanoparticles, where large nanoparticles were segregated to the center of A domain of a lamella phase while small particles were embedded within a polymer domain.32 Note that in solution phase self-assembly studied here, a large area A-A polymer interface is obtained by adopting vesicle or bilayer structures. Therefore, the addition of large particles can induce effective micelle-to-vesicle morphology changes.
Figure 3.
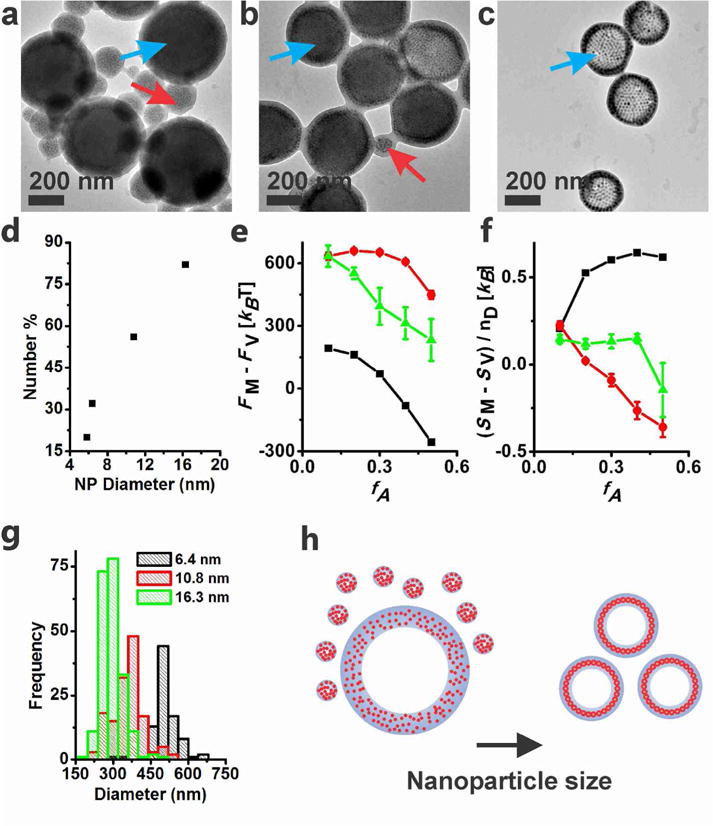
(a–c) TEM images of magneto-polymersomes assembled with (a) 6.4 nm, (b) 10.8 nm, and (c) 16.3 nm iron oxide particles at 25 np wt %. Blue and red arrows indicate polymersomes and micelles, respectively. (d) TEM analysis of polymersome number percent, which is defined by 100% times the number of polymersomes over the number of assemblies (polymersomes and micelles). Over 200 assemblies were counted for the analysis. (e) The calculated relative Helmholtz free energy of nanoparticle-free (black) or nanoparticle-loaded (green and red) polymer assemblies as a function of fA for two different nanoparticle radii (green: 1.34Rg, red: 2.24Rg). (f) The average entropy per diblock copolymer chain in vesicles (SV) minus that in micelles (SM). The error bars in (e) and (f) represent the standard error averaged over three different micelle configurations for the larger particle system (2.24Rg), and five configurations for the smaller particle system (1.34Rg). In all calculations, fA was varied while keeping the chain length N of the polymer constant. (g) Size histogram of magneto-polymersomes determined by TEM. (h) A pictorial description showing how the nanoparticle size affects the polymersome population and dimension.
Figure 4.
