Abstract
Introduction
Chronic alcohol abuse is associated with neurophysiological changes in brain activity; however, these changes are not well localized in humans. Non-human primate models of alcohol abuse enable control over many potential confounding variables associated with human studies. The present study utilized high-resolution magnetoencephalography (MEG) to quantify the effects of chronic EtOH self-administration on resting state (RS) brain function in vervet monkeys.
Methods
Adolescent male vervet monkeys were trained to self-administer ethanol (n=7) or an isocaloric malto-dextrin solution (n=3). Following training, animals received 12 months of free access to ethanol. Animals then underwent RS magnetoencephalography (MEG) and subsequent power spectral analysis of brain activity at 32 bilateral regions of interest associated with the chronic effects of alcohol use.
Results
Results demonstrate localized changes in brain activity in chronic heavy drinkers, including reduced power in the anterior cingulate cortex, hippocampus, and amygdala as well as increased power in the right medial orbital and parietal areas.
Discussion
The current study is the first demonstration of whole-head MEG acquisition in vervet monkeys. Changes in brain activity were consistent with human electroencephalographic studies; however, MEG was able to extend these findings by localizing the observed changes in power to specific brain regions. These regions are consistent with those previously found to exhibit volume loss following chronic heavy alcohol use. The ability to use MEG to evaluate changes in brain activity following chronic ethanol exposure provides a potentially powerful tool to better understand both the acute and chronic effects of alcohol on brain function.
Keywords: Alcohol, magnetoencephalography, vervet, primate
1. Introduction
Alcohol abuse is associated with widespread cortical atrophy and loss of white matter integrity (Buhler and Mann, 2011; Monnig et al., 2013), particularly in frontal cortical areas (Pfefferbaum et al., 1997). These changes may contribute to the characteristic patterns of brain activity associated with alcohol abuse seen both in the resting state (RS) and during task performance (Rangaswamy and Porjesz, 2008). However, it is not clear when during the disease process these patterns are manifested and which specific neurocircuits are affected.
Well-characterized non-human primate (NHP) drinking models are a powerful translational science tool that enable control of many variables, including environmental conditions, nutrition, intake patterns, etc. that are a source of uncontrolled variance in clinical populations. Studies of one such model (Grant et al., 2008; Vivian et al., 2001) have demonstrated functional and genomic consequences throughout the brain following 15 months of chronic daily drinking (Acosta et al., 2010; Ariwodola et al., 2003; Budygin et al., 2003; Carden et al., 2006; Cuzon Carlson et al., 2011; Floyd et al., 2004; Grant et al., 2008; Hemby et al., 2006; Mohr et al., 2013; Welsh et al., 2011). Recently, graph theoretical analyses of fMRI data from this model found that chronic heavy drinking altered RS brain network organization (Telesford et al., 2015). These findings are consistent with those from human studies (Zahr, 2013) and indicate that this NHP model of chronic alcohol consumption is a robust representation of the human condition of alcoholism.
Noninvasive electrophysiological measures of brain function, such as electroencephalography (EEG), are sensitive to differences in brain activity due to chronic alcohol consumption (Kamarajan and Porjesz, 2015; Pandey et al., 2012). EEG studies of humans with alcohol use disorders have most consistently reported increased power in the beta and theta bandwidths (Kamarajan and Porjesz, 2015), while findings in other bandwidths have been mixed. Magnetoencephalography (MEG) is another noninvasive method of directly measuring brain function with high temporal resolution and increased spatial resolution relative to EEG. However, MEG has not been previously used to record RS activity in the vervet brain. The present study was designed to assess the feasibility of measuring RS brain activity in the vervet brain using a MEG instrument designed for humans. We obtained RS MEG from vervet monkeys following 15 months of daily EtOH consumption and control animals. We hypothesized that power would be increased in the beta and theta bandwidths in EtOH animals, consistent with human EEG findings. We anticipated being able to extend current literature by localizing observed differences to particular brain regions due to the superior spatial resolution provided by MEG.
