Abstract
Background
Neurotoxicity associated with amyloid and tau protein aggregation could represent a pathophysiological cascade that, along with vascular compromise, may predispose individuals to late-life depression (LLD). In LLD, apathy is common, leads to worsening of functioning, and responds poorly to antidepressant treatment. Better understanding of the pathophysiological mechanisms of apathy in LLD would facilitate development of more effective diagnostic and treatment approaches. In this cross-sectional pilot study, we performed positron emission tomography scans after injection of 2-(1-{6-[(2-[18F]fluoroethyl)(methyl)-amino]-2-naphthyl}ethylidene) malononitrile ([18F]FDDNP), an in vivo amyloid and tau neuroimaging study, in patients with LLD to explore neural correlates of apathy.
Methods
Sixteen depressed elderly volunteers received clinical assessments and [18F]FDDNP positron emission tomography scans. The cross-sectional relationship of [18F]FDDNP binding levels with depression (Hamilton Depression Rating Scale) and apathy (Apathy Evaluation Scale) were studied using Spearman's correlation analyses because of the relatively small sample size. Age, sex, and years of education were partialed out. Significance levels were set at P ≤ 0.05.
Results
[18F]FDDNP binding in the anterior cingulate cortex was negatively associated with the Apathy Evaluation Scale total (r = -0.62, P = 0.02; where low Apathy Evaluation Scale score equals greater severity of apathy). This suggests that apathy in LLD is associated with higher amyloid and/or tau levels in the anterior cingulate cortex. None of the regional [18F]FDDNP binding levels was significantly associated with the Hamilton Depression Rating Scale total.
Conclusion
This pilot study suggests that increased apathy in subjects with LLD may be associated with greater amyloid and/or tau burden in certain brain regions. Future studies in larger samples would elucidate the generalizability of these results, which eventually could lead to improved diagnostic and treatment methods in LLD.
Keywords: amyloid, apathy, [18F]FDDNP, late-life depression, PET, tau
Introduction
Amyloid and tau are key neuropathological hallmarks in the development of Alzheimer's disease (AD),1,2 but they are increasingly studied in LLD. Our group has developed and used a 2-(1-{6-[(2-[F18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile ([18F]FDDNP) in vivo probe that binds to cerebral aggregates of amyloid-β (Aβ) plaques and tau neurofibrillary tangles.3 The [18F]FDDNP bio-marker has been found to differentiate individuals with mild cognitive impairment (MCI), AD, and normal cognition, wherein global [18F]FDDNP binding is highest in patients with AD, intermediate in those with MCI, and lowest in normal comparison subjects.3 Our group is the first to explore this probe in LLD. In a 2011 paper, Kumar et al. compared [18F]FDDNP levels between 20 LLD patients and 19 healthy controls.4 [18F]FDDNP binding was significantly higher overall and in the posterior cingulate cortex (PCC) and lateral temporal regions in the major depressive disorder (MDD) group than in controls. The authors suggested neurotoxicity due to amyloid and tau protein aggregation may represent a pathophysiological cascade, which, along with vascular compromise, may predispose individuals to LLD.
Apathy is a common feature of LLD and afflicts more than 30% of individuals with LLD.5 It is defined as a primary motivational impairment resulting in diminished goal-oriented behaviour, lack of intellectual interest, and flattening of affect.6 Clinically, this leads to poor engagement in treatment, greater disability of functioning,5 a greater burden on caregivers, and an increased risk for functional and possibly cognitive impairment.7,8 Understanding biomarkers underpinning the comorbidity of LLD and apathy are therefore important for improving treatment outcomes.
We are aware of two studies that have examined the neural underpinnings of apathy in LLD subjects with any modality of neuroimaging. The first study was performed by our group and used structural magnetic resonance imaging (MRI) to compare 43 patients with MDD and 41 normal comparison subjects.9 A higher degree of apathy was associated with decreased grey matter volumes in the right anterior cingulate cortex (ACC). It has been suggested that the ACC is a functional intersection of emotion, cognition, drive, and motor control, making it highly relevant to apathy. These findings in the ACC can be contextualized to a frontolimbic network dysfunction, which is often noted in LLD. The affective/frontolimbic network is a set of interconnected neural structures with the main functions of emotional processing, modulating motivated behaviours, and regulating the emotion–mood relationship to visceral functions.10,11 The second study was conducted by Yuen et al.12 This study performed structural and diffusion tensor imaging of the ACC and associated white matter tracts on 45 non-demented elderly subjects with MDD and 43 elderly, psychiatrically healthy comparison individuals. There were no significant differences in white matter fractional anisotropy between controls, non-apathetic depressed subjects, and apathetic depressed subjects. Bilateral dorsal and rostral ACC volumes distinguished controls from apathetic depressed subjects and controls from non-apathetic depressed subjects. There were no other significant differences in ACC volumes from this three-group comparison. Although these studies are informative on structural brain changes related to apathy, they do not add to the understanding of the amyloid- and tau-related pathology relevant to LLD. We are not aware of any study exploring the relationship between of apathy in LLD and tau and amyloid biomarkers using positron emission tomography (PET) imaging.