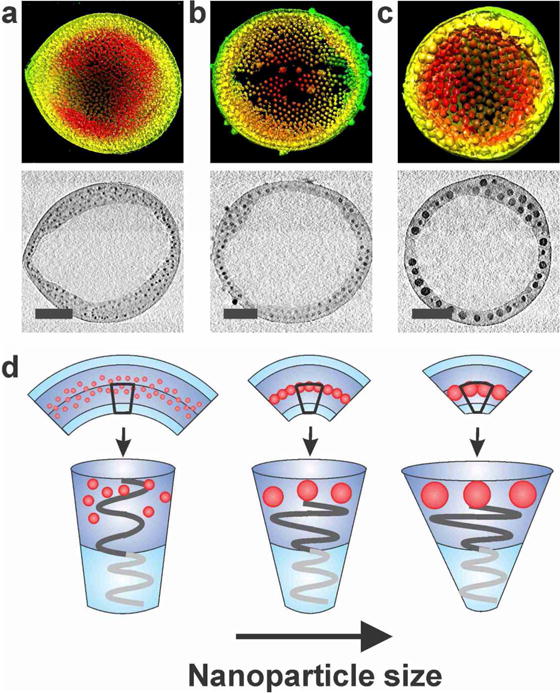
Reconstructed 3-D surface rendering of the tomographic volume and X-Y computational slices (0.23 nm) at the mid-point of magneto-polymersomes assembled with (a) 5.8 nm, (b) 9.9 nm, and (c) 16.3 nm iron oxide particles. Scale bars are 100 nm. (d) Pictorial representation showing how the nanoparticle size affects the curvature of polymersome membranes.
To further understand the nanoparticle-induced morphology change, we modeled our system in two dimensions using a variation of polymer self-consistent field theory (SCFT)33, 34 known as the hybrid particle-field theory.35 Polymer chains were treated on a coarse-grained scale as A-B diblock copolymers immersed in a solvent that is chemically identical to the hydrophilic A block of the copolymer. The interactions between the hydrophilic components (A), hydrophobic polymer block (B), and the nanoparticles were each captured through a set of distinct Flory χ parameters to ensure strong phase separation between A and B components and preferential wetting of nanoparticles by the hydrophobic B block of the copolymer. The relative free energy and polymer chain entropy were calculated under a variety of nanoparticle loading conditions for two different nanoparticle sizes and varying volume fraction of the hydrophilic polymer (fA). Representative images of nanoparticle-loaded micelles and polymersomes used in the calculations are shown in supporting information (Figures S5 and S6). The calculated free energy and entropy values are plotted as a function of fA in Figures 3e and 3f. In the absence of nanoparticles, micelles were found to be more stable than vesicles for a wide range of fA (fA > 0.3) (Figure 3e, black). Upon the incorporation of nanoparticles, the free energy of vesicles (FV) became substantially lower than that of micelles (FM) at all calculated values of fA (Figure 3e, green and red), indicating that the vesicle structure becomes more stable than micelles with the addition of nanoparticles. The entropic penalty of forming polymersomes relative to that of forming micelles is significantly reduced with the incorporation of nanoparticles (Figure 3f), supporting that the polymer entropy indeed contributes to the nanoparticle-induced polymersome formation. While these calculations do not necessarily predict that the simulated polymersomes are the most stable structure, they clearly show how the polymersome formation can be favored over micelles in the presence of nanoparticles.
Furthermore, the stabilization of polymersomes was found to be more pronounced for larger particles (Figure 3e, f), which explains the experimentally observed size-dependent polymersome yield shown in Figure 3d. When the nanoparticle radius (Rp) was increased from 1.34 Rg to 2.24 Rg, where Rg is the radius of gyration of the block copolymer, the free energy difference between the polymersomes and micelles became significantly larger (Figure 3e, green and red). The entropic cost for incorporating nanoparticles in micelles compared to that in vesicles was also found to be substantially larger for larger particles for a wide range of fA (Figure 3f). These results are consistent with our hypothesis that the entropic cost of incorporating nanoparticles in a polymer matrix is responsible for the micelle-to-vesicle morphology change.
