Abstract
Protease-activated receptors (PARs) have been implicated in a variety of physiological functions, as well as somatosensation and particularly itch and pain. Considerable attention has focused on PARs following the finding they are upregulated in the skin of atopic dermatitis patients. The present review focuses on recent studies showing that PARs are critically involved in itch and sensitization of itch. PARs are expressed by diverse cell types including primary sensory neurons, keratinocytes, and immune cells and are activated by proteases that expose a tethered ligand. Endogenous proteases are also released from diverse cell types including keratinocytes and immune cells. Exogenous proteases released from certain plants and insects contacting the skin can also induce itch. Increased levels of proteases in the skin contribute to inflammation that is often accompanied by chronic itch which is not predominantly mediated by histamine. The neural pathway signaling itch induced by activation of PARs is distinct from that mediating histamine-induced itch. In addition, there is evidence that PARs play an important role in sensitization of itch signaling under conditions of chronic itch. These recent findings suggest that PARs and other molecules involved in the itchsignaling pathway are good targets to develop novel treatments for most types of chronic itch that are poorly treated with antihistamines.
Keywords: Brain, GPCR, Human, Mouse, Pain, Pruritus, Rat, Scratching, Spinal cord
1 Protease-Activated Receptors and Itch
Protease-activated receptors (PARs) are unique among G-protein-coupled receptors. This uniqueness stems from the fact that PARs are activated following protease cleavage of part of the extracellular domain. Cleavage exposes a new amino terminus which acts as a ligand to activate PARs. PARs have various physiological and pathophysiological roles in cardiovascular and respiratory systems, nervous systems, the gastrointestinal tract, musculoskeletal systems, renal systems, embryogenesis, and cancer (Adams et al. 2011). In the skin, physiological functions of PARs include skin barrier homeostasis, inflammation, as well as itch and pain (Lee et al. 2010; Vellani et al. 2010; Adams et al. 2011; Akiyama and Carstens 2013; Kempkes et al. 2014). This review will highlight the role of PARs in itch and sensitization of itch.
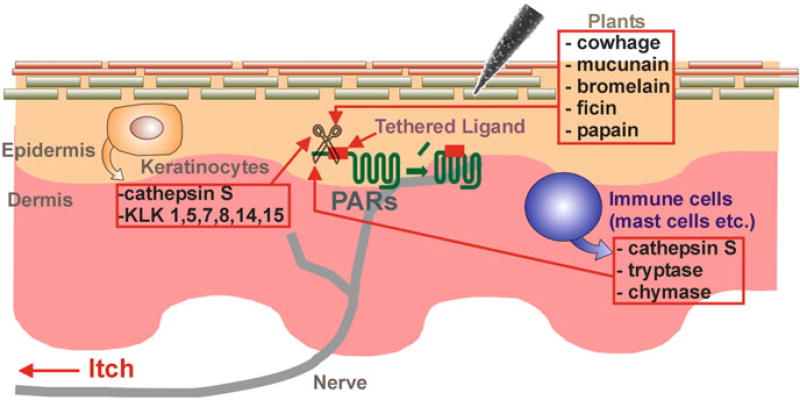
PARs consist of four members: PAR-1, PAR-2, PAR-3, and PAR-4. PARs other than PAR-3 are likely involved in acute itch. Hexapeptide agonists derived from the tethered ligand sequences, including TFLLR (PAR-1), SLIGRL (PAR-2), and AYPGKF (PAR-4), are known to elicit scratching in mice, but SFNGGP (PAR-3) failed to elicit scratching (Tsujii et al. 2008; Akiyama et al. 2009a, b, c, 2010b, 2012a). Although proteases efficiently lead to PAR activation, the hexapeptides are weak pruritogens in mice, being active at micromolar levels. This weakness may explain why neither SLIGRL nor AYPGKF elicits scratching in Sprague-Dawley rats (Klein et al. 2011). The activity of PARs is controlled by activating and deactivating proteases (Adams et al. 2011). The proteases originate from endogenous sources including keratinocytes, mast cells, macrophages, dendritic cells, B cells, T cells, and neutrophils, as well as from external sources including mites, fungi, cockroaches, bacteria, and plants (Shpacovitch et al. 2007; Lee et al. 2010; Reddy and Lerner 2010; Meyer-Hoffert 2012; Page 2012) (Fig. 1). As proteases activate PARs and injection of proteases into human skin can cause itch, it is possible that tryptase and chymase, serine proteases released from mast cells, contribute to itch (Hägermark et al. 1972; Kivinen et al. 2001; Steinhoff et al. 2003; Moormann et al. 2006; Sharma et al. 2007; Groschwitz et al. 2013). Intradermal injection of tryptase elicits scratching in mice (Ui et al. 2006). Several kallikreins, also serine proteases, including kallikrein (KLK) 1, 4, 5, 6, 7, 8, 9, 10, 11, 13, and 14, are expressed in the stratum corneum of the epidermis (Komatsu et al. 2003, 2005). KLK5 and KLK14, but not KLK7 or KLK8, act on PAR-2, while KLK1 acts on PAR-1 (Stefansson et al. 2008; Gao et al. 2010). Intradermal injection of KLK elicits itch in humans (Hägermark 1974). Cathepsin S, a cysteine protease, is expressed in antigen-presenting cells, including B cells, macrophages, and dendritic cells as well as keratinocytes (Schwarz et al. 2002; Lutzner and Kalbacher 2008). Cathepsin S delivered by inactivated spicules of cowhage elicited itch through PAR-2 and PAR-4 (Reddy et al. 2010). Cowhage spicules, which constitute itch powder, contain the active component mucunain, a cysteine protease that acts at PAR-2 and PAR-4 to produce itch (Reddy et al. 2008). Other plant cysteine proteases activate PAR-2 and PAR-4 to produce itch, such as bromelain (pineapple stem), ficin (fig tree latex), and papain (papaya) (Reddy and Lerner 2010).