2. Material and methods
All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest School of Medicine (WFSM) and conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) in research.
2.1 Animals
Adult male vervet monkeys (Chlorocebus aethiops, n=10, 7 years old; 28 year human equivalent) were acquired from the Primate Center at Wake Forest School of Medicine and currently participating in a separate EtOH self-administration study. The monkeys were trained to self-administer EtOH (EtOH, n=7) or an isocaloric maltose-dextrin solution (Control, n=3) (Grant et al., 2008).
2.2 Preparation for MEG scans
Animals were fasted overnight from EtOH and food prior to scans. Duration between the onset of fasting and beginning of scan was 927.3 minutes (SD=134.7) for the EtOH group and 879.7 minutes (SD=30.5) for the Control group. Across the ten days prior to imaging, the EtOH group consumed an average of 87.5% (SD=7.3) of their total daily intake by the fasting time. Only one animal consumed less than 85% by this point, consuming on average 74% (SD=11.69) by the fasting time. Animals were sedated with ketamine (10 mg/kg, i.m.) for transport. Anesthesia was induced with 3.0–4.0 mg/kg propofol and then maintained by continuous infusion of 200 μg/kg/min via syringe pump (Sage, Orion Research Corporation, Cambridge, Mass). The animals were placed in a supine position and artificially ventilated. Vital signs were monitored throughout the scan and recovery sessions.
MEG was acquired under propofol anesthesia due to concerns regarding head motion artifact during scanning if the animals were conscious. A known linear relationship exists between resting-state activity and propofol anesthesia (Boveroux et al., 2010). Notably, electrophysiological studies suggest propofol sedation is associated with increased power in the beta frequencies (Mahon et al., 2008). Changes in resting state networks observed with fMRI and associated with EtOH consumption have been previously demonstrated in these same animals using an identical preparation (Telesford et al., 2015).
2.3 MEG acquisition
Data were acquired using a whole head CTF Systems Inc. MEG 2005 neuromagnetometer system equipped with 275 first-order axial gradiometers coils. Data were sampled at 600 Hz over a DC-150 Hz bandwidth for 8 minutes. Following data acquisition, fiducial coils were replaced with a lipid marker to facilitate MEG co-registration with T1-weighted MRI scans.
2.4 MEG data processing
Data preprocessing, head model creation, and beamforming were performed using CTF MEG™ Software (MISL, Coquitlam, BC, Canada). MEG data were pre-processed using synthetic 3rd order gradient balancing with whole trial DC offset. Data were band pass filtered from 1–80 Hz with a 60 Hz notch filter (4 Hz width). Three spherical shell, multiple local-sphere head models were created using the fiducial information and T1-weighted MRI (Huang et al., 1999).
2.5 ROI Identification
Using the animal-specific MRI, 32 non-adjacent regions of interest (ROIs, 2 mm3; see Table 1) were identified in native brain space for each animal. Figures visually displaying the location of ROIs are available as supplementary materials1 (S1–S12). ROIs were chosen based on existing literature (Buhler and Mann, 2011) and evidence from our laboratories using an identical drinking model that chronic alcohol use affects these areas (cited above).
Table 1.
Percent change in power associated with EtOH group membership relative to the Control group. This was calculated as the parameter estimate for EtOH group membership divided by the intercept. Positive values indicate higher levels of power in drinkers, negative values indicate lower levels of power in drinkers.