This study aimed to explore the neural correlates of apathy with amyloid and tau PET imaging in a cohort with LLD. We hypothesized that greater severity of apathy will correlate with greater [18F]FDDNP binding in the ACC region based on our previous reports with other imaging modalities.9,13
Methods
From December 2013 to December 2014, we recruited 16 older adults (age 55 and older) to participate in an ongoing study of geriatric depression (NCT01902004). After the details of the study were described to interested and eligible subjects, written informed consent was obtained in accordance with the procedures set by the University of California, Los Angeles Institutional Review Board.
Participants
Inclusion criteria were as follows: (i) a current episode of unipolar MDD according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, criteria; (ii) a score ≥16 on the 24-item Hamilton Depression Rating Scale (HAM-D-24); and (iii) a Mini-Mental State Exam score ≥24.
Exclusion criteria were as follows: (i) a history of any other psychiatric disorders (other than unipolar MDD, with or without comorbid anxiety symptoms); (ii) a severe or acute unstable medical illness; (iii) acute suicidal or violent behaviour or a history of suicide attempt within the last year; or (iv) any other central nervous system diseases. Subjects were free of psychotropic medications for at least 2 weeks before participating in the study.
Mood and apathy measures
Mood was assessed by the HAM-D-24).14 Apathy was measured by the self-rated Apathy Evaluation Scale (AES) (score range: 18–72);15 lower AES scores correlate to greater apathy. The AES also measures behavioural subcomponents of apathy including cognitive (i.e. level of goal-directed cognition), emotional (i.e. level of emotional responsivity), behavioural (i.e. goal-directed motor behaviour), and other domains (i.e. combined insight and motivation). The AES is a psychometrically validated instrument in older normal individuals and psychiatric patients.15,16 The Mini-Mental State Exam was also used to assess cognitive impairment.17
PET neuroimaging methods
The radio-fluorinated imaging probe [18F]FDDNP was prepared at high specific activities (>37 GBq/μmol), as described elsewhere.18 All brain scans were performed at the University of California, Los Angeles, Ahmanson Biological Imaging Center with the EXACT HR+ tomograph (Siemens Medical Solutions, Munich, Germany; CTI Molecular Imaging, Knoxville, TN, USA), with individuals in the supine position and the imaging plane placed parallel to the orbito-meatal line. After the injection of a PET tracer (320–410 MBq) as a bolus via an indwelling venous catheter, the consecutive dynamic scans via PET were performed for as long as 2 h. All scans via PET were decay corrected and reconstructed using filtered back-projection (Hann filter, 5.5-mm full width at half maximum) with scatter correction and measured attenuation correction. The resulting images contained 63 contiguous sections with a plane-to-plane separation of 2.42 mm.
Image data were analyzed and regions of interest (ROI) determined, with investigators masked to clinical findings. Quantification of the data regarding [18F] FDDNP binding was performed with the Logan graphic method, with the cerebellum as the reference region for time points between 30 and 125 min.19,20 Similar results were obtained when analyses were performed in intervals of between 30 and 60 min. The slope of the linear portion of the Logan plot is the relative distribution volume (DVR), which is equal to the distribution volume of the tracer in an ROI divided by the distribution volume of the tracer in the reference region. Early frame [18F]FDDNP images via PET (sum of 0-5 min) were oriented in anterior commissure-posterior commissure orientation by rigid co-registration with the SPM2 software package (MathWorks, Natick, MA, USA) to the template for PET provided in the package. The parameters determined in this step were used to orient the [18F] FDDNP DVR images in the same, co-registered orientation.
