Abstract
Objective
The potential influence of dietary factors on inflammation is important for cancer prevention. Utilizing data from control participants (312 men, 911 women) in two nested case-control studies of cancer within the Multiethnic Cohort, we examined the associations of red and processed meat intake with serum levels of leptin, adiponectin, C-reactive protein (CRP), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 and the mediator effect of body mass index (BMI) on the above associations (if present).
Methods
Multivariable linear models were applied to assess the association between red and processed meat intake at cohort entry and serum biomarker levels measured 9.1 years later after adjusting for covariates and to determine the mediator effect of BMI.
Results
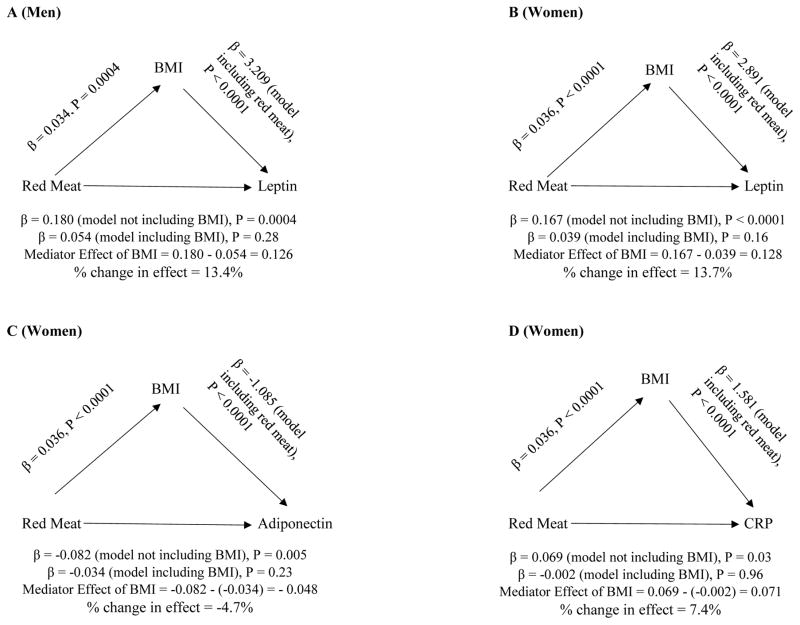
Overall red and processed meat intake was positively associated with serum leptin levels in men (β=0.180, P=0.0004) and women (β=0.167, P<0.0001). In women, higher red and processed meat consumption was significantly associated with higher CRP (β=0.069, P=0.03) and lower adiponectin levels (β=−0.082, P=0.005). In mediation analyses with red and processed meat intake and BMI as predictors, the associations of red and processed meat with biomarkers decreased substantially (as indicated by % change in effect: leptin in men, 13.4%; leptin in women, 13.7%; adiponectin in women, −4.7%; CRP in women, 7.4%) and were no longer significant (P>0.05), whereas BMI remained significantly associated with serum leptin (men: β=3.209, P<0.0001; women: β=2.891, P<0.0001), adiponectin (women: β= −1.085, P<0.0001) and CRP (women: β=1.581, P<0.0001).
Conclusion
The current data suggest that the amount of excess body weight or the degree of adiposity may mediate the relations between dietary red and processed meat intake and serum biomarkers associated with obesity and inflammation.
INTRODUCTION
Diet and inflammation play important roles in the development of cancer [1, 2]. Several possible associations of macro- and micronutrients with chronic inflammation, a risk factor for various cancers, have been described [3] in addition to the role of obesity in chronic low grade inflammation marked by altered levels of inflammatory markers and adipokines [4, 5]. Markers such as leptin and adiponectin may modify the risk of cancer either directly by activating signal transduction pathways involved in carcinogenesis or indirectly by affecting insulin sensitivity and inflammatory processes [6]. While leptin participates in pro-inflammatory responses and serves as an important growth factor for cancer [7, 8], adiponectin has a strong anti-inflammatory function [9]. C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor- α (TNF-α) are markers of systematic inflammation and have been linked to increased risk of cancer [1, 10].
On the basis of available evidence, the American Institute for Cancer Research (AICR) and the World Cancer Research Fund (WCRF) released lifestyle recommendations for the prevention of the most common cancers [11, 12], which include the advice to limit the intake of red meat to less than 500 g per week, to avoid processed meat, and to consume at least 400 g of vegetables per day [11, 12]. Red and processed meat consumption may increase oxidative stress and inflammation as suggested by associations of red meat with CRP [13–17]. However, in most studies the associations were attenuated after adjusting for body mass index (BMI), and the mediator effect of BMI was not tested [14–17]. One study reported that processed meat consumption was borderline associated with higher IL-6, but not with CRP, and with lower TNF-α [18]. Further, no study has yet assessed the direct effect of red meat intake alone on circulating leptin levels, although a positive correlation between a Western dietary pattern and higher serum leptin concentration was reported without determining the relative contributions of the various dietary constituents [19]. Given the increasing evidence that adiposity and inflammation are key etiologic factors for cancer development, it is important to understand the potential influence of dietary components on adipokines and inflammatory markers. Therefore, the current study examined the associations of dietary red and processed meat intake with serum levels of CRP, TNF-α, IL-6, leptin, and adiponectin among control participants in two nested case-control studies of cancer in the Multiethnic Cohort (MEC). In addition, we sought to determine whether the associations between red and processed meat intake and biomarker levels, if present were mediated by BMI, and were consistent across race/ethnic groups in the MEC.
