Abstract
Objectives
To evaluate the safety of a novel silver-impregnated Foley catheter system designed to prevent catheter-associated bacteriuria and funguria, assess recruitment feasibility for a future pivotal trial, and preliminarily assess efficacy.
Methods
This single-center, randomized controlled trial at a university hospital involved adult neurosurgical patients expected to have a urinary catheter for ≥ 24 hours. Subjects were randomized to a novel silver-impregnated (test) Foley catheter system or a control system. They were followed for 30 days (or until discharge) while catheterized, and for up to 48 hours after catheter removal, with daily bacteriuria testing and assessment for symptoms of infection and catheter intolerance.
Results
Ninety-five subjects were randomized (intention-to-treat [ITT] population), of whom 61 (64%) had a catheter for ≥ 24 hours without peri-operative antibiotics beyond 24 hours (evaluable population). In the ITT population, 11/95 (12%) subjects had an asymptomatic bacteriuria (ABU) event. Compared with controls, test system recipients had a trend toward longer time to ABU in the ITT population (p = 0.08, log-rank test) and a longer time to ABU in the evaluable population (p = 0.03). All 6 ABU events caused by Gram-negative bacilli occurred in the control group.
Conclusions
In this pilot randomized trial, the test system was well tolerated and seemingly effective in preventing catheter-associated bacteriuria, especially with Gram-negative bacilli. A pivotal study is warranted.
Keywords: Ionic silver, Foley catheter, catheter-associated bacteriuria, urinary tract infection
INTRODUCTION
Indwelling urinary catheters are placed in 16–25% of hospitalized patients.1, 2 Despite their necessity, indwelling urinary catheters have associated infection risks ranging from asymptomatic bacteriuria to bacteremia.3–5 Bacteriuria develops in up to 25% of subjects requiring a urinary catheter for ≥ 5 days, with a daily acquisition risk of 1–5%. 6, 7
Adverse consequences of catheter-associated bacteriuria (which can be symptomatic or asymptomatic) include increased mortality, higher costs, and prolonged hospitalization.8 The high estimated cost of healthcare-associated urinary tract infection (UTI), which is 400–500 million dollars annually in the U.S., has led to renewed interest in prevention of catheter-associated bacteriuria.9–13 Reducing the rate of bacteriuria in catheterized patients could help to lower rates of symptomatic infection and the associated costs and complications.
Bacteriuria (used hereafter to denote both bacteriuria and funguria) can develop in a patient with a urinary catheter either by intra-luminal spread of organisms introduced via a break in a closed urine collection system or by extra-luminal spread of organisms introduced at the time of catheter insertion or that migrate along the catheter’s external surface.6, 14 Sterile closed drainage systems reduce but do not eliminate the risk of bacteriuria, due to breaks in the system and the extra-luminal route of microbial entry.6, 15, 16 Therefore, other mechanisms of bacteriuria prevention have been pursued.
Silver is a broad-spectrum antiseptic that exhibits activity against both bacteria and fungi without inducing resistance.17, 18 Studies to date of silver alloy-coated catheters for bacteriuria prevention have yielded mixed results,19–21 with the most recent large, randomized trial showing no difference between the main currently marketed silver-containing Foley catheter and control.22 Another recent before-after non-randomized cohort study showed a reduction in symptomatic UTI.23 However, these studies do not prove that ionic silver is an ineffective biocide for use with urinary catheter systems; their inconsistent results may simply indicate that available ionic silver delivery features for urinary catheters are inadequate, indicating the need for an improved delivery system.
The ICET™ TIC system (hereafter, test system) is a silver-based urine drainage system that utilizes a novel (proprietary) sustained-release technology and is designed to prevent both intra-luminal and extra-luminal routes of infection. It includes an ionic silver-releasing Foley catheter attached to an accessory device that contains an antimicrobial silver and copper matrix. The test system has not been tested in an animal model, but has passed functionality and biocompatibility tests. This pilot study was the first study of the system in humans. It was a prospective, single site, randomized controlled trial (RCT) comparing the test system with the standard silver-alloy-coated catheter system currently in use at the study site with the primary objective of demonstrating feasibility of recruiting eligible patients to assess the incidence and time to ABU.