Interestingly, we found that the size of polymersomes can be effectively controlled by varying the size of incorporated nanoparticles. The TEM (Figure 3g) and DLS (Figure S2) measurements revealed that the polymersome size gradually decreased with increasing the size of nanoparticles, as illustrated in Figure 3h. We attribute the size-controlled assembly of polymersomes to the asymmetric layer structure of vesicle membranes and the size-dependent nanoparticle distribution in the membrane. The bilayer making up the vesicle membrane is composed of an inner and an outer layer of amphiphilic polymers with distinct molecular packing structures; the outer layer has a smaller relative volume for the hydrophobic block while the inner layer has a larger relative volume for the hydrophobic block. This distinct layer structure is shown to be important for the formation of vesicles. For example, Eisenberg and coworkers have reported that a mixture of different length PAA-b-PS tend to assemble into polymersomes by segregating polymers with longer PS chains (or shorter PAA chains) in the inner layer of polymersomes.36, 37 In the binary self-assembly of nanoparticles and amphiphilic polymers studied here, nanoparticles are expected to preferentially partition in the inner layer to support the asymmetric layer structure of polymersome membranes. As mentioned above, small particles (5.8 nm, Figure 4a) are dispersed throughout the membrane presumably with more particles concentrated in the inner layer. For large nanoparticles forming a monolayer at the PS-PS interface of the polymersome wall, a larger fraction of each nanoparticle should be immersed in the inner layer to support the asymmetric layer structure (Figure 4c, Figure S3). The selective localization of nanoparticles in the inner layer should be more pronounced for larger particles. Therefore, the curvature of the membrane becomes higher with the incorporation of larger particles, resulting in smaller polymersomes, as illustrated in Figure 4d. In a related work, Einsenberg and coworkers previously showed that the self-assembly of poly(amido amine) dendrimers and PAA-b-PS resulted in the incorporation of PS-coated dendrimers into vesicle wall.38, 39 In that work, the size of polymersomes became larger with larger dendrimers due to thicker vesicle wall. The size trend observed with a vesicle forming polymer and PS-coated dendrimers is the opposite of the size dependence observed here with a short polymer and alkyl-terminated hard nanoparticles. It indicates that the polymer entropy plays an important role in the morphology change and the unusual size dependence observed here with a micelle-forming polymer with short PS. On the other hand, the nanoparticle weight percent did not significantly affect the size of polymersomes (Figure 5), supporting that the change in the polymersome size with the dimension of incorporated nanoparticles is not due to the simple hydrophobic volume effect.
Figure 5.
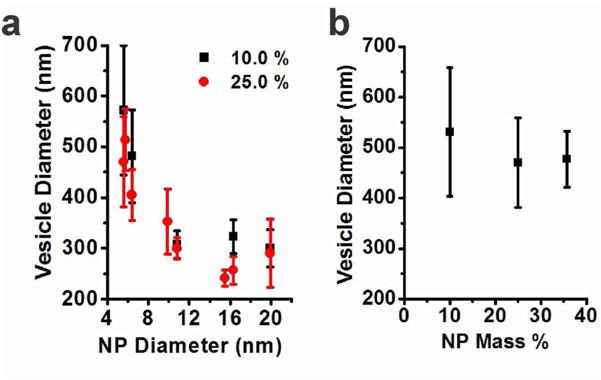
(a) The diameters of magneto-polymersomes incorporating 5.6, 5.8, 6.4, 9.9, 10.8, 15.5, 16.3, and 19.9 nm particles determined by DLS at two different nanoparticle weight percent. The standard deviation was calculated from three different measurements. (b) The hydrodynamic diameter of magneto-polymersomes formed with 5.6 nm particles as a function of nanoparticle weight percent.
To evaluate the magneto-polymersomes for their potential use as an MRI contrast agent, transverse relaxivity rates (r2) were measured for magneto-polymersomes prepared from different sized nanoparticles and are plotted in Figure 6 along with the size of polymersomes. The r2 of water surrounding nanoparticle assemblies is known to increase with increasing the nanoparticle size40 or increasing the size of nanoparticle assemblies.29, 41–43 In magneto-polymersomes studied here, the r2 values gradually increased with increasing the nanoparticle diameter and decreasing the polymersome size, indicating that the nanoparticle size effect is dominant.26 This result is ideal as both smaller sized polymersomes and higher r2 values are desirable for MRI applications. Polymersomes assembled with 15.5 nm particles had an average hydrodynamic diameter of 241 ± 16 nm (DLS), which is comparable to the size of typical polymersomes (~ 300 nm) made by the thin film hydration and membrane extrusion.15 The r2 value of the 241 nm magneto-polymersomes was determined to be 555 ± 24 s−1mM−1, which is the highest value reported thus far for superparamagnetic iron oxide particles.26
Figure 6.