MATERIALS AND METHODS
Study population and data collection
The current study used data collected from control participants (312 men and 911 women), free of cancer, who participated in nested case-control studies of breast cancer and non-Hodgkin lymphoma conducted within the MEC [20–22], a longitudinal study designed to investigate the association of dietary, lifestyle, and genetic factors with the incidence of cancer. The cohort was assembled in Hawaii and Los Angeles in 1993–1996, and details on recruitment and baseline information were reported previously [20]. Briefly, subjects from 5 main race/ethnic groups (whites, Japanese Americans, Latinos, African Americans, and Native Hawaiians) were identified primarily through drivers’ license files, supplemented with voter registration lists in Hawaii and Medicare files in California, and were recruited by mailing a self-administered, 26-page questionnaire on diet, anthropometric measures, medical history, family history of cancer, and lifestyle. A total of 215,251 men and women aged 45 to 75 years were included at baseline and formed a representative group of the general population as verified by a comparison of the cohort distributions across educational levels and marital status with corresponding census data for the two geographical areas [20]. During 2003–2007, approximately 50% of cohort members completed a full questionnaire (Qx3) asking about diet and body weight within 2 years of the collection of blood specimens, which were analyzed for serum biomarker levels. The study protocol was approved by the Institutional Review Boards of the University of Hawaii and the University of Southern California.
A biospecimen subcohort of 67,594 cohort members was established in 2001–2006 by asking surviving cohort members to provide blood and urine specimens [23]. When comparing the characteristics at cohort entry of individuals who provided specimens with those who did not, there were no substantial differences between the two groups in BMI, dietary fat and vegetable intake, physical activity, and family history of cancer, suggesting that the biospecimen repository participants are broadly representative of the cohort members. For nested case-control studies of breast cancer and non-Hodgkin lymphoma, incident cancer cases within the MEC were identified by routine linkages to the Hawaii Tumor Registry, the Los Angeles County Cancer Surveillance Program, and the State of California Cancer Registry, which are participants of the Surveillance, Epidemiology, and End Results (SEER) program. Controls were identified from the biospecimen subcohort who were alive and free of cancer at the age of the case’s diagnosis and matched on sex, birth date (±1 year), race/ethnicity (white, Japanese American, Latino, African American, or Native Hawaiian), location (California or Hawaii), date (± 6 months) and time of blood draw (±2 hours), fasting hours prior to blood draw (0 to <6, 6 to <8, 8 to <10, and ≥10 hours), and hormone replacement therapy use (for breast cancer controls) [21, 22]. The current study only included cancer-free, control participants from the above two nested case-control studies. As a higher number of participants were selected from nested case-control study of breast cancer, more women than men were part of the current analysis.
Dietary intakes were calculated based on a self-reported food frequency questionnaire (FFQ) at cohort entry validated for a multiethnic population [24] and, for a subset of participants, also at the time of Qx3. Participants reported their average intake in seven categories and serving sizes of specific foods during the last year; dietary intakes were calculated using a food composition table that included habitual foods for all race/ethnic groups in the study. The mean time interval between cohort entry and the collection of blood samples was 9.1 years. The mean time between Qx3 and blood draw was 2.1±2.6 years. The final sample size for this analysis was 1223 including 312 men and 911 women who had data available for biomarkers examined in the study. Two male participants had missing CRP values and one male participant had missing leptin value.
Laboratory assays
All assays were performed by the Analytical Biochemistry Shared Resource at the University of Hawaii Cancer Center as reported for the original nested-case control studies following the manufacturer’s protocol unless noted otherwise [22, 25]. Briefly, frozen serum samples from the MEC biorepository were analyzed in duplicate to quantify leptin and adiponectin using a double-antibody enzyme-linked-immunosorbent-assay (R&D Systems, Minneapolis, MN, U.S.A.). CRP was assessed using a Cobas MiraPlus clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN) and a latex particle enhanced immunoturbidimetry-based kit from Pointe Scientific (Lincoln Park, MI). TNF-α and IL-6 were included in the Luminex panel and were measured using a slight modification of an Invitrogen (Carlsbad, CA) magnetic high sensitivity 10-plex assay (LHC0001) and quantified on a Luminex 200 plate reader [22, 25]. As reported previously [22, 25], intra-batch coefficients of variation based on 96 blinded duplicate and 9 triplet samples for leptin, adiponectin, CRP, TNF-α, and IL-6 were 3.4 –6.4%, 2.5–9.4 %, 3.5–5.0%, 10.0%, and 8.9% respectively.