METHODS
Setting
This study was conducted over 12 months at the University of Minnesota Medical Center (UMMC), an 885-bed tertiary care teaching hospital in Minneapolis, MN. Subjects were recruited from among patients at least 18 years old who were scheduled for a neurosurgical or spinal surgery procedure and were expected (i) to have a urinary catheter in place for ≥ 24 hours, (ii) to receive no post-operative antibiotics, and (iii) to receive prophylactic perioperative antibiotics for < 24 hours. Exclusionary criteria included known UTI at the time of screening, receipt of systemic antibiotics within 48 hours prior to enrollment, having used an indwelling urinary catheter within 48 hours of the time of enrollment, and surgery involving the genitourinary tract. These inclusion and exclusionary criteria were stipulated by the Food and Drug Administration (from which approval was needed before the trial could be conducted), which considered the resulting population to be less likely to receive ongoing antibiotics than the general medical/surgical population, thereby allowing a non-confounded assessment of the bacteriuria-preventing effect of the test catheter. Operating room staff inserted all catheters in the operating room.
Catheter systems
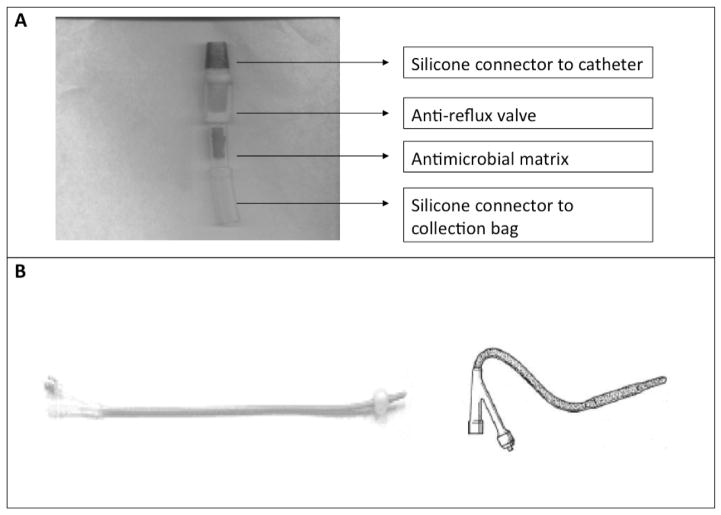
The control system was a commercially available, LUBRI-SIL® I.C. antimicrobial silicone Foley catheter, which incorporates a silver alloy coating in a closed system (CR Bard, Covington, GA). This system was chosen because it was the standard of care at UMMC at the time of the study. The test system includes a novel catheter designed to release continuous ionic silver into the immediate body fluid environment at the device-tissue interface at a steady concentration that is inhibitory to microbes but still low enough to avoid toxicity to surrounding tissues. Both the intra-luminal and extra-luminal surfaces of the test catheter are coated with ionic silver. To provide additional barriers to ascending infections from the collection bag, the catheter is connected to the drainage tubing by an interposed accessory device that contains, in series, an anti-reflux valve and an antimicrobial matrix made of polyurethane polymer impregnated with silver and copper (Figure 1). The combination of the novel silver elution system coating the Foley catheter and the combined anti-reflux/antimicrobial accessory device was designed to prevent both the intra-luminal and extra-luminal routes of infection.
Figure 1. Catheter configuration.
A) Components of the accessory device positioned between the catheter and the collection system. The device includes, in series, an anti-reflux valve and an antimicrobial matrix made of polyurethane polymer impregnated with silver and copper B) Photograph (left) and diagram (right) of the silver-coated catheter. The shaded portion of the diagram represents the areas that are coated in silver.
The test system was assembled by using a CR Bard commercial closed drainage Foley tray (Kit # 902816) and replacing the CR Bard catheter with the test catheter and antimicrobial filter matrix. It was produced, assembled, and supplied by ICET, Inc. and Primrose Medical, Inc. (Walpole, MA) under the investigational device exemption approval from the FDA.
Allocation and blinding
Subjects were assigned randomly to the test system or control system group based on an interactive web-based randomization system, with stratification by gender, diabetes diagnosis, and use of cefazolin vs. clindamycin as perioperative prophylaxis. Since the test and control catheter systems differed in appearance, neither the subject nor the health care worker who inserted the catheter could be blinded to treatment assignment, which was also the case in multiple previous studies of novel urinary catheters.19, 20, 24 However, the primary study endpoint was bacteriuria, the presence of which was determined based on culture results generated by the clinical microbiology laboratory staff, who were blinded to treatment assignments.