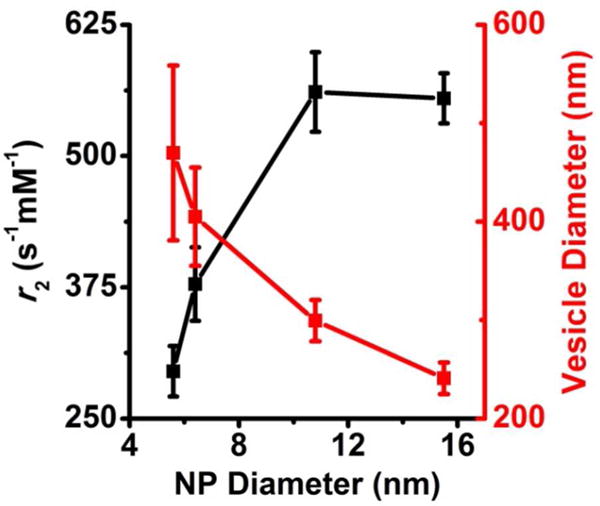
Transverse relaxivity rates (r2) for magneto-polymersomes loaded with different sized iron oxide nanoparticles at 25 np wt %. The r2 values (black) were calculated to be 295 ± 24 s−1mM−1, 378 ± 35 s−1mM−1, 561 ± 38 s−1mM−1, and 555 ± 24 s−1mM−1 for magneto-polymersomes made from iron oxide nanoparticles of diameter 5.6 nm, 6.4 nm, 10.8 nm, and 15.5 nm, respectively. The sizes of the magneto-polymersomes (red) from Figure 5b were plotted along with the r2 values. The standard deviations of the r2 values were determined from at least three separate samples. The plots of inverse transverse relaxation time (1/T2) versus iron molar concentration are provided in the supporting information.
CONCLUSIONS
In summary, polymersomes densely loaded with iron oxide nanoparticles were fabricated by the cooperative self-assembly of nanoparticles and amphiphilic polymers of PAA-b-PS. It was found that the yield of polymersomes increases with the size of incorporated nanoparticles as well as nanoparticle weight fraction when a micelle-forming polymer was used for the self-assembly. The size-dependent morphology change was explained by the entropic cost caused by incorporating large nanoparticles in a polymer domain. Furthermore, we found that the size of nanoparticles determines the size of polymersomes. This size-controlled self-assembly was explained by the partitioning of large nanoparticles towards the inner layer of polymersome membranes, which results in a large membrane curvature. This behavior is reminiscent of how protein binding and clustering affect the curvature of cell membranes. Based on the approach, we were able to reduce the polymersome diameter from 513 ± 76 nm to 257 ± 90 nm by increasing the nanoparticle diameter from 5.8 nm to 16.3 nm. Note that controlling the size of polymersomes is a challenging task because typical fabrication techniques rely on kinetic processes. The nanoparticle-induced self-assembly of polymersomes reported here provides a new and reliable way to form size-controlled hollow polymer assemblies possessing the functionalities of nanoparticles.
METHODS AND MATERIALS
Materials and instrumentation
Sodium oleate (TCI, 95%), oleic acid (Aldrich, 90%), 1-octadecene (Aldrich, 90%), 1,4-dioxane (99 +%, Extra Pure, Stabilized, Acros Organics) were used as received without further purification. THF was freshly distilled prior to use from sodium/benzophenone under nitrogen. Iron oxide nanoparticles were synthesized by a modified literature procedure.28, 29 Block copolymers of PAAm-b-PSn were synthesized by the reversible addition fragmentation chain transfer (RAFT) polymerization following a previously reported procedure.44 Magnetic relaxivity measurements and iron concentrations were determined following a previously reported procedure.27 Magneto-polymersomes were characterized by cryo-TEM following a previously reported procedure.45 Conventional TEM images were taken using a JEOL 1400 electron microscope, cryo-TEM images where taken using both a JEOL 2010 and JEOL 2010F, and STEM images were acquired using a JEOL 2010F electron microscope. Dynamic light scattering (DLS) measurements were taken with a Malvern Zetasizer Nano Series.