Statistical analyses
The SAS software version 9.3 (SAS Institute, Inc., Cary, NC) was used to perform all analyses. Multivariable linear models were applied to evaluate the association between red and processed meat intake at cohort entry and serum biomarker (leptin, adiponectin, CRP, TNF-α, and IL-6) levels measured 9.1±2.5 years later. Dietary intake variables and serum biomarkers were log-transformed to satisfy model assumptions. The models were adjusted for total energy intake (log-transformed, continuous), laboratory batch (categorical) to correct for possible difference across batches, and month of blood draw [binary, winter (October–March) or non-winter (April–September) months] to account for seasonal variations in biomarker levels based on previous findings showing seasonal variation of inflammatory marker levels with the highest levels during the winter months [26, 27]. In addition, further adjustment for BMI (log-transformed, continuous) at cohort entry was evaluated. The associations between BMI (log-transformed) and serum biomarker levels were also assessed using multivariable linear models adjusted for the aforementioned covariates, with and without adjustment for red and processed meat intake. All analyses were repeated after stratification by race/ethnic group. Interaction terms of red and processed meat intake with race/ethnicity were also examined. As age at blood draw, race/ethnicity, fasting hours prior to blood draw, smoking status, physical activity, and diabetes status did not significantly change the results they were not included in the final models. For 906 (74%) cohort members with Qx3 data (completed within 2 years of blood collection), the overall analyses were repeated to examine if diet and BMI (we used both dietary intake and BMI values assessed in Qx3) closer to blood draw would result in different findings (sensitivity analysis). Spearman’s rank correlation was used to assess the correlation between red and processed meat intake at cohort entry and that at Qx3.
Mediation analysis was performed to determine if BMI mediates the association of red and processed meat intake with biomarker levels. Three multivariable linear models were tested: the first model (Model 1) examined red and processed meat intake alone (predictor variable) as predictor of biomarker levels (outcome variable); the second model (Model 2) tested if red and processed meat intake (predictor variable) significantly predicts BMI (mediator); and in the third model (Model 3), red and processed meat intake (predictor variable) and BMI (mediator) were entered simultaneously to predict biomarker levels (outcome variable). Mediation is established if significant associations (P<0.05) are observed in the first and second model and two additional criteria are met in the third model: BMI (mediator) must significantly predict outcome variable biomarker levels (P<0.05) and the direct relation between the predictor and the outcome variables [as indicated by as estimated slope (β) for red and processed meat in Model 3] decreases to 0 in case of full mediation or is reduced substantially but different from 0 in case of partial mediation [28]. The mediator effect of BMI was calculated as the difference in estimated slopes: Mediator effect of BMI=β for red and processed meat in Model 1 - β for red and processed meat in Model 3. We also estimated percent (%) change in effect for mediator effect of BMI: % change in effect=[Exp (β in Model 1) – Exp (β in Model 3)]/ Exp (β in Model 3) x 100%.
RESULTS
The average age at blood draw was 70.2±7.9 years for men and 68.4±7.5 years for women. The mean BMI values at cohort entry were 26.5±3.7 kg/m2 and 26.0±5.1 kg/m2 for men and women, respectively. Among men, 31.4% were white, 26.0% Japanese American, 18.6% Latino, 18.3% African American, and 5.8% Native Hawaiian. The respective race/ethnic distributions among women were 22.2%, 33.0%, 20.8%, 15.3%, and 8.8%. At cohort entry, 9.4% of men and 9.2% women were current smokers, and 4.8% and 7.6% reported diabetes (Table 1). Mean estimates of dietary intake of red and processed meat at cohort entry were 65 g/day in men and 50 g/day in women, both meeting the AICR/WCRF recommendation; fruit and vegetable intakes in men (577 g/day) and women (629 g/day) also adhered to the AICR and WCRF guidelines [11] (Table 1).
Table 1.