Data Collection
Urine dipstick screening was done at the time of enrollment as a rapid screen for presence of bacteriuria, as indicated by positive leukocyte esterase and/or nitrite. After enrollment, randomization, and catheter insertion subjects were assessed daily to ensure proper catheter placement (i.e., position of the bag, tubing, seal, and clamp). Urine specimens for culture were collected aseptically each day from the catheter and the collection bag. An additional urine specimen was obtained 48 hours after catheter removal, if possible. Urine samples were assessed by the clinical microbiology laboratory at UMMC. Urine specimens were plated on MacConkey and sheep’s blood agar, per the clinical microbiology laboratory’s protocol. Species identity was determined using the VITEK 2 automated system (BioMérieux, Durham, NC) for all isolates, regardless of the number of colonies isolated.
Any blood cultures obtained were also recorded and reviewed. Randomized subjects were followed with the catheter indwelling for up to 30 days from catheter placement or until discharge, whichever came first, and were followed for 48 hours after catheter removal if the catheter was removed in-hospital within 30 days of its placement. All test system catheters were removed prior to hospital discharge.
For the duration of Foley catheter use, subjects were assessed daily for vital signs and signs/symptoms of infection, including temperature > 38°C, suprapubic tenderness, and costovertebral angle pain or tenderness. Subjects were also questioned daily about symptoms of catheter intolerance, including dysuria, itching, urethral/pelvic pain, urgency, redness, or swelling. Medical records were reviewed for adverse events such as dysuria, oliguria, or urinary retention. Adverse events were assessed individually by the study team based on evaluation of the patient and medical record review. Each event was determined to be “unrelated” or “possibly related” to the catheter after evaluation of the situation.
Definitions
Asymptomatic bacteriuria (ABU) was defined as a urine culture with ≥ 105 CFU/ml of no more than 2 species, from a urine sample collected either via the catheter (if during the catheterization period) or by voiding or in-out catheterization (if within 48 hours after catheter removal) in the absence of the symptoms outlined below.
Symptomatic UTI (SUTI) was defined as a positive urine test (see below) plus at least 1 of the following: temperature > 38°C, suprapubic tenderness, or costovertebral angle pain or tenderness. A positive urine test was defined as at least one of the following: i) dipstick-positive for leukocyte esterase or nitrite, ii) pyuria (≥ 10 white blood cells [WBC]/mm3, or ≥ 3 WBC/high power field of unspun urine), iii) positive Gram stain of unspun urine and a urine culture with ≥ 103 CFU/mL but < 105 CFU mL of no more than 2 species, or iv) a urine culture with ≥ 105 CFU/mL of no more than 2 species.
Approvals
The clinical protocol and definitions were approved by the FDA Center for Devices and Radiological Health under an investigational device exemption. The study was conducted in accordance with the FDA-approved protocol. The study was reviewed and approved by the Institutional Review Board of the University of Minnesota.
Statistical analysis
The study was designed to enroll up to 120 subjects, with the goal of having 30 evaluable subjects in each arm. Since the primary study objective was to demonstrate the feasibility of recruiting eligible patients for assessing the incidence of and time to ABU, as needed to inform the design of a future pivotal study, no formal power calculations were done.
Secondary study objectives were to evaluate the safety and tolerance of the test system and rates of SUTI. Statistical analyses were done using SAS® software. Incidence of ABU was evaluated using a logistic regression model. Time to first ABU event was evaluated using a log-rank test.