A set of ROI was drawn bilaterally on the frontal, parietal (PA), posterior cingulate (PC), anterior cingulate, mesial temporal, and lateral temporal lobe areas and on the cerebellum on each co-registered early frame [18F]FDDNP image via PET separately using the ROI set outlined previously.4 The resulting ROI sets were imported in their corresponding [18F] FDDNP DVR images, and DVR values were extracted. Drawing of ROI and extraction of DVR values were performed using the AMIDE Medical Image Data Examiner (UCLA Crump Institute for Molecular Imaging, Los Angeles, California, USA) software package.19 Each regional DVR or binding value was expressed as the mean of the left and right regions, and global DVR values were calculated as means of the values for all these regions. Rules for ROI drawing were based on the identification of gyral and sulcal landmarks with respect to the atlas by Talairach and Tournoux.21
Brain MRI was obtained for co-registration for all study participants using a 3T scanner (Siemens Medical Solutions). For each individual, coronal sections that were 1.6 mm thick were obtained (repetition time, 20 ms; echo time, 6 ms; field of vision, 22 cm; 256 × 256 matrix; number of excitations, 1.5; and flip angle, 45°). Axial sections that were 3 mm thick were also obtained (repetition time, 4000 ms; echo time, 14/112 ms; field of vision, 24 cm; 256 × 256 matrix; and number of excitations, 1). All MRI results were examined for space-occupying and other focal lesions, including stroke. Patients described in this study were free of overt neuroanatomical abnormalities.
The [18F]FDDNP DVR parametric images of 16 patients with MDD with available T1-weighted MRI results were co-registered to the T1-weighted MRI results. This was done using the transformation parameters determined during the co-registration of [18F] FDDNP images, summed for the first 5 min after injection, to the T1 -weighted MRI results using statistical parametric mapping software. The T1 -weighted MRI results and co-registered images via PET were further transformed into the common space with statistical parametric mapping software. The ROI were drawn on the normalized T1-weighted MRI results bilaterally on the superior and middle frontal gyri on the frontal lobe; the middle temporal gyrus in the lateral temporal lobe; the hippocampus proper, the entorhinal cortex, and the parahippocampal gyrus in the medial temporal lobe; the inferior lobule in the PA lobe; the anterior cingulate gyrus; and the PC gyrus.
The ROI sets were used to extract the DVR values from co-registered [18F]FDDNP parametric images. The DVR values for each brain region are given as the means of the left and right hemisphere DVR values. We imported PET-drawn ROI into co-registered MRI results and found good matching of ROI with grey matter areas on MRI results.
Data analysis
Data were checked for outliers, and descriptive statistics were obtained. The relationship of [18F]FDDNP DVR binding levels with depression levels (HAM-D total) and apathy (AES total) were studied using Spearman's correlation analyses because of the relatively small sample size. Age, sex, and years of education were partialed out. As this was an exploratory study to examine how regional [18F]FDDNP binding is related to the outcome measures in depressed individuals, we did not correct for multiple comparisons and significance levels were set at 0.05.
Results
Study participants ranged in age from 63 to 83 years (mean ± SD: 72.8 ± 6.8 years). The sample was well educated (mean ± SD: 15.8 ± 2.3 years of education), and depression scores were indicative of moderate depression (mean HAM-D ± SD: 17.5 ±2.2; range: 16-23) (Table 1).
Table 1. Clinical and demographic characteristics at baseline.
| Variables | Mean ± SD |
|---|---|
| Age (years) | 72.8 ± 6.8 |
| Education (years) | 15.8 ± 2.3 |
| HAM-D-17 | 17.5 2.2 |
| MMSE | 28.0 ± 2.0 |
| AES | 32.5 ± 11.0 |
| n (%) | |
| Men | 8 (50%) |
| Women | 8 (50%) |
AES, apathy evaluation scale; GDS, geriatric depression scale; HAM-D, hamilton depression rating scale; HAMA, hamilton anxiety rating scale; MMSE, mini-mental state exam.
[18F]FDDNP binding in the ACC was negatively associated with the AES total (r = -0.62, P = 0.02; where low AES score equals greater severity of apathy), suggesting apathy in LLD is associated with higher amyloid and/or tau levels in the ACC (Figure 1). None of the regional [18F]FDDNP binding levels was significantly associated with HAM-D total.
Figure 1.