Characteristics of study participantsa
| All | Men | Women | |
|---|---|---|---|
| N | 1223 | 312 | 911 |
| Age at blood draw (y) | 68.9±7.6 | 70.2±7.9 | 68.4±7.5 |
| Race/ethnicity, n (%) | |||
| African American | 196 (16.0) | 57 (18.3) | 139 (15.3) |
| Latino | 247 (20.2) | 58 (18.6) | 189 (20.8) |
| Japanese American | 382 (31.2) | 81 (26.0) | 301 (33.0) |
| Native Hawaiian | 98 (8.0) | 18 (5.8) | 80 (8.8) |
| White | 300 (24.5) | 98 (31.4) | 202 (22.2) |
| Time from cohort entry to blood draw | 9.1±2.5 | 8.8±2.5 | 9.2±2.4 |
| Fasting hours prior to blood drawb | 12.6±5.0 | 11.9±4.2 | 12.8±5.2 |
| Blood sample collected in winter months (October–March), n (%) | 566 (46.3%) | 147 (47.1%) | 419 (46.0%) |
| Body mass index at cohort entry (kg/m2) | 26.2±4.8 | 26.5±3.7 | 26.0±5.1 |
| Current smoking at cohort entry, n (%)b | 112 (9.3%) | 29 (9.4%) | 83 (9.2%) |
| Diabetes at cohort entry, n (%) | 84 (6.9%) | 15 (4.8%) | 69 (7.6%) |
| Total energy (kcal/day) | 2,017±958 | 2,279±990 | 1,927±930 |
| Dietary intake at cohort entry (g/day) | |||
| Unprocessed red meat | 37±34 | 43±33 | 35±35 |
| Processed meat (including red and other meats) | 17±18 | 22±22 | 15±16 |
| Poultry | 46±42 | 49±43 | 44±41 |
| Fish | 17±18 | 19±20 | 17±18 |
| Dairy | 223±210 | 219±184 | 224±218 |
| Vegetable | 339±224 | 331±217 | 342±226 |
| Fruit (not including fruit juice) | 277±263 | 246±234 | 287±271 |
| Dietary fiber | 25±14 | 25±14 | 25±15 |
| Serum biomarker levels | |||
| C-reactive protein (mg/L)b | 3.3±4.1 | 2.9±3.8 | 3.4±4.1 |
| Tumor necrosis factor-α (pg/mL) | 26.4±157.9 | 17.3±106.3 | 29.5±171.9 |
| Leptin (ng/mL)b | 22.3±23.3 | 9.1±8.4 | 26.9±25.0 |
| Adiponectin (μg/mL) | 11.4±8.9 | 8.0±5.6 | 12.6±9.5 |
| Interleukin-6 (pg/mL) | 11.2±62.4 | 13.0±78.6 | 10.6±55.8 |
Data are given as means ± standard deviation unless otherwise specified; percentages may not add to 100 due to rounding.
Men had missing data for fasting hours prior to blood draw (N=3), current smoking at cohort entry (N=4), C-reactive protein (N=2), and leptin (N=1), and women had missing data for fasting hours prior to blood draw (N=3) and current smoking at cohort entry (N=11).
Statistically significant, positive associations between red and processed meat intake and serum leptin levels were observed in men (β=0.180, P=0.0004) and women (β=0.167, P<0.0001). In women but not men, consumption of red and processed meat was positively associated with serum CRP levels (β=0.069, P=0.03) and inversely associated with serum adiponectin levels (β= −0.082, P=0.005). The associations between red and processed meat consumption and serum levels of TNF-α and IL-6 were not significant for men or women (Table 2). Similar results were observed in models restricted to red or processed meat although associations between processed meat intake and serum CRP levels were not significant (data not shown).
Table 2.
Red and processed meat intake (g/day) at cohort entry and serum biomarkers after 9 years by sexa
| Serum Marker | Men
|
Women
|
|||||
|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | ||
| Diet at cohort entry (1993–96) | N=312 | N=911 | |||||
| Leptin (ng/mL) | Model Ib | 0.180 | 0.062 | 0.0004 | 0.167 | 0.035 | <0.0001 |
| Model IIc | 0.054 | 0.049 | 0.28 | 0.039 | 0.027 | 0.16 | |
| Adiponectin (μg/mL) | Model Ib | −0.033 | 0.052 | 0.52 | −0.082 | 0.029 | 0.005 |
| Model IIc | −0.021 | 0.053 | 0.70 | −0.034 | 0.028 | 0.23 | |
| C-reactive protein (mg/L) | Model Ib | 0.026 | 0.066 | 0.70 | 0.069 | 0.031 | 0.03 |
| Model IIc | −0.021 | 0.066 | 0.75 | −0.002 | 0.050 | 0.96 | |
| Tumor necrosis factor-α (pg/mL) | Model Ib | −0.124 | 0.095 | 0.19 | −0.005 | 0.049 | 0.92 |
| Model IIc | −0.092 | 0.097 | 0.34 | −0.003 | 0.039 | 0.95 | |
| Interleukin-6 (pg/mL) | Model Ib | −0.047 | 0.095 | 0.62 | 0.036 | 0.041 | 0.38 |
| Model IIc | −0.054 | 0.097 | 0.58 | 0.019 | 0.041 | 0.64 | |
Values are estimates from multivariable linear models; red and processed meat intake and all serum biomarkers were log-transformed in all models.
Model I was adjusted for total energy intake at cohort entry (log-transformed, continuous), laboratory batch number (categorical), and month of blood draw [binary, winter (October–March) or non-winter (April–September) months].
Model II was further adjusted for body mass index at cohort entry (log-transformed, continuous).
Table 3 shows the association between BMI and serum biomarker levels. Significant, positive associations of BMI with serum leptin and CRP levels were observed in men (Leptin: β=3.262, P<0.0001; CRP: β=1.173, P=0.0003) and women (Leptin: β=2.923, P<0.0001; CRP: β=1.579, P<0.0001). In women, BMI was inversely associated with serum adiponectin (β= −1.113, P<0.0001) and positively associated with serum IL-6 levels (β=0.393, P=0.03). These associations between BMI and biomarkers remained significant when the model was adjusted for red and processed meat intake (P<0.05).