RESULTS
Study population
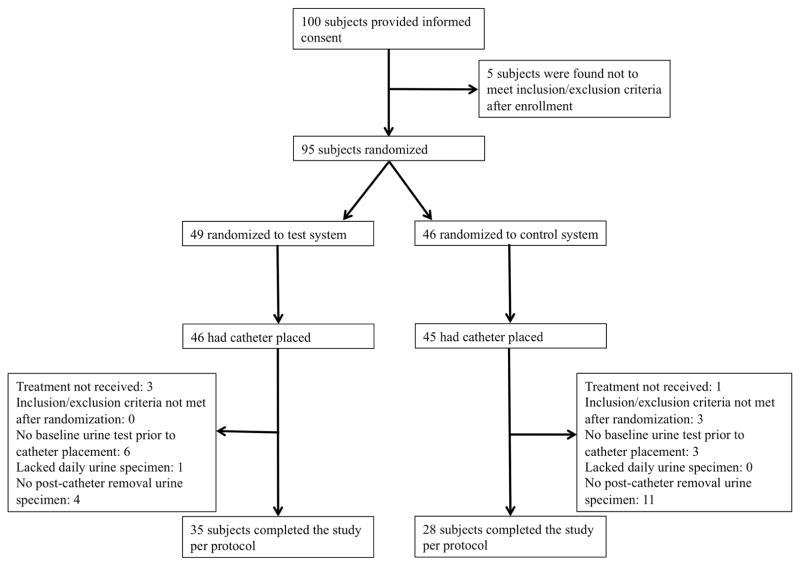
Overall, 100 subjects provided informed consent, 95 underwent randomization, and 91 had a catheter placed. One subject had a positive baseline dipstick screen, so was excluded prior to randomization. Of randomized subjects who had a catheter placed, 63 (69%) completed the study per protocol; the other 28 (31%) experienced one or more protocol deviations. The most common protocol deviation was the lack of a post-catheter removal urine specimen (Figure 2), which resulted from subjects being unable to give a urine specimen “on demand” post-catheter removal or being discharged from the hospital prior to specimen collection. The characteristics of subjects who were randomized and had a catheter placed were similar in the two study groups (details available from authors upon request).
Figure 2.
Clinical trial overview.
Incidence of ABU
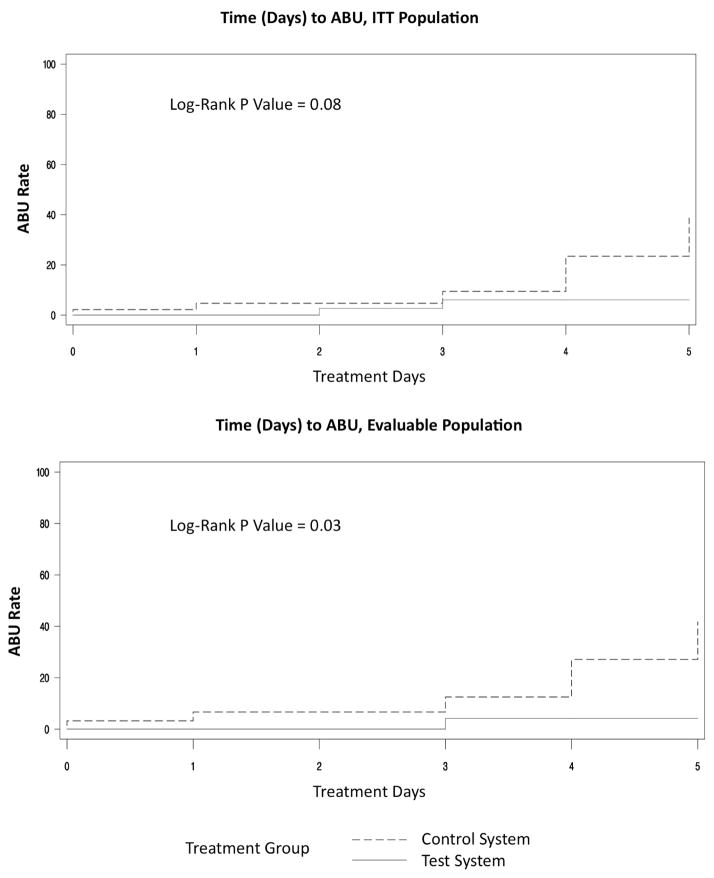
The 95 subjects who underwent randomization were included in the ITT analysis. Of these, 49 (52%) were randomized to the test system and 46 (48%) to the control system. Overall, 11 ABU events occurred, including 3 (6%) among subjects with the test system, vs. 8 (17%) among control subjects (p = 0.11, logistic regression). The only SUTI event occurred in the test system group (Table 1). There was a trend toward longer time to ABU among test system recipients (p = 0.08, log-rank test) (Figure 3).
Table 1.