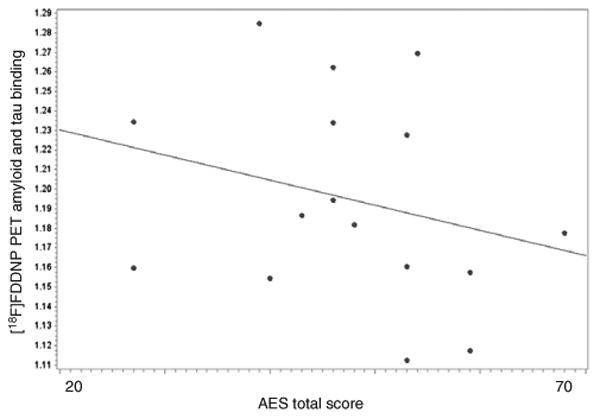
Associations between amyloid and tau binding in the anterior cingulate cortex (ACC) and apathy. This figure shows associations between amyloid and tau binding in the ACC from the [18F]FDDNP PET ligand and apathy scores as assessed by Apathy Evaluation Score (AES). [18F]FDDNP binding in the ACC was negatively associated with the AES total (r = -0.62, P = 0.02). [18F]FDDNP, 2-(1-{6-[(2-[18F]fluor-oethyl)(methyl)-amino]-2-naphthyl}ethylidene) malononitrile; PET, positron emission tomography.
Discussion
Our pilot study in an LLD population is the first to report the associations between the severity of apathy and [18F]FDDNP binding in the ACC. This research is important given apathy in LLD is associated with poorer functioning and reduced anti-depressant response.
ACC involvement in LLD
The ACC is a key part of the frontolimbic networks involved in LLD. The ACC is divided into dorsal and perigenual ACC regions. The rostral and subgenual regions control cognitive and emotional processes, respectively.22–25 More specifically, the perigenual ACC assesses the salience of emotional input and regulates emotional responses.23,26 The dorsal ACC controls aspects of executive function, including conflict detection, cognitive inhibition, and conflict resolution.27,28 The ACC is suggested to be a functional intersection of emotion, cognition, drive, and motor control and hence highly relevant to apathy. The dysfunction in the frontolimbic region may be due to the disruption outlined in the disconnection hypothesis and/or the dysfunction suggested by the hypoperfusion hypothesis. The disconnection hypothesis suggests ischaemia and white matter pathology may disrupt neural connections among regions modulating mood and cognition.29 In this model, widespread cerebral white matter hyperintensity severity is less relevant to LLD than is focal damage to tracts and circuits. The hypoperfusion hypothesis is suggested given that vascular dysfunction is common in LLD and cerebral blood flow reductions can alter brain function, contributing to depression-related symptomatology.30–35
Neuroimaging studies exploring the ACC in LLD
Several MRI-based neuroimaging studies have explored the effect of apathy in LLD. The first study found the severity of apathy was associated with decreased grey matter volumes in the right ACC.9 This is consistent with our [18F]FDDNP results with increased amyloid and tau binding in the ACC. The second study found no significant differences in white matter fractional anisotropy between controls, non-apathetic depressed subjects, and apathetic depressed subjects.12 Bilateral dorsal and rostral ACC volumes distinguished controls from apathetic depressed subjects and controls from non-apathetic depressed subjects. There were no other significant differences in ACC volumes from this three-group comparison. The lack of ACC findings from this study may be due to small sample size.
Amyloid and tau imaging studies in LLD
While we are not aware of any amyloid or tau PET studies exploring binding in relation to apathy in subjects with LLD, two studies have explored associations between amyloid and tau PET binding and depressive symptoms. One was by Kumar et al.,4 who compared [18F]FDDNP binding in 20 LLD patients and 19 healthy controls. Binding was significantly higher overall and in the posterior cingulate cortex and lateral temporal regions in the MDD group. The second study by Lavretsky et al. explored [18F]FDDNP binding in 23 MCI patients and 20 cognitively normal;36 depressed subjects were excluded, but depression scores were measured. The MCI and comparison subjects did not differ according to the depression scores. However, in the MCI group, depression scores correlated with lateral temporal binding, and in the comparison group, depression scores correlated with medial temporal binding.