Table 3.
Body mass index (kg/m2) at cohort entry and serum biomarkers after 9 years by sexa
| Serum Marker | Men (N=312)
|
Women (N=911)
|
|||||
|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | ||
| Leptin (ng/mL) | Model Ib | 3.262 | 0.239 | <0.0001 | 2.923 | 0.117 | <0.0001 |
| Model IIc | 3.209 | 0.244 | <0.0001 | 2.891 | 0.119 | <0.0001 | |
| Adiponectin (μg/mL) | Model Ib | −0.328 | 0.256 | 0.21 | −1.113 | 0.120 | <0.0001 |
| Model IIc | −0.308 | 0.261 | 0.24 | −1.085 | 0.122 | <0.0001 | |
| C-reactive protein (mg/L) | Model Ib | 1.173 | 0.321 | 0.0003 | 1.579 | 0.125 | <0.0001 |
| Model IIc | 1.179 | 0.328 | 0.0003 | 1.581 | 0.127 | <0.0001 | |
| Tumor necrosis factor-α (pg/mL) | Model Ib | −0.901 | 0.468 | 0.06 | −0.039 | 0.214 | 0.86 |
| Model IIc | −0.813 | 0.477 | 0.09 | −0.036 | 0.219 | 0.87 | |
| Interleukin-6 (pg/mL) | Model Ib | 0.124 | 0.471 | 0.79 | 0.393 | 0.177 | 0.03 |
| Model IIc | 0.176 | 0.470 | 0.71 | 0.377 | 0.180 | 0.04 | |
Values are estimates from multivariable linear models; body mass index (BMI) and all serum biomarkers were log-transformed in all models.
Model I was adjusted for total energy intake at cohort entry (log-transformed, continuous), laboratory batch number (categorical), and month of blood draw [binary, winter (October–March) or non-winter (April–September) months].
Model II was further adjusted for red and processed meat intake at cohort entry (log-transformed, continuous).
In mediation analysis, red and processed meat intake was significantly, positively associated with BMI in men (β = 0.034, P=0.0004) and women (β = 0.036, P<0.0001). When red and processed meat and BMI were simultaneously included as predictors of biomarker levels, the significant associations of red and processed meat intake with serum levels of leptin, adiponectin and CRP decreased substantially (as indicated by % change in effect: leptin in men, 13.4%; Leptin in women, 13.7%; adiponectin in women, −4.7%; CRP in women, 7.4%) and were no longer significant (P>0.05), suggesting either full or partial mediation by BMI (Figure 1). Meanwhile, BMI remained significantly, positively associated with serum leptin and CRP in men and women, and inversely associated with serum adiponectin levels in women with and without the inclusion of total intake of red and processed meats in the model (P<0.05) (Figure 1).
Figure 1.
Figure 1(A, B, C, D) shows the mediator effect of body mass index (BMI) on associations between red and processed meat intake (red meat) and serum leptin, adiponectin, and C-reactive protein (CRP) levels. Three multivariable linear models were applied: 1) In Model 1, biomarker levels (outcome variable) were regressed on red meat (predictor variable); 2) In Model 2, BMI (mediator) was regressed on red meat (predictor variable); 3) In Model 3, biomarker levels (outcome variable) were regressed simultaneously on red meat (predictor variable) and BMI (mediator). The mediator effect of BMI was estimated as follows: (β for red meat in Model 1 [model not including BMI]) - (β for red meat in Model 3 [model including BMI]). The percent (%) change in effect for mediator effect of BMI was estimated as follows: % change in effect = [Exp (β in Model 1) – Exp (β in Model 3)]/ Exp (β in Model 3) x 100%. Variables for red meat, BMI, leptin, adiponectin and CRP were log-transformed in all analyses.
Table 4 shows associations of red and processed meat intake with serum biomarkers by race/ethnicity. No significant interactions of meat intake with race/ethnicity in relation to biomarkers were observed. In women, the associations between red and processed meat intake and leptin were significant in African Americans (P=0.03), Latinas (P=0.006), and Japanese Americans (P=0.004) but not in whites (P=0.34) and Native Hawaiians (P=0.39). Similarly in women, the significantly inverse associations of red and processed meat intake with adiponectin levels were observed in African Americans (P=0.03) and Latinas (P=0.03) but not in Japanese Americans (P=0.77), Native Hawaiians (P=0.91), and whites (P=0.69). Red and processed meat intake was positively associated with serum CRP levels only in Latinas (P=0.04) but not in other race/ethnicity groups among women. In men, with the exception of an inverse association of red and processed meat intake with serum TNF-α levels in whites (P=0.0007), no statistically significant relations were observed across race/ethnic groups.
Table 4.