| Delta | Theta | Alpha | Beta | Gamma | |
|---|---|---|---|---|---|
| Left Anterior Cingulate | −6.6 | −22.8 | −36.8* | −25.8 | −31.9* |
| Right Anterior Cingulate | −12.6 | −22.4 | −39.3* | −30.3* | −39.9* |
| Bilateral Anterior Cingulate | −10.2 | −23.5 | −39.0* | −28.8* | −36.5* |
| Left Medial Orbital Sulcus | 36.5 | 11.1 | 2.4 | 6.7 | 22.7 |
| Right Medial Orbital Sulcus | 253.1* | 200.2* | 139.2* | 152.4* | 137.0* |
| Left Lateral Orbital Sulcus | 50.8 | 16.9 | −17.5 | −18.5 | −18.9 |
| Right Lateral Orbital Sulcus | 13.9 | 16.3 | −12.0 | −2.3 | −10.1 |
| Left Principle Sulcus | 60.4 | 22.9 | 4.8 | −0.1 | −6.6 |
| Right Principle Sulcus | −16.4 | −23.2 | −35.9* | −26.0 | −29.5 |
| Left Nucleus Accumbens | 35.6 | 12.9 | −14.4 | −7.2 | −3.4 |
| Right Nucleus Accumbens | 42.4 | 39.3 | 27.2 | 37.2 | 25.1 |
| Left Caudate | 12.9 | −2.0 | −14.8 | −9.3 | −10.2 |
| Right Caudate | −9.6 | −16.5 | −8.6 | −1.1 | −10.6 |
| Left Putamen | 10.1 | 2.8 | −11.2 | −9.8 | −19.6 |
| Right Putamen | 0.6 | 3.9 | 5.4 | −0.5 | −14.1 |
| Left Parietal | 15.4 | 8.4 | 4.1 | −0.6 | 1.5 |
| Right Parietal | 84.8* | 108.0* | 124.2* | 103.0* | 86.6* |
| Left Precuneus | −1.2 | 2.8 | 12.8 | −3.9 | 1.38 |
| Right Precuneus | 32.1 | 38.5 | 48.0 | 34.6 | 54.9* |
| Left Amygdala | 35.3* | 17.4 | 0.8 | −0.3 | −7.6 |
| Right Amygdala | −6.3 | −10.4 | −21.4* | −23.3* | −25.9* |
| Left Middle Hippocampus | 10.7 | 2.0 | −3.2 | −6.9 | −13.2 |
| Right Middle Hippocampus | −14.7 | −16.6 | −21.8* | −29.8* | −31.9* |
| Left Anterior Hippocampus | 32.5 | 18.6 | 0.9 | −3.2 | −12.2 |
| Right Anterior Hippocampus | −3.3 | −9.2 | −23.7* | −29.6* | −32.9* |
| Left Posterior Hippocampus | 19.1 | 8.5 | 6.6 | −1.8 | −4.6 |
| Right Posterior Hippocampus | −6.2 | −6.2 | −4.8 | −11.4 | −10.3 |
| Vermis of Cerebellum | 0.5 | 0.6 | −3.5 | −7.5 | −2.0 |
| Left Anterior Lobe Cerebellum | 9.0 | 8.8 | 3.0 | −3.3 | 2.2 |
| Right Anterior Lobe Cerebellum | 8.9 | 16.2 | 27.3 | 21.3 | 47.8 |
| Left Posterior Lobe Cerebellum | −25.7 | −31.6 | −28.8 | −34.0* | −27.5 |
| Right Posterior Lobe Cerebellum | −32.5 | −30.1 | −25.7 | −27.9 | −22.5 |
n=10, EtOH group n=7, Control group n=3;
p<.05 corrected.
2.6 Source Series
Synthetic Aperture Magnetometry (SAM; Robinson and Vrba, 1998) as implemented in CTF MEG™ Software was used to return source series for each ROI. Source series represent the unique weighted sum of MEG sensor output for a specific location in the brain, and are strongly correlated with the local field potential at that location. Source series retain the same time-frequency characteristics as the original MEG sensor data; here they were constructed for the full time domain of the original scans. SAM source series have been effective in analyzing both RS and event related brain activity (Beal et al., 2010; Cheyne et al., 2007; Cornwell et al., 2012; Cornwell et al., 2008; Douw et al., 2013; Hillebrand et al., 2012; Hung et al., 2010; Hung et al., 2012; Luo et al., 2007; Moses et al., 2009; Quraan et al., 2011; Riggs et al., 2009; Stapleton-Kotloski et al., 2014).