Self-assembly
To generate magneto-polymersomes, iron oxide nanoparticles and PAA38-b-PS73 were self-assembled by the slow addition of water to dioxane/THF (96.8% dioxane) solution of nanoparticles and polymers. In typical experiments, a THF solution of 5.8 nm nanoparticles (50 μL of a 3.0 mg/mL) was mixed with a PAA38-b-PS73 in dioxane (500 μL, 0.9 mg/mL) at 25 np wt%. Here, the weight percent of nanoparticles is defined by 100 % times the mass of nanoparticles over the combined mass of nanoparticles and polymers. The total volume of the nanoparticle/polymer solution was adjusted to 1.55 mL by adding additional dioxane. Then, water (600 μL) was slowly added (10 μL per 30 s) to the mixture of nanoparticles and block copolymers while stirring. The mixture was kept under stirring for 15 hours before adding additional water (1.5 mL) over 15 minutes. Then, the sample was dialyzed against water for 24 hours, and concentrated by centrifugation (14,000 rpm, 30 min). The purified assemblies were redispersed in 200 μL of deionized water.
TEM tomography
For electron tomography, tilted projection images collected at 1° tilt intervals from −76° to 78° were recorded with a Gatan 4K × 4K charge-coupled device (CCD) camera (Gatan Inc., Pleasanton, CA) mounted on a Tecnai F20 electron microscope (FEI Corporation, Hillsboro, OR) equipped with a field emission gun (FEG) operating at 200 kV. An ultra-high tilt room temperature tomography holder Gatan 916 (Gatan Inc., Pleasanton, CA) was used to access high tilt angles. A series of images were recorded at a nominal magnification of 29,000 × and at under-focus value of 1.5μm along the tilt axis. A back-projection algorithm, as implemented in the IMOD reconstruction package,46 was used to convert the information present in the series of tilted projection images into three-dimensional density maps. The surface rendering was generated using the Chimera software.47
Self-consistent field theory
A two-dimensional polymer field theory was used to model nanoparticle-loaded vesicles and micelles.33, 34 We used a coarse-grained model of A-B diblock copolymers in a solvent that is chemically identical to the hydrophilic A block of the copolymer. As we are primarily concerned with relatively short polymers, we employed the discrete Gaussian chain model for the diblock copolymers. Each chain was consisted of 30 total segments (N = NA+NB). The composition of the diblock copolymer given as fA (= NA / N) was varied by changing NA while keeping N constant. Nanoparticles were introduced using the hybrid particle-field theory reported by Sides et al.35 The repulsion between A and B components was captured by setting the Flory chi parameter of the A/B pair (χAB) to 2.0. The chi parameters of A/nanoparticle pair (χAP) and B/nanoparticle pair (χBP) were set to 0 and 5.0 respectively to enforce the preferential wetting of nanoparticles by the hydrophobic polymer block. The detailed derivation of our model and our numerical approach is presented in the Supporting Information.
Supplementary Material
Acknowledgments
S.J.P. acknowledges the support from NSF career award (DMR 0847646), ARO young investigator award, and the Camille Dreyfus teacher scholar award. This work was supported in part by the National Institutes of Health (GM085043 to PZ). Cryo-TEM images were taken at the Penn Regional Nanotechnology Facility. The authors thank Dr. Doug Yates for helpful discussions on the TEM analysis. The authors thank Kevin Vargo and Eric Johnston from Professor Daniel Hammer’s group for cryo-TEM training and assistance. The authors thank Professor Adrew Tsourkas and his group for their help with the relaxivity and DLS measurements. The authors thank Dr. David Vann for his help with the ICP.