Red and processed meat intake at cohort entry and serum biomarkers after 9 years by race/ethnicity and sexa
| Race/ethnicity | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Latino | Japanese American | Native Hawaiian | White | ||||||||||||
|
| ||||||||||||||||
| Serum biomarker | N | β | P value | N | β | P value | N | β | P value | N | β | P value | N | β | P value | |
| Leptin (ng/mL) | ||||||||||||||||
| Menb | 57 | 0.178 | 0.51 | 58 | −0.239 | 0.13 | 81 | 0.185 | 0.10 | 18 | 0.357 | 0.53 | 98 | 0.191 | 0.09 | |
| Womenb | 139 | 0.201 | 0.03 | 189 | 0.195 | 0.006 | 301 | 0.178 | 0.004 | 80 | 0.144 | 0.39 | 202 | 0.100 | 0.34 | |
| Adiponectin (μg/mL) | ||||||||||||||||
| Menb | 57 | 0.221 | 0.23 | 58 | 0.018 | 0.88 | 81 | −0.066 | 0.56 | 18 | −0.353 | 0.21 | 98 | 0.061 | 0.53 | |
| Womenb | 139 | −0.161 | 0.03 | 189 | −0.106 | 0.03 | 301 | −0.020 | 0.77 | 80 | 0.016 | 0.91 | 202 | −0.027 | 0.69 | |
| CRP (mg/L) | ||||||||||||||||
| Menb | 57 | 0.22 | 0.38 | 58 | −0.084 | 0.62 | 81 | −0.043 | 0.70 | 18 | −0.129 | 0.63 | 98 | 0.063 | 0.36 | |
| Womenb | 139 | 0.152 | 0.07 | 189 | 0.126 | 0.04 | 301 | 0.021 | 0.72 | 80 | 0.074 | 0.65 | 202 | 0.063 | 0.36 | |
| TNF-α (pg/mL) | ||||||||||||||||
| Menb | 57 | 0.214 | 0.68 | 58 | 0.211 | 0.29 | 81 | −0.205 | 0.25 | 18 | 0.719 | 0.39 | 98 | −0.504 | 0.0007 | |
| Womenb | 139 | −0.152 | 0.11 | 189 | −0.073 | 0.56 | 301 | −0.17 | 0.64 | 80 | −0.125 | 0.68 | 202 | 0.192 | 0.10 | |
| IL-6 (pg/mL) | ||||||||||||||||
| Menb | 57 | −0.065 | 0.90 | 58 | −0.084 | 0.64 | 81 | −0.229 | 0.26 | 18 | 0.110 | 0.74 | 98 | −0.191 | 0.16 | |
| Womenb | 139 | 0.087 | 0.45 | 189 | 0.036 | 0.38 | 301 | 0.144 | 0.07 | 80 | −0.197 | 0.33 | 202 | 0.112 | 0.25 | |
Note: CRP: C-reactive protein; TNF-α: Tumor necrosis factor-α; IL-6: interleukin 6
Values are estimates from multivariable linear models; red and processed meat intake and serum biomarkers were log-transformed in all models.
Model was adjusted for total energy intake at cohort entry (log-transformed, continuous), laboratory batch number (categorical), and month of blood draw [binary, winter (October–March) or non-winter (April–September) months].
The mean intakes of red and processed meat for the 231 men and 675 women with a valid diet in Qx3 were 70±58 g and 51±40 g, respectively and the values were highly correlated (rs=0.56, p<0.0001) with those at cohort entry. Sensitivity analyses among participants with Qx3 data (within two years of blood collection) yielded similar results to those across all participants (data not shown). In particular, significant associations in models without BMI were no longer significant after including BMI (assessed in Qx3) in the model. For example, the respective results before and after BMI adjustment for leptin in women were β=0.109, P=0.02 and β= −0.033, P=0.30, respectively. Similarly, the corresponding results for adiponectin in women were β= −0.080, P=0.04 and β= −0.052, P=0.16 and for CRP in women were β=0.040, P=0.27 and β=0.008, P=0.84.
DISCUSSION
One of the AICR/WCRF cancer prevention recommendations is to limit intake of red meat and to avoid eating processed meat [11, 12]. In the present study, red and processed meat consumption was positively associated with serum leptin in men and women, and positively associated with CRP and inversely associated with serum adiponectin levels in women after nine years of follow-up. However, adjustment for BMI and a mediation analysis indicated that these associations were fully or partially mediated by BMI, suggesting that a diet high in red and processed meats affects serum obesity-related inflammatory markers through its effect on body weight. Using dietary intake and BMI data closer to the time of blood draw did not substantially change the results. Recently, a Working Group of the International Agency for Research on Cancer (IARC) classified processed meat as carcinogenic based on sufficient epidemiologic data for colorectal cancer. The 2016 IARC Working Group further concluded that the mechanistic evidence for the carcinogenicity was strong for red meat but moderate for processed meat [29]. High iron contents of red meat, particularly heme iron, meat processing resulting in formation of carcinogenic chemicals including N-nitroso-compounds (NOC) and polycyclic aromatic hydrocarbons (PAH), and the production of carcinogens such as heterocylic aromatic amines (HAA) and PAH through high-temperature cooking may account for the association between red and/or processed meat and cancer [29, 30]. However, since inflammation is a key risk factor for cancer [1], our results may suggest that red and/or processed meat consumption could also affect cancer development through the inflammatory pathway.
