Abstract
A multitude of exogenous environmental stimuli and endogenous molecular and cellular components interface directly or indirectly with the free nerve endings of sensory nerves in the skin. Environmental stimuli include substances derived from the microbiome and materials, such as allergens, that otherwise come in contact with the skin. Endogenous stimuli include components of or mediators derived from the epidermal barrier, keratinocytes, mast cells, and additional resident and skin-homing immune cells. The sensation of itch is ultimately provoked by mediators that interact with and activate pruriceptors on the sensory nerve fibers. These peripheral fibers convey signals from the skin to the dorsal root and trigeminal ganglia and on to the spinal cord and brain where central processing of the itch sensation occurs. A discussion of the nature and sources of itch stimuli and receptors in the periphery form the basis of this chapter. The development of drugs that target these processes is in the process of revolutionizing therapeutic approaches to itch.
Graphical abstract
A simple view of peripheral itch is that a stimulus generates an action potential in a sensory nerve fiber that the brain interprets as the sensation of itch. This view, while convenient, does not reflect the remarkable complexity and redundancy in mechanisms that contribute to itch. Here, we address the panoply that contributes to peripheral itch. Our focus is on aspects that are most relevant to the practicing clinician so that mechanisms are appreciated while ongoing advances in therapies can be placed within an approachable context.
A multitude of exogenous environmental stimuli and endogenous molecular and cellular components interface directly or indirectly with sensory nerves in the skin. Environmental stimuli include substances derived from the microbiome, temperature, humidity, and materials that otherwise come in contact with the skin. Endogenous components include the epidermal barrier, pH, keratinocytes, and resident and skin-homing immune cells. It is now appreciated that communication between nerves, stimuli, and cutaneous components, including the dermal milieu of vessels and stroma, which provide a scaffold, is bi- or multidirectional such that each may serve to modulate the sensation of itch.
Nerve Fibers and Itch
Itch is sensed by nerve fibers called pruriceptors. Fibers that transmit pain and other noxious stimuli are called nociceptors. These are all afferent fibers that convey signals from the periphery to the dorsal root and trigeminal ganglia and on to the spinal cord where they synapse with second-order neurons. Signals are then transmitted to the brain for interpretation as itch and then back to the skin via motor neurons instructing us to scratch, in an effort to relieve this sensation. Complex neurocircuitry at the level of the spinal cord, as discussed in the previous chapter, can serve to not only activate, but also to inhibit itch signaling.
The sensation of itch, but not pain, requires at least a portion of the epidermis. It was demonstrated more than 50 years ago that free nerve endings can be found in the epidermis [1]. It is likely that these free nerve endings are innate sensors probing the environment and communicating with adjacent skin cells. These sensory fibers are characterized and distinguished by a number of features. Two distinct types of fibers that transmit itch are recognized: A-fibers and C-fibers [2]. A-fibers are myelinated, rapidly conduct nerve impulses, and are divided further into Aδ- and Aβ-fibers. Aδ-fibers can function as pruriceptors and nociceptors. Aβ-fibers do not [2].
Being unmyelinated, C-fibers conduct impulses slowly. They are of smaller diameter as compared to A-fibers. There are two populations of C-fibers. One population can respond to mechanical stimulation and heat, and is thus referred to as CM or CMH fibers. The other class is insensitive to such stimulation and is referred to as CMi fibers. CMi fibers respond to histamine and, upon stimulation, release the neuropeptides substance P and calcitonin gene-related peptide. Substance P and calcitonin gene-related peptide participate in vascular flare and the activation of mast cells. CMH fibers do not respond to histamine, but they do respond to cowhage [3]. Cowhage refers to a tropical bean plant, the pods of which are covered with small needles, also called cowhage. Contact with the skin allows the active component, a protease, to interact with a receptor on sensory fibers to provoke itch, pricking, and stinging sensations, akin to many clinical itches, including that of atopic dermatitis [4]. While we may ask a patient if he or she itches, or they tell us that they itch, further questioning often reveals the presence of additional, but less intense, sensations of pricking or stinging. It is likely that most itches are mediated by CMH fibers.