2.7 Signal Power
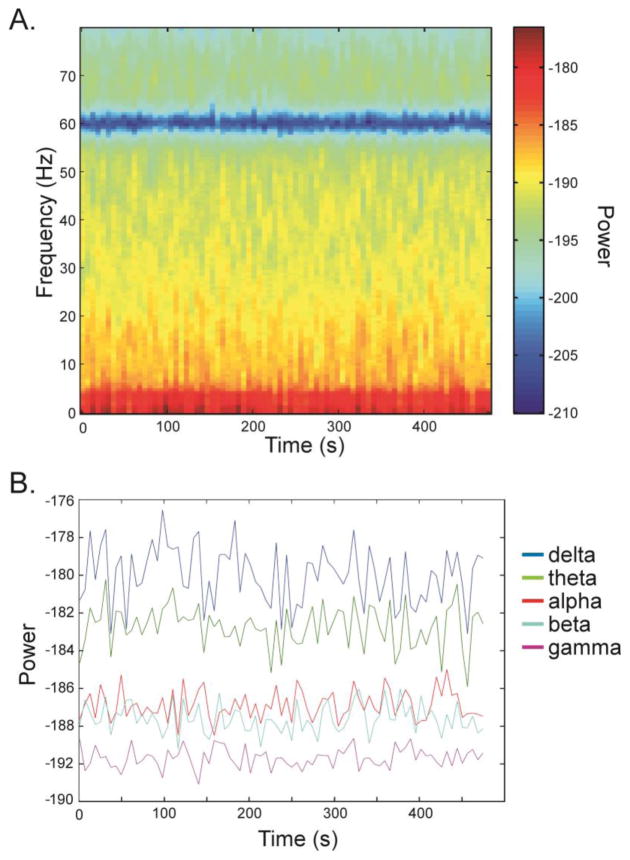
Signal power for each source series was calculated using multi-taper estimation implemented in Chronux (Mitra and Bokil, 2008); http://chronux.org). The mtspectrogramc function was used with a 1 second window and 500 ms overlap. A modified Welch’s method was implemented by averaging within each 6-second epoch of the resulting spectrum (See Figure 1 A). For comparison, the resulting spectrum from applying an identical analysis to sensor level data from a control animal can be seen in supplementary materials2 (S13–S18). Next, the maximum power within each of the classic bandwidths was identified for each source series (delta: 1–4 Hz, theta: 4–8 Hz, alpha: 8–13 Hz, beta: 13–30 Hz, gamma: 30–80 Hz) at each time point, creating a vector of values across time (See Figure 1 B). One subject was scanned for only six minutes; therefore, all subjects’ data was truncated to six minutes. Results were replicated calculating signal power using 750 ms and 900 ms overlap.
Figure 1.
This figure illustrates the creation of the outcome variables. Data are presented as 10*log10 for ease of viewing. First, the spectrogram is calculated using a 1 second window with 50% overlap. Then every twelve data points are averaged in a modified Welch’s method, as shown in panel A. From the result, the maximum value in each of the delta, theta, alpha, beta, and gamma bandwidths is extracted at each time point, creating a vector of maximum values as shown in panel B. These vectors are extracted from each ROI and used in the analyses.
2.8 Analyses
Analyses were conducted using SPSS version 21. Power in each bandwidth at each ROI was analyzed using the Generalized Linear Models approach. A linear model was selected with power in the appropriate bandwidth as the outcome. Group, Time, and the Group*Time interaction were entered into the model. Parameter estimates were calculated using maximum likelihood estimation. The False Discovery Rate (FDR; Benjamini and Hochberg, 1995) was used to control for Type-I error across analyses (160 comparisons) using α = 0.05 (step up method).