Incidence of asymptomatic bacteriuria (ABU) and symptomatic urinary tract infection (SUTI) in relation to type of Foley catheter system.
| Intention-to-treat populationa | Evaluable populationb | |||||
|---|---|---|---|---|---|---|
| Incidence, no. (column %) | Incidence, no. (column %) | |||||
| Endpoint | Test system (n = 49) | Control system (n = 46) | P value | Test system (n = 30) | Control system (n = 31) | P value |
| ABU | 3 (6) | 8 (17) | 0.11 | 2 (7) | 8 (26) | 0.06 |
| SUTI | 1 (2) | 0 (0) | 0.90 | 1 (3) | 0 (0) | 0.96 |
The intention-to-treat population included the 95 subjects who underwent randomization.
The evaluable population included the 61 subjects who had a catheter indwelling for at least 24 hours without post-operative systemic antibiotics.
Figure 3. Time to asymptomatic bacteriuria (ABU) for intention-to-treat (ITT) population (top) and evaluable population (bottom).
According to the log rank text, time to ABU among test system recipients (solid line) vs. control subjects (dashed line) was borderline significantly longer in the ITT population (top), and significantly longer in the evaluable population (bottom).
A total of 61 subjects qualified for the evaluable population, i.e., had a catheter indwelling for at least 24 hours without post-operative systemic antibiotics. These included 30 (49%) test system recipients and 31 (51%) control system recipients. Within this population, ABU occurred in 2 (7%) test system recipients and 8 (26%) control system recipients (p = 0.06, logistic regression) (Table 1). Similarly, time to ABU was significantly greater among test system recipients (p = 0.03, log-rank test) (Figure 3).
Organisms causing bacteriuria
A total of 12 organisms were recovered in association with the 11 ABU events. One ABU event involved Klebsiella pneumoniae and Escherichia coli, both at ≥ 105 cfu/mL. The remaining 10 events each involved 1 organism, also at ≥ 105 cfu/mL. Of the 12 ABU organisms, 6 (50%) were Gram-negative bacilli (4 E. coli, 1 K. pneumoniae, and 1 Pseudomonas aeruginosa), 5 (42%) were Gram-positive cocci (4 Enterococcus spp. and 1 coagulase-negative Staphylococcus), and 1 (8%) was yeast (Candida kefyr). Of note, all 6 ABU events involving Gram-negative bacilli occurred in the control system group; none occurred in the test group.
Adverse events
Events judged by the study team to be possibly catheter-related included mainly decreased urine output and urinary retention. These occurred in 6% of subjects overall and did not differ in frequency between treatment groups (Table 2).
Table 2.
Adverse events among subjects with a catheter placed
| Number of subjects with event (column %) | ||||
|---|---|---|---|---|
| Event | Subset | Test System (n = 49) | Control System (n = 46) | Total (n = 95) |
| Dysuria | Total | 1 (2.0) | 0 (0) | 1 (1.0) |
| Possibly relateda | 0 (0.0) | 0 (0) | 0 | |
| Oliguria | Total | 2 (4.0) | 0 (0.0) | 2 (2.1 |
| Possibly relateda | 0 (0.0) | 0 (0.0) | 0 | |
| Urinary retention | Total | 4 (8.2) | 2 (4.3) | 6 (6) |
| Possibly relateda | 2 (4.0) | 2 (4.3) | 4 (4.2) | |
| Decreased urine output | Total | 1 (2.0) | 1 (2.2) | 2 (2.1) |
| Possibly relateda | 1 (2.0) | 1 (2.2) | 2 (2.1) | |
| Total | Total | 8 (16.3) | 3 (6.5) | 11 (11.6) |
| Possibly relateda | 3 (6.1) | 3 (6.5) | 6 (6.3) | |
Whether adverse events were possibly related to the study catheter was determined by members of the study team based on the clinical context, without regard for study group.