Our findings are in agreement with previously published literature [14–17]. In a cross-sectional study, Ley et al. reported that red meat intake was positively associated with serum CRP and inversely associated with serum adiponectin levels in 3,690 diabetes-free females. However, the associations were attenuated for CRP after adjusting for BMI and for adiponectin after adjusting for medical and lifestyle factors [16]. Monton et al. assessed the association between the consumption of red meat and circulating high sensitive CRP levels with and without the adjustment of BMI in 2,198 men and women and reported similar results, but no significant associations between red meat intake and serum adiponectin levels were observed even without adjustment of BMI [17]. None of the aforementioned studies conducted mediation analysis to evaluate the role of BMI. Furthermore, in an 8-week, parallel-designed randomized study, researchers found that partially replacing dietary carbohydrate with protein from lean red meat in isoenergetic diets did not increase circulating levels of inflammatory markers, such as high sensitive CRP, fibrinogen and serum amyloid A protein [31]. The sum of the evidence indicates an indirect association between red meat and inflammation through body weight or fat.
As adipose tissue consists of adipocytes, immune cells, and nerve/connective tissue, it is an endocrine organ and plays an important role in regulating whole-body metabolism. The pro-inflammatory phenotype associated with excess fat mass leads to low production of adiponectin and high production of leptin, CRP, IL-6, and TNF-α [4]. Our results indicate that BMI was significantly, positively associated with serum leptin, CRP, and IL-6 levels and inversely associated with serum adiponectin levels, although the associations with IL-6 and adiponectin were restricted to women. Additionally, current results also suggest that BMI mediated the associations between red and processed meat intake and serum leptin, adiponectin and CRP levels, indicating that a diet high in red and processed meat may contribute to weight gain and body fat accumulation, which in turn induces the obesity-related inflammatory process. Therefore, in terms of influencing circulating levels of adipokines and inflammatory markers, reducing body fat may be more relevant than lowering intake of dietary red and processed meats.
After stratification by race/ethnic in women, the associations of red and processed meat intake with certain biomarkers (e.g., leptin, adiponectin, CRP) were stronger in some race/ethnicity groups compared to others, although there were no significant interactions with race/ethnicity in our study. However, due to the small number of the participants in each race/ethnic group, it would not be surprising that some of the associations were not statistically significant even when the directions of the associations appeared to be the same. The inverse association between red and processed meat intake and TNF-α levels observed in white men warrants future investigation with the involvement of more markers in the TNF pathway. Previous work reported that processed and unprocessed red meat were inversely associated with TNF receptors (sTNF-R1 and sTNF-R2, both considered as pro-inflammatory cytokines). However, unprocessed red meat consumption was positively associated with bioavailable TNF-α, and processed meat intake was inversely associated with total TNF-α [18].
To the best of our knowledge, this is the first study to examine the associations of dietary intakes of red and processed meat with various circulating inflammatory and adiposity biomarkers. The current study is also unique in that we examined a population of five race/ethnic groups. The multiethnic population and the population-based cohort design strengthen the generalizability of the study results. Study limitations include the fact that dietary intake was estimated by self-reported FFQs and the long time interval of 9.1 years between cohort entry and the collection of blood samples. However, the associations remained similar in a subset of participants who provided an updated diet history and BMI within 2 years of the blood draw. The fact that the current study population consisted of controls in two nested case-control studies of cancer may have introduced selection bias. Another limitation of our study was the study design, which prevents the unequivocal determination of temporal causal relations between red and processed meat intake, BMI, and serum biomarkers associated with obesity and inflammation.
CONCLUSION
Consistent with AICR/WCRF cancer prevention recommendations, the current findings suggest that dietary intakes of red and processed meats may be associated with adverse health effects such as cancer through an inflammatory pathway in some population groups. The current data suggest that the amount of excess body weight or the degree of adiposity mediates the association between dietary red and processed meat intake and levels of obesity-related serum inflammatory markers.
Acknowledgments
The authors thank William Cooney and Jennifer Lai for their technical performance of serum biomarker assays.
FINANCIAL SUPPORT
The Multiethnic Cohort Study has been supported by grants R37 CA 54281, R01 CA 63464, P01 CA033619, and UM1 CA164973 from the National Cancer Institute. Partial support was also provided by NCI grant P01 CA138338. The Analytical Biochemistry Shared Resource of the University of Hawaii Cancer Center is supported, in part, by grant P30 CA71789 from NCI.