Fig. 1.
Schematic diagram of origins of PAR ligands in the skin. Figure shows cross section through the skin. The proteases originate from endogenous sources including keratinocytes and immune cells as well as from external sources, such as plants
Whether the activation of PAR-2 contributes to initiating itch in mice remains controversial. SLIGRL- and trypsin-evoked scratching remained in PAR-2 knockout mice (Liu et al. 2011). In contrast, tryptase-evoked scratching was inhibited by genetic knockout of PAR-2 as well as pharmacological blockade of PAR-2 (Ui et al. 2006). Different types of agonists (e.g., SLIGRL, tryptase, and trypsin) may activate different signaling pathways. Alternatively, roles of PAR-2 may be different between neurons and keratinocytes.
PARs are involved in pathological conditions accompanied by chronic itch. The number of tryptase-containing mast cells in the upper dermis is increased in atopic dermatitis as well as psoriatic skin (Harvima et al. 1990; Jarvikallio et al. 1997). Mutations in the serine protease inhibitor Kazal type 5 (spink5) gene, which encodes the protease inhibitor lymphoepithelial Kazal-type-related inhibitor, result in upregulation of KLK5 activity which is involved in the formation of atopic dermatitis-like skin lesions via PAR-2 (Briot et al. 2009) (Table 1). Consistent with this, transgenic KLK5 overexpressor mice displayed signs of severe inflammation and pruritus (Furio et al. 2014). Cathepsin S is mainly present in the dermis of normal human skin. In contrast, cathepsin S is upregulated and detected in keratinocytes in psoriatic skin (Schonefuss et al. 2010). Overexpression of cathepsin S induces atopic dermatitis-like skin through an increase in PAR-2 expression in dendritic cells (Kim et al. 2012). Transgenic expression of the serine protease channel-activating protease-1 in the skin induces atopic dermatitis-like skin accompanied by increased spontaneous scratching (Frateschi et al. 2011). These phenotypes are completely negated when superimposed on a PAR-2 null background. In the skin of NC mice, an animal model of atopic dermatitis, the activity of serine proteases as well as the number of PAR-2-positive keratinocytes is increased (Tsujii et al. 2009). These findings imply that PARs play an important role in chronic itch under pathological conditions.
Table 1.
Protease-related mutant mice with atopic dermatitis-like skin
| Mutations | Upregulated proteases | Subtype of PARs |
References |
|---|---|---|---|
| Serine protease inhibitor Kazal type 5 | KLK5 | PAR-2 | Briot et al. (2009) |
| KLK5 | KLK5 | Not tested | Furio et al. (2014) |
| Cathepsin S | Cathepsin S | PAR-2 | Kim et al. (2012) |
| Serine protease channel-activating protease-1 | Serine protease channel-activating protease-1 | PAR-2 | Frateschi et al. (2011) |
2 Mechanisms of Protease-Activated Receptor-Mediated Itch
PARs are expressed broadly in neuronal as well as nonneuronal cells (Shpacovitch et al. 2002, 2007; Steinhoff et al. 2003; Zhu et al. 2005; Moormann et al. 2006; Vellani et al. 2010). The activation of PAR-2 on keratinocytes induces the release of LTB4 (Zhu et al. 2009b), which elicits itch through BLT1-expressing neurons (Andoh and Kuraishi 1998, 2005). Activation of PAR-2 in keratinocytes also induces release of thymic stromal lymphopoietin, a pruritogenic cytokine that excites TRPA1-expressing sensory neurons (Wilson et al. 2013). PARs are expressed by a variety of different immune cells, including neutrophils, eosinophils, monocytes, macrophages, and mast cells (Shpacovitch et al. 2007). In particular, PAR-1 is expressed in mast cells. Considering the partial inhibition of the PAR-1 agonist-evoked scratching by an H1 histamine receptor antagonist (Tsujii et al. 2008), PAR-1 agonist-evoked itch can be partially attributed to histamine released from mast cells. Although PAR-2 and PAR-4 are expressed by mast cells, an intradermal injection of PAR-2 or PAR-4 agonist presumably does not activate these receptors expressed by mast cells, since H1 histamine receptor antagonists failed to inhibit scratching evoked by the PAR agonists (Tsujii et al. 2008; Akiyama et al. 2012a). In addition to mast cells, other immune cells may be activated through PARs under pathophysiological conditions to release certain pruritogens. PAR-1, PAR-2, and PAR-4 have been found to be expressed in primary sensory neurons (Steinhoff et al. 2003; Zhu et al. 2005; Vellani et al. 2010). Proteases such as trypsin and thrombin and hexapeptide ligands of PAR-1, PAR-2, and PAR-4 can activate primary sensory neurons (Amadesi et al. 2004; Akiyama et al. 2010a; Vellani et al. 2010). Direct activation of primary sensory neurons through these receptors might contribute to itch. A PAR-2 agonist and either a PAR-1 or PAR-4 agonist apparently activate different subpopulations of primary sensory neurons (Vellani et al. 2010).