It is not clear whether humans have fibers that specifically sense itch as opposed to pain, although such a distinction has been shown in mice. There are theories that can account for the differentiation between itch and pain. For our purposes, the stimulation of overlapping populations of cutaneous sensory fibers combined with interpretation of signals in the spinal cord and brain result in the sensation of itch.
Acute versus Chronic Itch
Acute itches are those that last anywhere from an instant to 6 weeks. Examples range from a spontaneous itch that necessitates a simple scratch, to the itch, scratch, and dermatographism occurring in some people while changing into pajamas at night or the itch from poison ivy that lasts approximately 3 weeks. These are self-limited itches although treatment is often indicated.
Chronic itch is that which persists for 6 weeks or longer. Chronic itches associated with inflammation include atopic dermatitis, psoriasis, or the persistence of contact with an unknown allergen. Systemic diseases are also associated with chronic itch but may not include apparent inflammation. Examples include chronic kidney disease and the itch of cholestasis. Neurogenic and neuropathic chronic itches include notalgia paresthetica and postherpetic neuralgia.
Itch mediators that turn on pruriceptors in the skin may be present in both acute and chronic itches. Blocking the mediators, treatment of the inflammation, or a kidney transplant in a patient with renal itch may ameliorate these itches. However, both the peripheral and central nervous systems can become sensitized over time. When this happens, the neural architecture is altered such that despite removal of the initial itch stimulus, itch may persist. We do not yet understand the role of mediators and pruriceptors in these situations, nor do we know how to reverse the effect of sensitization.
Sources of Mediators of Itch
As noted in the Introduction, exogenous environmental stimuli and endogenous molecular and cellular components interface in a direct or indirect manner with sensory nerves in the skin to provoke itch (fig. 1). Direct stimuli include mediators, such as proteases, released by members of the microbiome. These stimuli and how they work are discussed in more detail below. An indirect stimulus could be a contact allergen that generates an allergic response leading to the release, from immune cells, of itch mediators which then stimulate sensory nerves. Using atopic dermatitis as another example, there is a complex interplay between bacteria, the epidermal barrier, keratinocytes, immune cells, and sensory nerves, which together leads to itch, inflammation, and the itch-scratch cycle. This interplay is being teased apart as manifest by the development of new therapeutics for atopic dermatitis. Ironically, with the exception of some urticarias associated with histamine, there is not a single clinical situation in which the mediators of itch are defined.
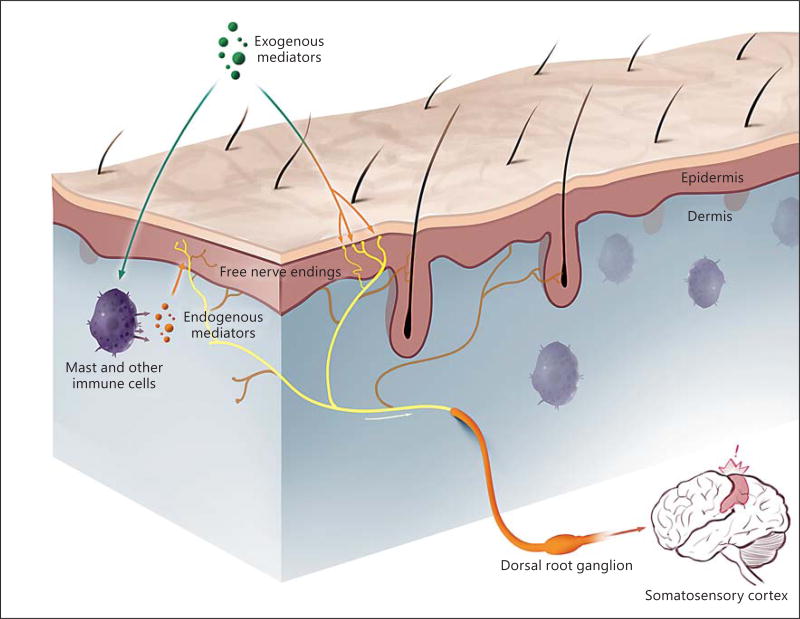
Fig. 1.