A recent systematic review by Harrington et al. aimed to examine the relationship between Aβ, a key biomarker of AD, and depression in older adults.37 Studies were also required to include an outcome variable that was a direct measure of Aβ levels in either blood or cerebrospinal fluid samples, or via neuroimaging techniques such as PET. Nineteen studies were identified, 15 of which found significant differences in Aβ levels between depressed and non-depressed older adults. Five studies used PET neuro-imaging as a primary outcome measure, with three observing statistically significant relationships between neuroimaging results and depression status. Of the statistically significant studies, two used [18F] FDDNP binding and one used [18F]florbetapir. The non-significant studies used the Pittsburgh Compound B (PiB) binding compound. Therefore, variations in results may be due to differences in these compounds.
Comparing neuroimaging and clinical data on apathy and LLD as risk factors for progression of cognitive decline
Data suggest that 1 in 10 cases of dementia worldwide can be attributed to depression.38 There is some variation in the literature exploring the role of depression as a risk factor for dementia.37 This may be partly due to the heterogeneity of depression phenotypes studied, including vascular, melancholic, and atypical depression. One way to enhance the research is by investigating commonly shared clinical and neural features between depression and dementia.
Apathy comorbid with LLD may increase rates of cognitive decline, but data are conflicting from clinical studies. A prospective cohort study of 397 subjects explored the effect of apathy on progression from MCI to AD.39 The presence of symptoms of apathy without symptoms of depressive affect increased the risk of progression, whereas apathy in the context of depressive affect did not increase the risk of progression. This study used the 15-item Geriatric Depression Scale to measure depressive symptoms, which is a significant limitation. A small, 2-year prospective cohort study of 124 MCI patients found rates of conversion to dementia were highest in apathetic individuals (60%).40 However, depression and apathy appeared to reduce the rates of conversion from MCI to dementia with rates of conversion higher for MCI normal (24%) than for MCI depressed and apathetic (19%) and MCI depressed (7.9%). These findings may be due to a small sample size.
To help discern the role of apathy in LLD in affecting rates of cognitive decline, the [18F]FDDNP amyloid and tau marker has been explored in AD patients with apathy. Apathy in AD was found to correlate with neurofibrillary tangle density in the ACC and reduced grey matter volume in the ACC.41,42 In vivo studies with PET markers of amyloid or tau did not implicate ACC in the progression of cognitive impairment. Findings from the [18F]FDDNP study differentiating individuals with MCI, AD, and normal cognition did not analyze ACC binding values;3 this study found that global values of [18F]FDDNP binding (average of the values for the temporal, parietal, posterior cingulate, and frontal regions) were lower in the control group than in the MCI group (P < 0.001) and that values in the MCI group were lower than in the AD group (P < 0.001). Interestingly, other functional neuroimaging modalities have found hypometabolism in the ACC to be associated with apathy in AD,43–45 but other data are conflicting.46
Our data showing apathy in LLD is correlated with increased amyloid and tau binding in the ACC may suggest apathy and LLD together increase the rate of cognitive decline. This, however, must be carefully explored in a larger population with more robust neuropsychological analyses.
The findings of our pilot study must be considered in the context of a number of limitations. Our sample size was small and included only 16 individuals with LLD. There was no control group in this study, which means only within-group, not between-group, analyses were possible. It is therefore difficult to distinguish between whether apathy is a symptom of depression or a preclinical symptom of late-life depression. This study did not include comprehensive neuropsychological assessments, which is a significant limitation and an area for improvement in future studies. Additionally, computed tomography and MRI findings were not available in this cohort, meaning the presence of white matter hyperintensities, lacunar infarcts, and vascular disease markers could not be determined. Finally, the ligand used in this study binds to both amyloid and tau proteins, which means further studies are required to understand if the associations between apathy in LLD and binding are related to amyloid, tau, or both proteins. We and others are not aware of any tau PET studies in LLD.
This pilot study suggests that increased apathy in subjects with LLD may be associated with greater amyloid and/or tau burden in certain brain regions. Future prospective studies in larger samples should elucidate the generalizability of these results and correlate with cognitive assessment, which eventually could lead to improved diagnostic and treatment methods in LLD.
Footnotes
Disclosure: JRB and GWS are co-inventors of the [18F]FDDNP PET technology, which is covered under University of California, Los Angeles patents and licensed to TauMark, LLC.
There are no other conflicts of interest.