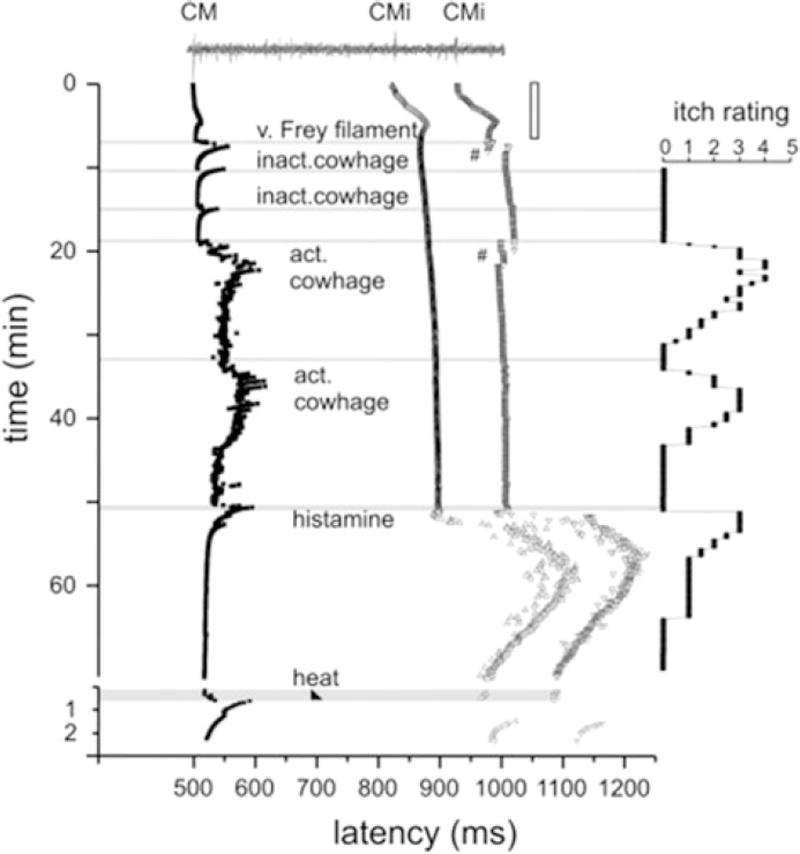
The neuronal pathway for PAR-mediated itch has been studied mainly using cowhage and PAR-2 tethered ligands such as SLIGRL. Itch elicited by intradermal insertion of cowhage spicules is considered to be non-histaminergic based on the following observations. (1) In contrast to histamine, cowhage spicules elicit itch without accompanying flare (Johanek et al. 2007; Sikand et al. 2009). (2) While histamine-elicited itch is described as mosquito bite-like, cowhage-elicited itch is described as stinging, sharp, and prickly (Kosteletzky et al. 2009). (3) Desensitization of the skin with topical capsaicin abolished cowhage-induced itch but not histamine-induced itch (Johanek et al. 2007). (4) Cowhage-evoked itch was not inhibited by pretreatment with an H1 histamine receptor antagonist (Johanek et al. 2007). Itch elicited by cowhage spicules is apparently mediated by populations of C- as well as Aδ-fibers that are distinct from those mediating histamine-elicited itch. Mechano-insensitive C-fibers preferentially respond to histamine but not cowhage (Schmelz et al. 1997; Namer et al. 2008) (Fig. 2). In contrast, mechanosensitive, polymodal C-fibers readily responded to cowhage with lesser or no responses to histamine (Johanek et al. 2008; Namer et al. 2008). Mechanosensitive A-fibers also responded more vigorously to cowhage than to histamine, but some exclusively responded to histamine (Ringkamp et al. 2011). At the level of the spinal cord, cowhage and histamine activated separate subpopulations of primate spinothalamic tract neurons (Davidson et al. 2007, 2012). In the mouse, a majority of spinal neurons were activated by both SLIGRL and histamine (Akiyama et al. 2009a, b). This difference between murine and primate spinal neurons might be due to the agonistic activity of SLIGRL on Mas-related G-protein-coupled receptors C11 (MrgprC11) (Liu et al. 2011). In the brain, cowhage and histamine activated largely overlapping areas including thalamus, primary and secondary somatosensory cortices, posterior parietal cortex, superior and middle temporal cortices, PCC, ACC, precuneus, and cuneus. However, some areas exhibited more extensive activation by cowhage, including the insular cortex, claustrum, basal ganglia, putamen, thalamic nuclei, and pulvinar (Papoiu et al. 2012).
Fig. 2.