Itch is provoked by the interaction of exogenous and endogenous stimuli with free nerve endings in the epidermis. Itch-selective free nerve endings reach all layers of the epidermis and are depicted in yellow. Pain-selective free nerve endings are depicted in a darker color. The pain and itch nerve endings may be part of the same sensory nerve with cell bodies in dorsal root ganglia (figure generated by Jimmy Xia).
Receptors, Channels, and Mediators of Itch
There are three classes of receptors that can be activated by itch mediators. These include members of the G protein-coupled receptor (GPCR), Toll-like receptor (TLR), and cytokine families, respectively. There is one class of channels broadly associated with itch. This is the transient receptor potential (TRP) channel family. Generation of an action potential and transmission of an itch signal ultimately depends upon activation of sodium channels.
G Protein-Coupled Receptors
Histamine and the H1 receptor remain the most widely known mediator and receptor in itch, but they are no longer considered the most important. Their diminished importance arises from the clinical observation that antihistamines are not effective in most itches, including atopic dermatitis. Histamine is important in some urticarias. A number of other mediators and their cognate receptors are in the process of replacing the classic view of the importance of histamine and the H1 receptor in itch. There are four histamine receptors. One of these, H4, is also involved in itch [5]. H4 antagonists are helpful in itch, but side effects have limited their use for now [5].
Most of the currently known endogenous and exogenous pruritogens activate GPCRs. GPCR activation does not lead directly to the generation of an action potential. GPCR activation is coupled via intracellular signaling pathways to TRP channels, the activation of which allows for sufficient current influx to generate action potentials. As a general rule, histamine and activation of the histamine receptor is linked to TRPV1 sensitization and activation, whereas histamine-independent itch works through other GPCRs and is linked to TRPA1 [6, 7].
Nonhistamine receptors of importance in itch include protease-activated receptors, Mas-related G protein-coupled receptors, the neurokinin 1 receptor, and those for serotonin, endothelin, and lysophatidic acid. Protease-activated receptors are activated by proteases, including tryptase from mast cells and kallikreins and cathepsin S from keratinocytes. Mas-related G protein-coupled receptors are activated by some proteases but also by many other substances, including antimicrobial peptides [8, 9]. Substance P, a neuropeptide associated with neurogenic inflammation and atopic dermatitis, activates both Mas-related G protein-coupled receptors and the neurokinin 1 receptor [10]. Drugs that the target neurokinin 1 receptor are in clinical trials for itch. Autotaxin is the enzyme responsible for the production of lysophosphatidic acid. Autotaxin and the lysophosphatidic acid family of receptors may be involved with cholestatic itch [11].
Prostaglandins and leukotrienes can induce itch in human skin and can signal through GPCRs. However, it appears that their function is to potentiate itch rather than evoke it directly. Gastrin-releasing peptide and its receptor as well as brain natriuretic peptide and its receptor are important in itch, but these seem to be primarily in the spinal cord, not the skin [12, 13]. Morphine and other opiates cause itch via activation of certain opiate receptors. These itches are not relieved by scratching and the receptors are thus in the central, not peripheral, nervous system.
There is also a protective side to GPCR signaling. Activation of certain GPCRs has the benefit of inhibiting itch. Cannabinoids, which are agonists of CB1 and CB2 receptors, compounds that are agonists of the κ-opioid receptors including dynorphin, an endogenous κ-agonist, and stimulation of H3 receptors all can inhibit itch [14, 15]. The extent to which these effects are peripheral, central, or a mix is an active area of investigation.
Toll-Like Receptors
TLRs function as innate sensors in the immune system. They may have a similar role in the nervous system but this possibility has not been demonstrated conclusively. TLR3, TLR7, and potentially TLR4 are expressed on small-sized primary sensory neurons. Direct activation of TLRs by any of the classic pruritogens has not been demonstrated. TLRs can thus facilitate itch transmission, but a direct role in itch has not yet been elucidated [16].