COMMENT
In this pilot RCT we evaluated the feasibility of studying a novel antimicrobial urinary catheter system within an FDA-mandated study design, while generating exploratory data regarding the system’s safety and efficacy in preventing ABU. We found a trend toward lower incidence of ABU in the test system group. Similarly, time to first ABU event was longer with the test system, to a borderline significant extent in the ITT population and significantly so in the evaluable population. With current hospital protocols aimed at decreasing the duration of catheter use in post-operative patients, a catheter system that increases the time to colonization with microorganisms presumably could significantly decrease rates of SUTI.25
Of particular interest was the absence of Gram-negative bacteriuria episodes among test system recipients. Previous studies have suggested that marketed silver-coated Foley catheters may be no more (and possibly less) active both in vitro and in vivo against Gram-negative bacilli than against Gram-positive organisms.26, 27 In contrast, a study of silver wound dressings found a greater effect on Gram-negative bacilli than on Gram-positive organisms, with differences noted between dressing brands.28 Although it would be premature to draw conclusions based on this pilot study, the absence among test system recipients of ABU due to Gram-negative bacilli, which usually are the most common cause of catheter-associated bacteriuria and are the highest-risk organisms for causing catheter-associated urosepsis, is promising.3, 29 It suggests that the test system might be a timely addition to the arsenal of tools to prevent healthcare-associated Gram-negative bacteriuria.
Overall, the test system was well tolerated and had equivalent adverse effects to the (silver-alloy-coated) control antimicrobial catheter system currently in use at the study hospital. Only about 6% of subjects in each group had a suspected study catheter-related adverse event, usually urinary retention or decreased urine output.
An important strength of this study was the RCT study design. Since previous studies of antimicrobial catheters have shown mixed results, it is especially important to use a strong study design to evaluate novel catheter systems. Additionally, this pilot study was able to recruit and enroll 100 subjects from the target population, thereby demonstrating the feasibility of a future, larger pivotal RCT to evaluate the test system.
Study limitations included the relatively small sample size, the focus on bacteriuria rather than SUTI, the lack of blinding to catheter assignment (for subjects and study team members), the comparatively large number of protocol deviations, and missing data points (as outlined in Figure 2). However, the study size was appropriate for the immediate goals of this pilot project, which were primarily to assess the feasibility of recruiting eligible subjects and rates of ABU, as parameter estimates for design of a future pivotal trial. The lack of blinding of subjects and study team members, although not ideal, was unlikely to have influenced the main study outcome, ABU, since this endpoint was defined based on objective laboratory results, and the laboratory workers who generated these results were unaware of study group assignment.
Regarding missing samples, this study was conducted in the “real-life” hospital setting. Some patients were unable to provide a voided specimen prior to catheterization and 15% of randomized subjects lacked a post-catheter removal urine specimen. For a future pivotal trial, additional safeguards should be put into place to prevent missed urine specimens, including involving the clinical care nurses in obtaining the specimens, along with the clinical research coordinators.
CONCLUSIONS
In this pilot study of a novel antimicrobial Foley catheter system the test system was safe and well tolerated, and appeared to delay the onset of bacteriuria overall, particularly that due to Gram-negative bacilli. Given the potential for silver to act as a broad-spectrum biocide, it is worthwhile to explore novel ionic silver delivery technologies. Therefore, a future pivotal study is warranted to confirm these promising preliminary findings.
HIGHLIGHTS.
We present a pilot randomized controlled trial to evaluate a novel Foley catheter
Novel silver catheter system includes an antimicrobial matrix and antireflux valve
The test system was tolerated and seemingly effective in preventing bacteriuria
The test system seemed especially effective against Gram-negative bacilli
A pivotal study is warranted
Acknowledgments
Financial support
This pilot study was supported by a small business innovation research grant awarded to ICET, Inc. (R44 DK55891-06) by the National Institute of Diabetes and Digestive and Kidney Diseases. A.M.L. is supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant [5T32AI055433-09]. This material is also based in part upon work supported by Department of Veterans Affairs (J.R.J.).
Potential conflicts of interest
A.M.L., S.K., K.D., K.K., and P.F. report no conflicts of interest. J.R.J reports that he has been a paid independent consultant to ICET (the manufacturer of the test study catheter) and also has received research grants or contracts from Rochester Medical, Merck, Crucell, and Syntiron. M.A.H. consults on education projects for DePuy Synthes.