Specimen of a multifiber recording from 1 mechano-responsive (CM) and 2 mechano-insensitive nociceptors (CMi). A trace of the raw signal containing the C-fiber action potentials is shown on top. Conduction latencies of these three marked fibers (filled square, open triangle) in response to successive electrical stimulation at the receptive field are plotted from top to bottom. Top traces were recorded during stimulation with increasing frequencies (see open square on right side), followed by traces recorded during stimulation with mechanical stimuli (v. Frey filament), inactive (inact.) and active (act.). Adapted from Namer et al. (2008)
The downstream signal transduction molecules involved in PAR-mediated itch have not yet been specified. Histaminergic itch requires TRPV1, whereas non-histaminergic itch via MrgprA3 and MrgprC11 agonists requires TRPA1 (Shim et al. 2007; Imamachi et al. 2009; Wilson et al. 2011). PAR-1-mediated itch may require TRPV1, but it is not currently known if itch mediated by PAR-2 and PAR-4 requires TRPV1 or TRPA1. Scratching evoked by trypsin, which acts at PAR-1, PAR-2, and PAR-4, was reduced in knockout mice lacking TRPV1 (Costa et al. 2008). Trypsin-evoked scratching was inhibited by an H1 histamine receptor antagonist as well as depletion of mast cells by repeated treatment with compound 48/80 (Costa et al. 2008), suggesting that mast cells play a major role in itch evoked by trypsin. Knockout mice lacking PAR-2 exhibited greater scratching compared to wild types (Liu et al. 2011), implying that PAR-2 is not involved in trypsin-evoked scratching. Considering that PAR-1 agonist-evoked scratching was partially inhibited by the H1 histamine antagonist, trypsin-evoked scratching is presumably mediated by PAR-1. It would be interesting to know whether scratching evoked by the PAR-2 and PAR-4 agonists requires TRPV1 or TRPA1. Phospholipase C (PLC) plays a key role in intracellular signaling by G-protein-coupled receptors. While PLCβ3 contributes to itch evoked by histamine and 5-HT, PLCβ3 does not appear to be involved in SLIGRL-evoked itch (Imamachi et al. 2009). Pirt (phosphoinositide-interacting protein) binds to phosphatidylinositol (4,5)-bisphosphate, TRPV1, and other ion channels to potentiate them. Knockout mice lacking Pirt exhibited a significant loss of scratching evoked by histamine, 5-HT, endothelin-1, and the MrgprA3 agonist chloroquine (Patel et al. 2011). On the other hand, SLIGRL-evoked scratching was not significantly inhibited in Pirt knockout mice.
3 Itch Sensitization via Protease-Activated Receptors
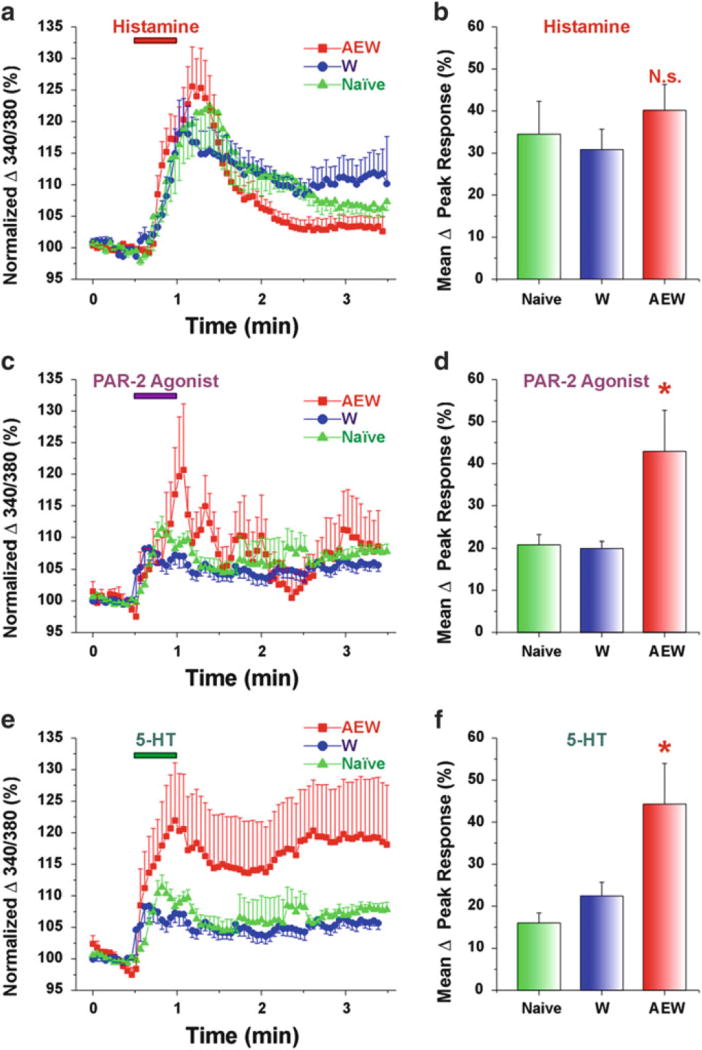
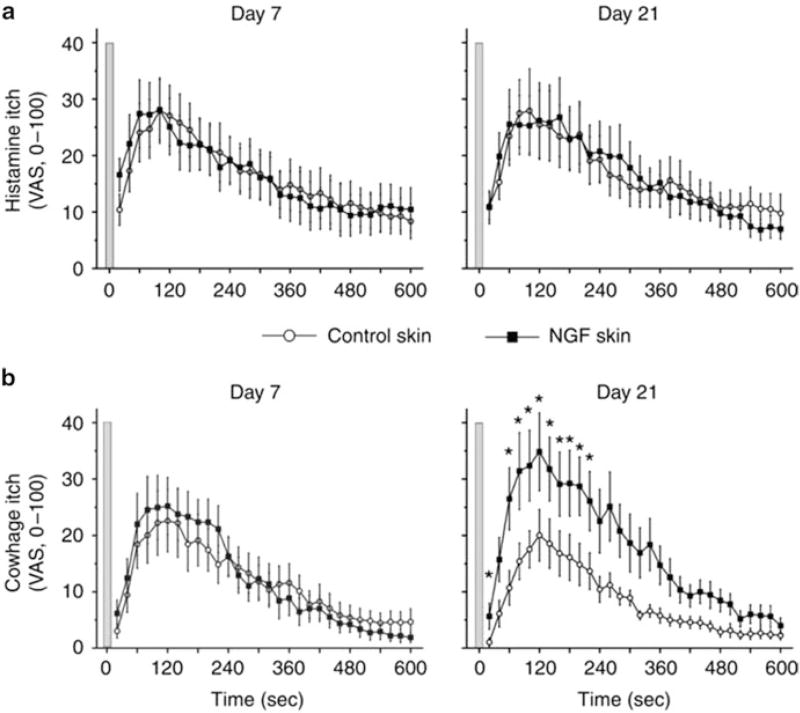
PARs are involved in sensitization of itch, with the features of spontaneous itch, hyperknesis (enhanced itch to a normally itchy stimulus), and alloknesis (itch elicited by an innocuous touch stimulus). These manifestations of itch sensitization are observed in patients suffering from chronic itch (Schmelz et al. 2003; Ikoma et al. 2004; Hosogi et al. 2006). In mice, hyperknesis has been demonstrated in a model of chronic dry skin itch. We reported that mice treated with drying agents exhibited significantly greater scratching following intradermal injections of 5-HT and SLIGRL delivered in the dry skin treatment area compared to control (water-) treated mice (Akiyama et al. 2010a). In contrast, histamine-evoked scratching was not significantly enhanced in dry skin-treated mice. DRG cells taken from the dry skin-treated mice exhibited significantly greater responses to 5-HT and SLIGRL, but not histamine, consistent with the behavioral results (Fig. 3). Superficial dorsal horn neurons receiving afferent input from a dry skin-treated hind paw exhibited significantly enhanced responses to SLIGRL, but not histamine, compared to units recorded in control animals (Akiyama et al. 2011). These findings suggest that the enhanced response to SLIGRL may be attributed to