Footnotes
The authors declare no conflicts of interest
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Kim YS, Milner JA. Dietary modulation of colon cancer risk. The Journal of nutrition. 2007;137(11 Suppl):2576S–2579S. doi: 10.1093/jn/137.11.2576S. [DOI] [PubMed] [Google Scholar]
- 3.Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutrition reviews. 2013;71(8):511–527. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- 4.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. The Proceedings of the Nutrition Society. 2011;70(4):408–417. doi: 10.1017/S0029665111000565. [DOI] [PubMed] [Google Scholar]
- 5.Bulcao C, Ferreira SR, Giuffrida FM, Ribeiro-Filho FF. The new adipose tissue and adipocytokines. Current diabetes reviews. 2006;2(1):19–28. doi: 10.2174/157339906775473617. [DOI] [PubMed] [Google Scholar]
- 6.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiological research / Academia Scientiarum Bohemoslovaca. 2006;55(3):233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 7.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2(2):131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 8.Garofalo C, Surmacz E. Leptin and cancer. Journal of cellular physiology. 2006;207(1):12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 9.Tilg H, Wolf AM. Adiponectin: a key fat-derived molecule regulating inflammation. Expert opinion on therapeutic targets. 2005;9(2):245–251. doi: 10.1517/14728222.9.2.245. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence T. Inflammation and cancer: a failure of resolution? Trends in pharmacological sciences. 2007;28(4):162–165. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition. Physical Activity, and the Prevention of Cancer: a, Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 12.Hastert TA, Beresford SA, Patterson RE, Kristal AR, White E. Adherence to WCRF/AICR cancer prevention recommendations and risk of postmenopausal breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(9):1498–1508. doi: 10.1158/1055-9965.EPI-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. The Journal of nutrition. 2009;139(2):335–339. doi: 10.3945/jn.108.096297. [DOI] [PubMed] [Google Scholar]
- 14.Damiao R, Castro TG, Cardoso MA, Gimeno SG, Ferreira SR Japanese-Brazilian Diabetes Study G. Dietary intakes associated with metabolic syndrome in a cohort of Japanese ancestry. The British journal of nutrition. 2006;96(3):532–538. [PubMed] [Google Scholar]
- 15.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. The Journal of nutrition. 2007;137(4):992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 16.Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, Grodstein F, Hu FB. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. The American journal of clinical nutrition. 2014;99(2):352–360. doi: 10.3945/ajcn.113.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montonen J, Boeing H, Fritsche A, Schleicher E, Joost HG, Schulze MB, Steffen A, Pischon T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. European journal of nutrition. 2013;52(1):337–345. doi: 10.1007/s00394-012-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwedhelm C, Pischon T, Rohrmann S, Himmerich H, Linseisen J, Nimptsch K. Plasma Inflammation Markers of the TNF Pathway but not C-Reactive Protein Are Associated with Processed Meat and Unprocessed Red Meat Consumption in Bavarian Adults. The Journal of nutrition. 2016 doi: 10.3945/jn.116.237180. [DOI] [PubMed] [Google Scholar]
- 19.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. The American journal of clinical nutrition. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 20.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. American journal of epidemiology. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto Y, Conroy SM, Ollberding NJ, Henning SM, Franke AA, Wilkens LR, Goodman MT, Hernandez BY, Le Marchand L, Henderson BE, et al. Erythrocyte membrane fatty acid composition, serum lipids, and non-Hodgkin’s lymphoma risk in a nested case-control study: the multiethnic cohort. Cancer causes & control : CCC. 2012;23(10):1693–1703. doi: 10.1007/s10552-012-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ollberding NJ, Kim Y, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, Maskarinec G, Hernandez BY, Henderson BE, Le Marchand L, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer prevention research. 2013;6(3):188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SY, Wilkens LR, Henning SM, Le Marchand L, Gao K, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer causes & control : CCC. 2009;20(2):211–223. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AM, Earle ME, Nagamine FS, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. American journal of epidemiology. 2000;151(4):358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conroy SM, Maskarinec G, Morimoto Y, Franke AA, Cooney RV, Wilkens LR, Goodman MT, Hernadez BY, Le Marchand L, Henderson BE, et al. Non-hodgkin lymphoma and circulating markers of inflammation and adiposity in a nested case-control study: the multiethnic cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(3):337–347. doi: 10.1158/1055-9965.EPI-12-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavri A, Guzic-Salobir B, Salobir-Pajnic B, Keber I, Stare J, Stegnar M. Seasonal variation of some metabolic and hemostatic risk factors in subjects with and without coronary artery disease. Blood Coagul Fibrinolysis. 2001;12:359–365. doi: 10.1097/00001721-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Sung KC. Seasonal variation of c-reactive protein in apparently healthy koreans. Int J Cardiol. 2006;107:338–342. doi: 10.1016/j.ijcard.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1989;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 29.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K International Agency for Research on Cancer Monograph Working G. Carcinogenicity of consumption of red and processed meat. The Lancet Oncology. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 30.Turner ND, Lloyd SK. Association between red meat consumption and colon cancer: A systematic review of experimental results. Experimental biology and medicine. 2017;0:1–27. doi: 10.1177/1535370217693117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgson JM, Ward NC, Burke V, Beilin LJ, Puddey IB. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. The Journal of nutrition. 2007;137(2):363–367. doi: 10.1093/jn/137.2.363. [DOI] [PubMed] [Google Scholar]