3. Results
3.1 Ethanol Self-administration
Average daily ethanol intake ranged from 0.73–2.57 g/kg (3–10 drink/day equivalent) across the 12-months of free access prior to the scans. Average daily intake across the last 3 months ranged from 1.06 to 3.11 g/kg (equivalent to 4–12 drinks/day). Lifetime intake ranged from 307.1–1109.3 g/kg.
3.2 Signal power
Results of the analysis can be seen in Table 1. Values indicate percent change in power due to EtOH group membership. Positive values indicate higher power and negative values indicate lower power in EtOH animals relative to Control animals. EtOH animals displayed significantly lower levels of power in the alpha, beta, and gamma bandwidths in the anterior cingulate cortices, right hippocampus, and right amygdala. Power was also lower in EtOH animals in the right principle sulcus in the alpha bandwidth and left posterior lobe of the cerebellum in the beta bandwidth. EtOH animals displayed significantly higher power across all bandwidths in the right medial orbital and parietal areas. Power was also higher in EtOH animals in the right precuneus in the gamma bandwidth and left amygdala in the delta bandwidth. There were no significant effects of time, or time*group interactions, indicating that patterns of brain activity did not meaningfully change over the duration of the scan for either group. Results were essentially identical when signal power was calculated using 750 ms and 900 ms overlaps.
4. Discussion
The current study provides the first demonstration of whole-head MEG acquisition in vervet monkeys. Results revealed differences in RS activity in specific brain regions between chronic EtOH consuming monkeys and age-matched controls after 15 months of daily drinking (See Table 1) that are consistent with previous findings in humans. These findings extend our previous report of altered hub connectivity using fMRI (Telesford et al., 2015) by examining changes in the frequency domain in the same animals using an imaging modality less sensitive to potential EtOH-related disruptions of the microvasculature (Altura and Altura, 1984). The current study supports the feasibility of using a human MEG instrument to record from cortical and subcortical brain regions in vervet monkeys.
Additionally, these findings help clarify previous reports examining changes in power associated with alcohol use disorders (Kamarajan and Porjesz, 2015). The relatively poor spatial resolution of EEG may have been a key factor producing inconsistent findings as recent studies have demonstrated unique spectral profiles across brain regions (Keitel and Gross, 2016). The current findings demonstrate that changes in brain activity due to EtOH exposure are dependent on the brain region being examined. Analyses utilizing sensor level EEG may be examining brain activity combined across regions of both increased and decreased activity. Using MEG to spatially localize brain activity, the current results suggest the right parietal and ventral frontal regions as sources of differences in the theta and beta bandwidths with the anterior cingulate and right limbic areas (amygdala and hippocampus) as generators underlying differences in the alpha bandwidth. Future studies may be able to utilize this NHP model of alcoholism and MEG during task performance to better understand the changes in brain function associated with chronic alcohol consumption.
Several limitations of this study should be considered. Although it was possible to detect and localize significant differences in brain activity resulting from chronic EtOH consumption, it is not possible to determine when during the course of EtOH exposure the changes occur due to the cross-sectional nature of the data. Additionally, data were acquired under propofol anesthesia and a known linear relationship exists between resting-state activity and propofol anesthesia (Boveroux et al., 2010). Previous research suggests that propofol has a greater effect on activity in higher frequency ranges, suggesting changes in brain activity in these ranges may not be solely due to EtOH consumption. The EtOH fast minimally interfered with drinking behaviors for all but one animal, suggesting disruption of drinking cannot fully explain the findings. However, propofol is a GABAA agonist that can be used to treat alcohol withdrawal syndrome (Brotherton et al., 2016), it is possible the two groups responded differently to anesthesia due to differences in EtOH exposure, or propofol could have mitigated potential acute withdrawal symptoms in EtOH animals. Overnight fasting from EtOH suggests that the changes in activity are a result of chronic exposure to EtOH, rather than an interaction between acute EtOH and propofol. The ability to acquire data in awake NHPs, particularly during task performance, will better utilize the translational value of these models. Finally, future studies should determine the optimal analytic approach for NHP data. It is possible that alternative approaches, particularly for head modeling, may be required for optimal results. The ability to use MEG to evaluate changes in brain activity in the NHP following chronic ethanol exposure provides a potentially powerful tool to better understand both the acute and chronic effects of ethanol on brain function.