peripheral sensitization of pruriceptors projecting to the recorded dorsal horn neurons. NGF might account for this peripheral sensitization. In dry skin, NGF levels are elevated and might contribute to peripheral sensitization of pruriceptors (Tominaga et al. 2007). Intradermally administered NGF enhanced itch induced by cowhage but not histamine in humans (Rukwied et al. 2013) (Fig. 4). PAR-2 may be sensitized in the skin in which NGF levels are elevated under chronic itch conditions. Moreover, not only may pruriceptors expressing PARs become sensitized, but PARs may also contribute to sensitization of non-histaminergic pruriceptors expressing transduction molecules such as MrgprA3 and MrgprC11. Pretreatment with SLIGRL, but not BAM8–22, resulted in an enhancement of responses of primary sensory neurons to MrgprA3 and MrgprC11 agonists, as well as enhanced scratching evoked by the MrgprA3 and MrgprC11 agonists (Akiyama et al. 2012b). Pretreatment with SLIGRL failed to enhance scratching evoked by histamine, 5-HT, the PAR-4 agonist, or SLIGRL (Akiyama et al. 2009c).
Fig. 3.
DRG cells from AEW-treated mice show enhanced responses to PAR-2 agonist and 5-HT but not histamine. (a) Histamine. Mean normalized ratiometric responses of DRG cells from each treatment group vs. time relative to histamine perfusion (red bar). Error bars: SEM. (b) Mean peak response (% change from baseline) of DRG cells to histamine for each treatment group. N.s.: no significant difference compared to W. (c, e) as in (a) for PAR-2 agonist and 5-HT, respectively. (d, f) as in (b) for PAR-2 agonist and 5-HT, respectively. Asterisk, significantly different compared to W (p < 0.05, unpaired t-test). (n = 14–23/group). Naïve data from cervical DRG cells (n = 719) obtained from untreated mice. Adapted from Akiyama et al. (2010a)
Fig. 4.
Nerve growth factor (NGF) sensitizes cowhage- but not histamine-induced itch. Itch sensation recorded upon (a) histamine iontophoresis and (b) cowhage spicule insertion at day 7 (left panel) and day 21 (right panel) after administration of 1 µg NGF (n = 12). In comparison with control skin, cowhage-induced itch was perceived significantly stronger at the NGF-treated sites at day 21 (marked by asterisks, Wilcoxon test, P < 0.05). Gray bars indicate the time point of histamine iontophoresis and gray cowhage application. Error bars indicate SEM. VAS, visual analog scale. Adapted from Rukwied et al. (2013)
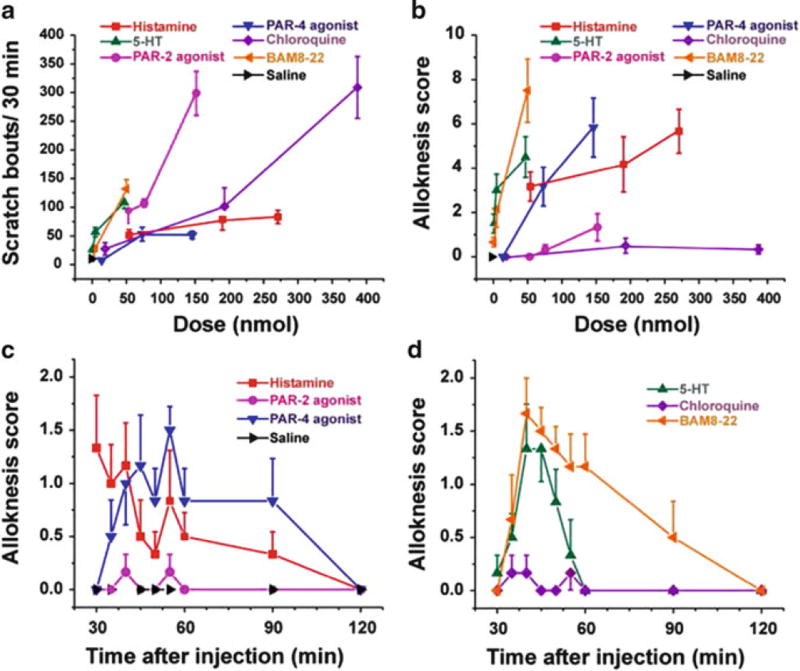
Certain PARs are also involved in alloknesis, the phenomenon in which itch is induced by low-threshold mechanical stimulation of skin surrounding the site of the pruritic stimulus. Cowhage spicule-elicited itch is accompanied by alloknesis (Sikand et al. 2009), implying the involvement of PAR-2 and/or PAR-4 since they are activated by mucunain in cowhage (Reddy et al. 2008). Alloknesis is a common and often distressing symptom for many patients suffering from chronic itch. The neural mechanisms underlying alloknesis are poorly understood, partly due to a lack of animal models for alloknesis. To begin to investigate alloknesis, we recently developed an animal model (Akiyama et al. 2012a). C57BL/6 mice do not normally respond to innocuous mechanical stimulation of the rostral back. However, following intradermal injection of histamine and certain other pruritogens, low-threshold mechanical stimuli delivered to the skin around the injection site reliably elicited discrete hind limb scratch bouts directed to the stimulus. The time course of touch-evoked scratching had a slower onset and longer duration compared to the pruritogen-evoked scratching that usually ceased within 30 min. Touch-evoked scratching was observed following histamine, 5-HT, the PAR-4 agonist, and BAM8–22 but not SLIGRL or chloroquine (Fig. 5). Considering that alloknesis was evoked by the PAR-4 agonist but not SLIGRL, alloknesis evoked by cowhage is presumably mediated by PAR-4.