Footnotes
Supporting Information. Additional characterization data, plots of inverse transverse relaxation time (1/T2) versus iron molar concentration, and a detailed description of SCFT calculations. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
References
- 1.Marguet M, Bonduelle C, Lecommandoux S. Multicompartmentalized Polymeric Systems: Towards Biomimetic Cellular Structure and Function. Chem Soc Rev. 2013;42:512–529. doi: 10.1039/c2cs35312a. [DOI] [PubMed] [Google Scholar]
- 2.Tanner P, Baumann P, Enea R, Onaca O, Palivan C, Meier W. Polymeric Vesicles: From Drug Carriers to Nanoreactors and Artificial Organelles. Acc Chem Res. 2011;44:1039–1049. doi: 10.1021/ar200036k. [DOI] [PubMed] [Google Scholar]
- 3.Kamat NP, Katz JS, Hammer DA. Engineering Polymersome Protocells. J Phys Chem Lett. 2011;2:1612–1623. doi: 10.1021/jz200640x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du J, O’Reilly RK. Advances and Challenges in Smart and Functional Polymer Vesicles. Soft Matter. 2009;5:3544–3561. [Google Scholar]
- 5.Bellomo EG, Wyrsta MD, Pakstis L, Pochan DJ, Deming TJ. Stimuli-Responsive Polypeptide Vesicles by Conformation-Specific Assembly. Nat Mater. 2004;3:244–248. doi: 10.1038/nmat1093. [DOI] [PubMed] [Google Scholar]
- 6.Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Oxidation-Responsive Polymeric Vesicles. Nat Mater. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 7.Yu S, Azzam T, Rouiller I, Eisenberg A. “Breathing” Vesicles. J Am Chem Soc. 2009;131:10557–10566. doi: 10.1021/ja902869q. [DOI] [PubMed] [Google Scholar]
- 8.Sanson C, Diou O, Thévenot J, Ibarboure E, Soum A, Brûlet A, Miraux S, Thiaudière E, Tan S, Brisson A, et al. Doxorubicin Loaded Magnetic Polymersomes: Theranostic Nanocarriers for MR Imaging and Magneto-Chemotherapy. ACS Nano. 2011;5:1122–1140. doi: 10.1021/nn102762f. [DOI] [PubMed] [Google Scholar]
- 9.De Oliveira H, Thevenot J, Lecommandoux S. Smart Polymersomes for Therapy and Diagnosis: Fast Progress Toward Multifunctional Biomimetic Nanomedicines. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2012;4:525–546. doi: 10.1002/wnan.1183. [DOI] [PubMed] [Google Scholar]
- 10.Lipowsky R, S E. Structure and Dynamics of Membranes - From Cells to Vesicles. Elsevier Science; Amsterdam: 1995. [Google Scholar]
- 11.Baumgart T, Capraro BR, Zhu C, Das SL. Thermodynamics and Mechanics of Membrane Curvature Generation and Sensing by Proteins and Lipids. Annu Rev Phys Chem. 2011;62:483–506. doi: 10.1146/annurev.physchem.012809.103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holowka EP, Pochan DJ, Deming TJ. Charged Polypeptide Vesicles with Controllable Diameter. J Am Chem Soc. 2005;127:12423–12428. doi: 10.1021/ja053557t. [DOI] [PubMed] [Google Scholar]
- 13.Hayward RC, Pochan DJ. Tailored Assemblies of Block Copolymers in Solution: It Is All about the Process. Macromolecules. 2010;43:3577–3584. [Google Scholar]
- 14.Discher DE, Eisenberg A. Polymer Vesicles. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 15.Ghoroghchian PP, Frail PR, Susumu K, Blessington D, Brannan AK, Bates FS, Chance B, Hammer DA, Therien MJ. Near-Infrared-Emissive Polymersomes: Self-Assembled Soft Matter for in vivo Optical Imaging. Proc Natl Acad Sci USA. 2005;102:2922–2927. doi: 10.1073/pnas.0409394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JCM, Bermudez H, Discher BM, Sheehan MA, Won Y-Y, Bates FS, Discher DE. Stability and in vitro Performance of Vesicles Made With Diblock Copolymers. Biotechnol Bioeng. 2001;73:135–145. doi: 10.1002/bit.1045. [DOI] [PubMed] [Google Scholar]