Author Contributions: HL, HAE, PS, KVD, NSC, JRB, and GWS designed the research. NSC, KVD, and HL performed the research. JRB, and GWS contributed the analytic tools. HAE, PS, KVD, NSC, BTB, HL, JRB, and GWS analyzed data. HAE, PS, KVD, BTB, HL, JRB, and GWS wrote the paper.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 3.Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Kepe V, Barrio JR, et al. Protein binding in patients with late-life depression. Arch Gen Psychiatry. 2011;68:1143–1450. doi: 10.1001/archgenpsychiatry.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuen GS, Bhutani S, Lucas BJ, et al. Apathy in late-life depression: common, persistent, and disabling. Am J Geriatr Psychiatry. 2015;23:488–494. doi: 10.1016/j.jagp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 7.Chase TN. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotox Res. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- 8.Holtta EH, Laakkonen ML, Laurila JV, Strandberg TE, Tilvis RS, Pitkala KH. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. J Am Med Dir Assoc. 2012;13:541–545. doi: 10.1016/j.jamda.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr Psychiatry. 2007;15:386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Yuen GS, Gunning FM, Woods E, Klimstra SA, Hoptman MJ, Alexopoulos GS. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. J Affect Disord. 2014;166:179–186. doi: 10.1016/j.jad.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavretsky H, Zheng L, Weiner MW, et al. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23:1040–1050. doi: 10.1002/gps.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 16.Clarke DE, Reekum Rv, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci. 2007;19(1):57–64. doi: 10.1176/jnp.2007.19.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Kepe V, Zabjek A, et al. High-yield, automated radiosynthesis of 2-(1-{6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl} ethylidene)malononitrile ([18F]FDDNP) ready for animal or human administration. Mol Imaging Biol. 2007;9:6–16. doi: 10.1007/s11307-006-0061-4. [DOI] [PubMed] [Google Scholar]
- 19.Kepe V, Barrio JR, Huang SC, et al. Serotonin 1A receptors in the living brain of Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2006;103:702–107. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Talairach J, Tournouz P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System: An Approach to Cerebral Imaging, ed. New York: Thieme Publishers; 1988. [Google Scholar]
- 22.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 23.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 24.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 26.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 28.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- 30.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133–139. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Greenstein AS, Paranthaman R, Burns A, et al. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56:734–740. doi: 10.1161/HYPERTENSIONAHA.110.152801. [DOI] [PubMed] [Google Scholar]
- 33.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–523. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–198. A7. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 35.Chen CS, Chen CC, Kuo YT, Chiang IC, Ko CH, Lin HF. Carotid intima-media thickness in late-onset major depressive disorder. Int J Geriatr Psychiatry. 2006;21:36–42. doi: 10.1002/gps.1420. [DOI] [PubMed] [Google Scholar]
- 36.Lavretsky H, Siddarth P, Kepe V, et al. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am J Geriatr Psychiatry. 2009;17:493–502. doi: 10.1097/jgp.0b013e3181953b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington KD, Lim YY, Gould E, Maruff P. Amyloid-beta and depression in healthy older adults: a systematic review. Aust N Z J Psychiatry. 2015;49:36–46. doi: 10.1177/0004867414557161. [DOI] [PubMed] [Google Scholar]
- 38.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 39.Richard E, Schmand B, Eikelenboom P, et al. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer's disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33:204–209. doi: 10.1159/000338239. [DOI] [PubMed] [Google Scholar]
- 40.Vicini Chilovi B, Conti M, Zanetti M, Mazzu I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2009;27:390–398. doi: 10.1159/000210045. [DOI] [PubMed] [Google Scholar]
- 41.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:144–147. doi: 10.1159/000090674. [DOI] [PubMed] [Google Scholar]
- 42.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131(Pt 9):2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- 43.Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64:1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- 44.Benoit M, Clairet S, Koulibaly PM, Darcourt J, Robert PH. Brain perfusion correlates of the apathy inventory dimensions of Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:864–869. doi: 10.1002/gps.1163. [DOI] [PubMed] [Google Scholar]
- 45.Benoit M, Koulibaly PM, Migneco O, Darcourt J, Pringuey DJ, Robert PH. Brain perfusion in Alzheimer's disease with and without apathy: a SPECT study with statistical parametric mapping analysis. Psychiatry Res. 2002;114:103–111. doi: 10.1016/s0925-4927(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 46.Delrieu J, Desmidt T, Camus V, et al. Apathy as a feature of prodromal Alzheimer's disease: an FDG-PET ADNI study. Int J Geriatr Psychiatry. 2015;30:470–477. doi: 10.1002/gps.4161. [DOI] [PubMed] [Google Scholar]