Fig. 5.
Scratching and alloknesis elicited by different pruritogens. (a) Dose-response curve for scratch bouts (assessed over 30 min) elicited by intradermal injection of pruritogens indicated in each figure. Error bars: SEM (n = 6/group). (b) Dose-response curve for alloknesis score elicited by the same pruritogens. (c) Time course of alloknesis for histamine (271 nmol/10 µl), PAR-2 agonist SLIGRL-NH2 (76 nmol/10 µl), PAR-4 agonist AYPGKF-NH2 (146 nmol/10 µl), and saline vehicle. (d) Time course of alloknesis for 5-HT (47 nmol/10 µl), chloroquine (193 nmol/10 µl), and BAM8–22 (50 nmol/10 µl). Adapted from Akiyama et al. (2012a)
4 Protease-Activated Receptors and Pain
PARs have been shown to play a role in modulating nociception. PAR-2 is expressed in nociceptive primary sensory neurons, and its activation leads to hyperalgesia (Amadesi et al. 2004; Dai et al. 2004, 2007; Wang et al. 2012). PAR-2 is involved in joint, visceral, and somatic pain through the sensitization of TRPV1, TRPA1, TRPV4, and P2X3 (Amadesi et al. 2004; Dai et al. 2004, 2007; Sipe et al. 2008; Helyes et al. 2010; Lam et al. 2012; Wang et al. 2012; Poole et al. 2013). In contrast, PAR-1 and PAR-4 agonists exert antinociceptive effects such as increased thermal and mechanical nociceptive withdrawal thresholds (Asfaha et al. 2002, 2007; Auge et al. 2009; Karanjia et al. 2009; Annahazi et al. 2012) with one exception (McDougall et al. 2009). High doses of PAR agonists can induce inflammation, presumably explaining the pro-nociceptive action of PAR-4 agonists in the study of joint pain (McDougall et al. 2009).
5 Protease-Activated Receptors as Target Molecules for Future Treatments of Chronic Itch
The studies discussed above provide strong evidence for the participation of PARs in acute itch as well as itch sensitization. Thus, the clinical treatment of chronic itch is likely to benefit from the development of drugs directed at PARs. There are two strategies to block PAR-mediated itch signaling: (1) inhibit the protease agonists of PARs and (2) antagonize PARs. Nafamostat mesilate, a serine protease inhibitor, inhibited scratching evoked by tryptase and compound 48/80 as well as spontaneous scratching in NC mice exhibiting atopic dermatitis-like skin lesions (Ui et al. 2006; Tsujii et al. 2009). Leupeptin, a protease inhibitor, inhibited scratching evoked by compound 48/80 as well as passive cutaneous anaphylaxis in mice (Ui et al. 2006; Zhu et al. 2009a). The chymase inhibitor SUN13834 inhibited spontaneous scratching in a mouse dermatitis model induced by repeated treatments of hapten (Terakawa et al. 2008). Similar to the serine protease inhibitors, PAR-2 antagonists have been used successfully to inhibit itch-related behavior in mice. Scratching evoked by tryptase and compound 48/80 was inhibited by the PAR-2 antagonist FSLLRY, as well as by an anti-PAR-2 antibody (Ui et al. 2006). Spontaneous scratching in dry skin-treated mice, as well as in NC mice, was inhibited by an anti-PAR-2 antibody (Tsujii et al. 2009; Akiyama et al. 2010a). FK506 is a drug that provides temporary itch relief in atopic dermatitis. Its antipruritic effect might be due to inhibition of PAR-2-mediated signaling (Nakano et al. 2008). Overall, PARs are attractive targets for the development of treatments for itch. PAR-2 antagonists, such as the novel low molecular weight, non-peptide PAR-2 antagonist GB88, have been developed recently. Their antipruritic effects await future testing (Suen et al. 2012). Further studies will reveal the detailed mechanisms underlying the role of PARs in acute and, especially, chronic itch.
Acknowledgments
The work was supported by grants from the National Institutes of Health DE013685, AR057194, and AR063228.