This trial was registered with ClinicalTrials.gov (identification number NCT01681511) and can be found at http://www.clinicaltrials.gov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476. doi: 10.1016/s0002-9343(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein JW, Mazon D, Pantelick E, et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 1999;20:543. doi: 10.1086/501675. [DOI] [PubMed] [Google Scholar]
- 3.Krieger JN, Kaiser DL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis. 1983;148:57. doi: 10.1093/infdis/148.1.57. [DOI] [PubMed] [Google Scholar]
- 4.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med. 2000;160:678. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 5.Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68. doi: 10.1016/s0196-6553(00)90015-4. [DOI] [PubMed] [Google Scholar]
- 6.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stark RP, Maki DG. Bacteriuria in the catheterized patient. What quantitative level of bacteriuria is relevant? N Engl J Med. 1984;311:560. doi: 10.1056/NEJM198408303110903. [DOI] [PubMed] [Google Scholar]
- 8.Scott R. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Centers for Disease Control and Prevention; 2009. http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. [Google Scholar]
- 9.Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17:552. doi: 10.1086/647371. [DOI] [PubMed] [Google Scholar]
- 10.Cope M, Cevallos ME, Cadle RM, et al. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis. 2009;48:1182. doi: 10.1086/597403. [DOI] [PubMed] [Google Scholar]
- 11.Trautner BW. Management of catheter-associated urinary tract infection. Curr Opin Infect Dis. 2010;23:76. doi: 10.1097/QCO.0b013e328334dda8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saint S, Meddings JA, Calfee D, et al. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877. doi: 10.7326/0003-4819-150-12-200906160-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S41. doi: 10.1086/591066. [DOI] [PubMed] [Google Scholar]
- 14.Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131. doi: 10.4065/74.2.131. [DOI] [PubMed] [Google Scholar]
- 15.Burke J, Riley D. Nosocomial urinary tract infection. In: Mayhall CG, editor. Hospital epidemiology and infection control. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 16.Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91:65S. doi: 10.1016/0002-9343(91)90345-x. [DOI] [PubMed] [Google Scholar]
- 17.Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother. 2007;59:587. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 18.Sardi JC, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62:10. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 19.Drekonja DM, Kuskowski MA, Wilt TJ, et al. Antimicrobial urinary catheters: a systematic review. Expert Rev Med Devices. 2008;5:495. doi: 10.1586/17434440.5.4.495. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JR, Kuskowski MA, Wilt TJ. Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med. 2006;144:116. doi: 10.7326/0003-4819-144-2-200601170-00009. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan A, Karchmer T, Richards A, et al. A prospective trial of a novel, silicone-based, silver-coated foley catheter for the prevention of nosocomial urinary tract infections. Infect Control Hosp Epidemiol. 2006;27:38. doi: 10.1086/499998. [DOI] [PubMed] [Google Scholar]
- 22.Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927. doi: 10.1016/S0140-6736(12)61380-4. [DOI] [PubMed] [Google Scholar]
- 23.Lederer JW, Jarvis WR, Thomas L, et al. Multicenter Cohort Study to Assess the Impact of a Silver-Alloy and Hydrogel-Coated Urinary Catheter on Symptomatic Catheter-Associated Urinary Tract Infections. J Wound Ostomy Continence Nurs. 2014 doi: 10.1097/WON.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Kim SW, Cho YH, et al. A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazone-coated and silicone catheters. Int J Antimicrob Agents. 2004;24(Suppl 1):S65. doi: 10.1016/j.ijantimicag.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Wald HL, Kramer AM. Feasibility of audit and feedback to reduce postoperative urinary catheter duration. J Hosp Med. 2011;6:183. doi: 10.1002/jhm.846. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JR, Johnston B, Kuskowski MA. In vitro comparison of nitrofurazone- and silver alloy-coated foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms. Antimicrob Agents Chemother. 2012;56:4969. doi: 10.1128/AAC.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maki DG, Knasinski V, Halvorson K, Tambyah PA. A novel silver-hydrogel impreganated indwelling catheter reduces CAUTIs: a prospective double-blind trial. In: Abstracts from the Eighth Annual Scientific Meeting of the Society for Healthcare Epidemiology of America. Infection Control and Hospital Epidemiology. 1998;19:680. [Google Scholar]
- 28.Ip M, Lui SL, Poon VK, et al. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol. 2006;55:59. doi: 10.1099/jmm.0.46124-0. [DOI] [PubMed] [Google Scholar]
- 29.Eykyn SJ. Urinary tract infections in the elderly. Br J Urol. 1998;82(Suppl 1):79. doi: 10.1046/j.1464-410x.1998.0820s1079.x. [DOI] [PubMed] [Google Scholar]