Supplementary Material
Highlights.
First application of Magnetoencephalography (MEG) to vervet monkeys
Utilized a well validated model of chronic heavy alcohol use
Examined changes in brain activity of naïve subjects to chronic alcohol use
Localized previously observed changes to specific brain regions
Increases and decreases in brain activity were observed dependent on the region
Acknowledgments
Role of Funding Source
This study was supported by AA019431 and AA016748 (JBD), AA016852 (DWG), AA014106 (DPF), 2T32NS073553-06 and 5F30AA023708-02 (GEA), Ignition Funds from the Wake Forest School of Medicine Translational Sciences Institute (JBD) and a TSI pilot award (DWG). These funding sources had no role in study design, data acquisition, data analysis, or manuscript preparation.
We wish to thank the Department of Neurology for providing scanner time on the MEG and the Center for Biomolecular Imaging for MRI pilot scans. We acknowledge the Wake Forest University Primate Center for providing animals for this study OD010965 (Jay Kaplan), as well as support from the W.G. (Bill) Hefner Veterans Affairs Medical Center and VA Mid-Atlantic Mental Illness, Research, Education, and Clinical Center. Conflicts of interest: none.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
JAR, JRSK, GEA, AD, and JBD participated in protocol design and data collection. JAR and JRSK conducted data analyses. All authors participated in hypothesis development, interpretation of results, and manuscript preparation. All authors have approved the final article.
Conflict of Interest
No conflict declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE. Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res. 2010;1318:144–154. doi: 10.1016/j.brainres.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:352–331. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp Res. 2003;27:1632–1639. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Beal DS, Cheyne DO, Gracco VL, Quraan MA, Taylor MJ, De Nil LF. Auditory evoked fields to vocalization during passive listening and active generation in adults who stutter. Neuroimage. 2010;52:1645–1653. doi: 10.1016/j.neuroimage.2010.04.277. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- Brotherton AL, Hamilton EP, Kloss HG, Hammond DA. Propofol for treatment of refractory alcohol withdrawal syndrome: A review of the literature. Pharmacotherapy. 2016;36:433–442. doi: 10.1002/phar.1726. [DOI] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Carden WB, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, Godwin DW. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res. 2006;1089:92–100. doi: 10.1016/j.brainres.2006.02.135. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bostan AC, Gaetz W, Pang EW. Event-related beamforming: A robust method for presurgical functional mapping using MEG. Clin Neurophysiol. 2007;118:1691–1704. doi: 10.1016/j.clinph.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22:1848–1859. doi: 10.1002/hipo.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Carver FW, Coppola R, Johnson L, Alvarez R, Grillon C. Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Res. 2008;1244:103–112. doi: 10.1016/j.brainres.2008.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L, de Groot M, van Dellen E, Aronica E, Heimans JJ, Klein M, Stam CJ, Reijneveld JC, Hillebrand A. Local MEG networks: The missing link between protein expression and epilepsy in glioma patients? Neuroimage. 2013;75:195–203. doi: 10.1016/j.neuroimage.2013.02.067. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, O’Connor JA, Acosta G, Floyd D, Anderson N, McCool BA, Friedman D, Grant KA. Ethanol-induced regulation of GABA-A subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol Clin Exp Res. 2006;30:1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR, Bosboom JL, Berendse HW, Stam CJ. Frequency-dependent functional connectivity within resting-state networks: An atlas-based MEG beamformer solution. Neuroimage. 2012;590:3909–3921. doi: 10.1016/j.neuroimage.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, Mosher JC, Leahy RM. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys Med Biol. 1999;44:423–440. doi: 10.1088/0031-9155/44/2/010. [DOI] [PubMed] [Google Scholar]