Abbreviations
- ACC
Anterior cingulate cortex
- AYPGKF
Ala-Tyr-Pro-Gly-Lys-Phe-NH2
- BAM8–22
Bovine adrenal medulla 8–22
- KLK
Kallikrein
- Mrgpr
Mas-related G-protein-coupled receptors
- NGF
Nerve growth factor
- PARs
Protease-activated receptors
- PCC
Posterior cingulate cortex
- PLC
Phospholipase C
- SLIGRL
Ser-Leu-Ile-Gly-Arg-Leu-NH2
- spink5
serine protease inhibitor Kazal type 5
Contributor Information
Tasuku Akiyama, Department of Dermatology, Anatomy and Cell Biology/Temple Itch Center, Temple University School of Medicine, Philadelphia, PA 19140, USA.
Ethan A. Lerner, Department of Dermatology/Cutaneous Biology Research Center, Massachusetts General Hospital, Building 149, 13th Street, Charlestown, Boston, MA 02129, USA, elerner@mgh.harvard.edu
E. Carstens, Department of Neurobiology, Physiology and Behavior, University of California, Davis, 1 Shields Avenue, Davis, CA 95616, USA, eecarstens@ucdavis.edu
References
- Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther. 2011;130(3):248–282. doi: 10.1016/j.pharmthera.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol. 2009a;102(4):2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci. 2009b;29(20):6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther. 2009c;329(3):945–951. doi: 10.1124/jpet.109.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic itch. Pain. 2010a;151(2):378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol. 2010b;104(5):2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J Neurophysiol. 2011;105(6):2811–2817. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012a;132(7):1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Cross-sensitization of histamine-independent itch in mouse primary sensory neurons. Neuroscience. 2012b;226:305–312. doi: 10.1016/j.neuroscience.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24(18):4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur J Pharmacol. 1998;353(1):93–96. doi: 10.1016/s0014-2999(98)00440-3. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Brain Res Mol Brain Res. 2005;137(1–2):263–266. doi: 10.1016/j.molbrainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Annahazi A, Dabek M, Gecse K, Salvador-Cartier C, Polizzi A, Rosztoczy A, Roka R, Theodorou V, Wittmann T, Bueno L, Eutamene H. Proteinase-activated receptor-4 evoked colorectal analgesia in mice: an endogenously activated feed-back loop in visceral inflammatory pain. Neurogastroenterol Motil. 2012;24(1):76–85. e13. doi: 10.1111/j.1365-2982.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- Asfaha S, Brussee V, Chapman K, Zochodne DW, Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br J Pharmacol. 2002;135(5):1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, Steinhoff M, Chapman K, Zamponi GW, Vergnolle N. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol. 2007;150(2):176–185. doi: 10.1038/sj.bjp.0706975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auge C, Balz-Hara D, Steinhoff M, Vergnolle N, Cenac N. Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motil. 2009;21(11):1189–e1107. doi: 10.1111/j.1365-2982.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, Dubus P, Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206(5):1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, Quintao NL, Juliano L, Brain SD, Calixto JB. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154(5):1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24(18):4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117(7):1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27(37):10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108(6):1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frateschi S, Camerer E, Crisante G, Rieser S, Membrez M, Charles RP, Beermann F, Stehle JC, Breiden B, Sandhoff K, Rotman S, Haftek M, Wilson A, Ryser S, Steinhoff M, Coughlin SR, Hummler E. PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nat Commun. 2011;2:161. doi: 10.1038/ncomms1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furio L, de Veer S, Jaillet M, Briot A, Robin A, Deraison C, Hovnanian A. Transgenic kallikrein 5 mice reproduce major cutaneous and systemic hallmarks of Netherton syndrome. J Exp Med. 2014;211(3):499–513. doi: 10.1084/jem.20131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Chao L, Chao J. A novel signaling pathway of tissue kallikrein in promoting keratinocyte migration: activation of proteinase-activated receptor 1 and epidermal growth factor receptor. Exp Cell Res. 2010;316(3):376–389. doi: 10.1016/j.yexcr.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz KR, Wu D, Osterfeld H, Ahrens R, Hogan SP. Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2013;304(5):G479–G489. doi: 10.1152/ajpgi.00186.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägermark O. Studies on experimental itch induced by kallikrein and bradykinin. Acta Derm Venereol. 1974;54(5):397–400. [PubMed] [Google Scholar]
- Hägermark D, Rajka G, Berqvist U. Experimental itch in human skin elicited by rat mast cell chymase. Acta Derm Venereol. 1972;52(2):125–128. [PubMed] [Google Scholar]
- Harvima IT, Naukkarinen A, Harvima RJ, Aalto ML, Neittaanmaki H, Horsmanheimo M. Quantitative enzyme-histochemical analysis of tryptase- and chymase-containing mast cells in psoriatic skin. Arch Dermatol Res. 1990;282(7):428–433. doi: 10.1007/BF00402617. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Sandor K, Borbely E, Tekus V, Pinter E, Elekes K, Toth DM, Szolcsanyi J, McDougall JJ. Involvement of transient receptor potential vanilloid 1 receptors in protease-activated receptor-2-induced joint inflammation and nociception. Eur J Pain. 2010;14(4):351–358. doi: 10.1016/j.ejpain.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126(1–3):16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62(2):212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997;136(6):871–877. [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27(28):7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28(30):7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanjia R, Spreadbury I, Bautista-Cruz F, Tsang ME, Vanner S. Activation of protease-activated receptor-4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol Motil. 2009;21(11):1218–1221. doi: 10.1111/j.1365-2982.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in neuroimmune communication and itch. In: Carstens E, Akiyama T, editors. Itch: mechanisms and treatment. Frontiers in Neuroscience. CRC Press; Boca Raton, FL: 2014. [PubMed] [Google Scholar]