- 17.LoPresti C, Lomas H, Massignani M, Smart T, Battaglia G. Polymersomes: Nature Inspired Nanometer Sized Compartments. J Mater Chem. 2009;19:3576–3590. [Google Scholar]
- 18.Stoenescu R, Meier W. Vesicles with Asymmetric Membranes from Amphiphilic ABC Triblock Copolymers. Chem Commun. 2002:3016–3017. doi: 10.1039/b209352a. [DOI] [PubMed] [Google Scholar]
- 19.Howse JR, Jones RAL, Battaglia G, Ducker RE, Leggett GJ, Ryan AJ. Templated Formation of Giant Polymer Vesicles with Controlled Size Distributions. Nat Mater. 2009;8:507–511. doi: 10.1038/nmat2446. [DOI] [PubMed] [Google Scholar]
- 20.Lorenceau E, Utada AS, Link DR, Cristobal G, Joanicot M, Weitz DA. Generation of Polymerosomes from Double-Emulsions. Langmuir. 2005;21:9183–9186. doi: 10.1021/la050797d. [DOI] [PubMed] [Google Scholar]
- 21.Hauschild S, Lipprandt U, Rumplecker A, Borchert U, Rank A, Schubert R, Förster S. Direct Preparation Loading of Lipid Polymer Vesicles Using Inkjets. Small. 2005;1:1177–1180. doi: 10.1002/smll.200500093. [DOI] [PubMed] [Google Scholar]
- 22.Brown L, McArthur SL, Wright PC, Lewis A, Battaglia G. Polymersome Production on a Microfluidic Platform using pH Sensitive Block Copolymers. Lab Chip. 2010;10:1922–1928. doi: 10.1039/c004036c. [DOI] [PubMed] [Google Scholar]
- 23.Sanson C, Schatz C, Le Meins JF, Soum A, Thévenot J, Garanger E, Lecommandoux S. A Simple Method to Achieve High Doxorubicin Loading in Biodegradable Polymersomes. J Control Release. 2010;147:428–435. doi: 10.1016/j.jconrel.2010.07.123. [DOI] [PubMed] [Google Scholar]
- 24.Sanson C, Schatz C, Le Meins JF, BruZlet A, Soum A, Lecommandoux S. Biocompatible and Biodegradable Poly(trimethylene carbonate)-block-Poly(L-glutamic acid) Polymersomes: Size Control and Stability. Langmuir. 2009;26:2751–2760. doi: 10.1021/la902786t. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz ME, Prud’homme RK, Robb I, Adamson DH. Formation Characterization of Polymersomes Made by a Solvent Injection Method. Polym Advan Technol. 2007;18:427–432. [Google Scholar]
- 26.Arosio P, Thevenot J, Orlando T, Orsini F, Corti M, Mariani M, Bordonali L, Innocenti C, Sangregorio C, Oliveira H, et al. Hybrid Iron Oxide-Copolymer Micelles and Vesicles as Contrast Agents for MRI: Impact of the Nanostructure on the Relaxometric Properties. J Mater Chem B. 2013;1:5317–5328. doi: 10.1039/c3tb00429e. [DOI] [PubMed] [Google Scholar]
- 27.Hickey RJ, Haynes AS, Kikkawa JM, Park S-J. Controlling the Self-Assembly Structure of Magnetic Nanoparticles and Amphiphilic Block-Copolymers: From Micelles to Vesicles. J Am Chem Soc. 2011;133:1517–1525. doi: 10.1021/ja1090113. [DOI] [PubMed] [Google Scholar]
- 28.Park J, An KJ, Hwang YS, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 29.Hickey RJ, Meng X, Zhang P, Park S-J. Low-Dimensional Nanoparticle Clustering in Polymer Micelles and Their Transverse Relaxivity Rates. ACS Nano. 2013;7:5824–5833. doi: 10.1021/nn400824b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray CB, Kagan CR, Bawendi MG. Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies. Annu Rev Mater Sci. 2000;30:545–610. [Google Scholar]