Cytokine Receptors: Interleukin-31 and Thymic Stromal Lymphopoietin
Interleukin (IL)-31 is produced by Th2 cells, each of which has been identified as having a role in atopic dermatitis [17]. The receptor for IL-31 is expressed on various cell types, including sensory neurons [18]. Although these observations suggest a role for this receptor in itch, the slow onset of pruritus following injection are consistent with IL-31 being primarily an indirect mediator of itch [19]. Antibodies to the receptor are in development for the treatment of itch.
Thymic stromal lymphopoietin (TSLP) is an IL-7-like cytokine produced primarily by epithelial cells. This cytokine has been linked to atopic dermatitis and the atopic march to asthma. TSLP mediates its effects via a receptor composed of a TSLP receptor chain and an IL-7 receptor α-chain. Itch evoked by TSLP is via its receptor on a subset of sensory nerves [20]. A monoclonal antibody directed to TSLP has demonstrated efficacy in allergen- induced asthmatic responses in humans [21]. Whether or not targeting TSLP or its receptor will be of benefit in itch in general or atopic dermatitis has not been reported.
Transient Receptor Potential Channels
Although a direct role for TRPs in itch remains elusive, TRPs facilitate itch transduction. More than 25 TRP family members have been described and these are broken down further into subfamilies. TRPs are distributed broadly across tissues. TRPs relevant to itch are TRPV1, TRPV3, TRPV4, TRPA1, and TRPM8 [22]. TRPV1 is best known as the receptor for capsaicin, but is also a heat sensor. TRPA1 is a chemosensor that is activated by allyl isothiocyanate, cinnamaldehyde, and allicin, the pungent compounds found in mustard, cinnamon, and garlic extracts, respectively. TRPM8 is a cold sensor and is activated by menthol. Extremes of heat and cold, via activation of TRPV1 and TRPM8, can distract from the sensation of itch. Although TRPV1 and TRPA1 may not be directly associated with most clinical itches, their threshold for activation is modulated by signaling following pruritogen activation of GPCRs.
Conclusions and Future Expectations
Many receptors, channels, and mediators are linked to itch. The importance of each of these in clinical conditions is being deciphered. The possibility of treating itch by targeting the nervous system, not just the immune system, is on the horizon. Drugs directed to some of these targets will be available within the next several years and will usher in a targeted approach for the treatment of many itches.
References
- 1.Shelley WB, Arthur RP. The neurohistology and neurophysiology of the itch sensation in man. AMA Arch Derm. 1957;76:296–323. doi: 10.1001/archderm.1957.01550210020004. [DOI] [PubMed] [Google Scholar]
- 2.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurmond RL, Kazerouni K, Chaplan SR, Greenspan AJ. Antihistamines and itch. Handb Exp Pharmacol. 2015;226:257–290. doi: 10.1007/978-3-662-44605-8_15. [DOI] [PubMed] [Google Scholar]
- 6.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA. Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nat Commun. 2015;6:7864. doi: 10.1038/ncomms8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 2011;286:44739–44749. doi: 10.1074/jbc.M111.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig Dis. 2011;29:66–71. doi: 10.1159/000324131. [DOI] [PubMed] [Google Scholar]
- 12.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 13.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haruna T, Soga M, Morioka Y, Hikita I, Imura K, Furue Y, et al. S-777469, a novel cannabinoid type 2 receptor agonist, suppresses itch-associated scratching behavior in rodents through inhibition of itch signal transmission. Pharmacology. 2015;95:95–103. doi: 10.1159/000371890. [DOI] [PubMed] [Google Scholar]
- 16.Taves S, Ji RR. Itch control by Toll-like receptors. Handb Exp Pharmacol. 2015;226:135–150. doi: 10.1007/978-3-662-44605-8_7. [DOI] [PubMed] [Google Scholar]
- 17.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. 2014;69:113–117. doi: 10.1111/all.12316. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 22.Lucaciu OC, Connell GP. Itch sensation through transient receptor potential channels: a systematic review and relevance to manual therapy. J Manipulative Physiol Ther. 2013;36:385–393. doi: 10.1016/j.jmpt.2013.05.018. [DOI] [PubMed] [Google Scholar]