- Hung Y, Smith ML, Bayle DJ, Mills T, Cheyne D, Taylor MJ. Unattended emotional faces elicit early lateralized amygdala-frontal and fusiform activations. Neuroimage. 2010;50:727–733. doi: 10.1016/j.neuroimage.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Hung Y, Smith ML, Taylor MJ. Development of ACC-amygdala activations in processing unattended fear. Neuroimage. 2012;60:545–552. doi: 10.1016/j.neuroimage.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B. Advances in electrophysiological research. Alcohol Res Curr Rev. 2015;37:53–87. [PMC free article] [PubMed] [Google Scholar]
- Keitel A, Gross J. Individual human brain areas can be identified from their characteristic spectral activation fingerprints. PLoS Biol. 2016;14:e1002498. doi: 10.1371/journal.pbio.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon P, Greene BR, Greene C, Boylan GB, Shorten GG. Behavior of spectral entropy, spectral edge frequency 90%, and alpha and beta power parameters during low-dose propofol infusion. Br J Anaesth. 2008;101:213–221. doi: 10.1093/bja/aen161. [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. Oxford University Press; New York: 2008. [Google Scholar]
- Mohr C, Kolotushkina O, Kaplan JS, Welsh J, Daunais JB, Grant KA, Rossi DJ. Primate cerebellar granule cells exhibit a tonic GABAAR conductance that is not affected by alcohol: A possible cellular substrate of the low level of response phenotype. Front Neur Circ. 2013;7:189. doi: 10.3389/fncir.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS. White matter volume in alcohol use disorders: A meta-analysis. Addict Biol. 2013;18:581–592. doi: 10.1111/j.1369-1600.2012.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Ryan JD, Bardouille T, Kovacevic N, Hanlon FM, McIntosh AR. Semantic information alters neural activation during transverse patterning performance. Neuroimage. 2009;46:863–873. doi: 10.1016/j.neuroimage.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Rangaswamy M, Porjesz B. Event-related oscillations in alcoholism research: A review. J Addict Res Ther Suppl. 2012;7 doi: 10.4172/2155-6105.S7-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Quraan MA, Moses SN, Hung Y, Mills T, Taylor MJ. Detection and localization of hippocampal activity using beamformers with MEG: A detailed investigation using simulations and empirical data. Hum Brain Mapp. 2011;32:812–827. doi: 10.1002/hbm.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. From event-related potential to oscillations: Genetic diathesis in brain (dys)function and alcohol dependence. Alcohol Res Health. 2008;31:238–242. [PMC free article] [PubMed] [Google Scholar]
- Riggs L, Moses SN, Bardouille T, Herdman AT, Ross B, Ryan JD. A complementary analytic approach to examining medial temporal lobe sources using magnetoencephalography. Neuroimage. 2009;45:627–642. doi: 10.1016/j.neuroimage.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vrba J. Functional Neuroimaging by Synthetic Aperture Magnetometry (SAM). Biomag98, 11th International Conference on Biomagnetism; Sendai. 1998. pp. 1–4. [Google Scholar]
- Stapleton-Kotloski JR, Kotloski RJ, Boggs JA, Popli G, O’Donovan CA, Couture DE, Cornell C, Godwin DW. Localization of interictal epileptiform activity using magnetoencephalography with synthetic aperture magnetometry in patients with a vagus nerve stimulator. Front Neurol. 2014;5:244. doi: 10.3389/fneur.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford QK, Laurienti PJ, Davenport AT, Friedman DP, Kraft RA, Daunais JB. The effects of chronic alcohol self-administration in nonhuman primate brain networks. Alcohol Clin Exp Res. 2015;39:659–671. doi: 10.1111/acer.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): Long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci U S A. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM. Graphs of brain networks. Alcohol Clin Exp Res. 2013;37:1813–1815. doi: 10.1111/acer.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.