- 31.Wang T, LaMontagne D, Lynch J, Zhuang J, Cao YC. Colloidal Superparticles from Nanoparticle Assembly. Chem Soc Rev. 2013;42:2804–2823. doi: 10.1039/c2cs35318k. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RB, Ginzburg VV, Matsen MW, Balazs AC. Predicting the Mesophases of Copolymer-Nanoparticle Composites. Science. 2001;292:2469–2472. doi: 10.1126/science.1060585. [DOI] [PubMed] [Google Scholar]
- 33.Fredrickson GH. The Equilibrium Theory of Inhomogeneous Polymers. Oxford University Press, USA; New York: 2006. [Google Scholar]
- 34.Matsen MW. The Standard Gaussian Model for Block Copolymer Melts. J Phys: Condens Matter. 2002;14:R21–R47. [Google Scholar]
- 35.Sides SW, Kim BJ, Kramer EJ, Fredrickson GH. Hybrid Particle-Field Simulations of Polymer Nanocomposites. Phys Rev Lett. 2006;96:250601. doi: 10.1103/PhysRevLett.96.250601. [DOI] [PubMed] [Google Scholar]
- 36.Luo L, Eisenberg A. Thermodynamic Stabilization Mechanism of Block Copolymer Vesicles. J Am Chem Soc. 2001;123:1012–1013. doi: 10.1021/ja005824v. [DOI] [PubMed] [Google Scholar]
- 37.Mai Y, Eisenberg A. Self-assembly of Block Copolymers. Chem Soc Rev. 2012;41:5969–5985. doi: 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- 38.Kroeger A, Li X, Eisenberg A. Dendrimer-Influenced Supramolecular Structure Formation of Block Copolymers. Langmuir. 2007;23:10732–10740. doi: 10.1021/la701334r. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Kroeger A, Azzam T, Eisenberg A. Dendrimer Influenced Supramolecular Structure Formation of Block Copolymers: II. Dendrimer Concentration Dependence. Langmuir. 2008;24:2705–2711. doi: 10.1021/la702614x. [DOI] [PubMed] [Google Scholar]
- 40.Jun Y-w, Huh Y-M, Choi J-s, Lee J-H, Song H-T, Kim S, Yoon S, Kim K-S, Shin J-S, Suh J-S, et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J Am Chem Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 41.Pöselt E, Kloust H, Tromsdorf U, Janschel M, Hahn C, Maßlo C, Weller H. Relaxivity Optimization of a PEGylated Iron-Oxide-Based Negative Magnetic Resonance Contrast Agent for T2-Weighted Spin–Echo Imaging. ACS Nano. 2012;6:1619–1624. doi: 10.1021/nn204591r. [DOI] [PubMed] [Google Scholar]
- 42.Lee N, Choi Y, Lee Y, Park M, Moon WK, Choi SH, Hyeon T. Water-Dispersible Ferrimagnetic Iron Oxide Nanocubes with Extremely High r2 Relaxivity for Highly Sensitive in Vivo MRI of Tumors. Nano Lett. 2012;12:3127–3131. doi: 10.1021/nl3010308. [DOI] [PubMed] [Google Scholar]
- 43.Yoon T-J, Lee H, Shao H, Hilderbrand SA, Weissleder R. Multicore Assemblies Potentiate Magnetic Properties of Biomagnetic Nanoparticles. Adv Mater. 2011;23:4793–4797. doi: 10.1002/adma.201102948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Gaytan BL, Cui WH, Kim YJ, Mendez-Polanco MA, Duncan TV, Fryd M, Wayland BB, Park SJ. Interfacial Assembly of Nanoparticles in Discrete Block-Copolymer Aggregates. Angew Chem Int Ed. 2007;46:9235–9238. doi: 10.1002/anie.200703032. [DOI] [PubMed] [Google Scholar]
- 45.Hickey RJ, Luo Q, Park S-J. Polymersomes and Multicompartment Polymersomes Formed by the Interfacial Self-assembly of Gold Nanoparticles Amphiphilic Polymers. ACS Macro Lett. 2013;2:805–808. doi: 10.1021/mz400301g. [DOI] [PubMed] [Google Scholar]
- 46.Kremer JR, Mastronarde DN, McIntosh JR. Computer Visualization of Three-Dimensional Image Data Using IMOD. J Struc Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 47.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—A Visualization System for Exploratory Research Analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