- Kim N, Bae KB, Kim MO, Yu DH, Kim HJ, Yuh HS, Ji YR, Park SJ, Kim S, Son KH, Yoon D, Lee DS, Lee S, Lee HS, Kim TY, Ryoo ZY. Overexpression of cathepsin S induces chronic atopic dermatitis in mice. J Invest Dermatol. 2012;132(4):1169–1176. doi: 10.1038/jid.2011.404. [DOI] [PubMed] [Google Scholar]
- Kivinen PK, Kaminska R, Naukkarinen A, Harvima RJ, Horsmanheimo M, Harvima IT. Release of soluble tryptase but only minor amounts of chymase activity from cutaneous mast cells. Exp Dermatol. 2001;10(4):246–255. doi: 10.1034/j.1600-0625.2001.100404.x. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106(3):1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Takata M, Otsuki N, Toyama T, Ohka R, Takehara K, Saijoh K. Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J Invest Dermatol. 2003;121(3):542–549. doi: 10.1046/j.1523-1747.2003.12363.x. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Saijoh K, Sidiropoulos M, Tsai B, Levesque MA, Elliott MB, Takehara K, Diamandis EP. Quantification of human tissue kallikreins in the stratum corneum: dependence on age and gender. J Invest Dermatol. 2005;125(6):1182–1189. doi: 10.1111/j.0022-202X.2005.23933.x. [DOI] [PubMed] [Google Scholar]
- Kosteletzky F, Namer B, Forster C, Handwerker HO. Impact of scratching on itch and sympathetic reflexes induced by cowhage (Mucuna pruriens) and histamine. Acta Derm Venereol. 2009;89(3):271–277. doi: 10.2340/00015555-0624. [DOI] [PubMed] [Google Scholar]
- Lam DK, Dang D, Zhang J, Dolan JC, Schmidt BL. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. J Neurosci. 2012;32(41):14178–14183. doi: 10.1523/JNEUROSCI.2399-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J. 2010;51(6):808–822. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4(181):ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzner N, Kalbacher H. Quantifying cathepsin S activity in antigen presenting cells using a novel specific substrate. J Biol Chem. 2008;283(52):36185–36194. doi: 10.1074/jbc.M806500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JJ, Zhang C, Cellars L, Joubert E, Dixon CM, Vergnolle N. Triggering of proteinase-activated receptor 4 leads to joint pain and inflammation in mice. Arthritis Rheum. 2009;60(3):728–737. doi: 10.1002/art.24300. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U. Epidermal serine proteases and their inhibitors in atopic dermatitis. In: Jorge Esparza-Gordillo., editor. Atopic dermatitis—disease etiology and clinical management. 2012. InTech. http://www.intechopen.com/books/atopic-dermatitis-disease-etiology-and-clinical-management/epidermal-serine-proteases-and-their-inhibitors-in-atopic-dermatitis.
- Moormann C, Artuc M, Pohl E, Varga G, Buddenkotte J, Vergnolle N, Brehler R, Henz BM, Schneider SW, Luger TA, Steinhoff M. Functional characterization and expression analysis of the proteinase-activated receptor-2 in human cutaneous mast cells. J Invest Dermatol. 2006;126(4):746–755. doi: 10.1038/sj.jid.5700169. [DOI] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Tayama M, Kosaka M, Lee JB, Kuraishi Y. Effects of topical application of tacrolimus on acute itch-associated responses in mice. Biol Pharm Bull. 2008;31(4):752–754. doi: 10.1248/bpb.31.752. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100(4):2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K. Role of cockroach proteases in allergic disease. Curr Allergy Asthma Rep. 2012;12(5):448–455. doi: 10.1007/s11882-012-0276-1. [DOI] [PubMed] [Google Scholar]
- Papoiu AD, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. NeuroImage. 2012;59(4):3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Liu Q, Meeker S, Undem B, Dong X. Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One. 2011;6(5):e20559. doi: 10.1371/journal.pone.0020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu T, Darby W, Liedtke W, Lew MJ, McIntyre P, Bunnett NW. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem. 2013;288(8):5790–5802. doi: 10.1074/jbc.M112.438184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Lerner EA. Plant cysteine proteases that evoke itch activate protease-activated receptors. Br J Dermatol. 2010;163(3):532–535. doi: 10.1111/j.1365-2133.2010.09862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V, Iuga A, Shimada S, LaMotte R, Lerner E. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28(17):4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Shimada SG, Sikand P, Lamotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130(5):1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp M, Schepers R, Shimada S, Johanek L, Hartke T, Borzan J, Shim B, LaMotte R, Meyer R. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31(42):14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage-but not histamine-induced itch in human skin. J Invest Dermatol. 2013;133(1):268–270. doi: 10.1038/jid.2012.242. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Hilliges M, Schmidt R, Orstavik K, Vahlquist C, Weidner C, Handwerker HO, Torebjork HE. Active “itch fibers” in chronic pruritus. Neurology. 2003;61(4):564–566. doi: 10.1212/01.wnl.0000078193.64949.08. [DOI] [PubMed] [Google Scholar]
- Schonefuss A, Wendt W, Schattling B, Schulten R, Hoffmann K, Stuecker M, Tigges C, Lubbert H, Stichel C. Upregulation of cathepsin S in psoriatic keratinocytes. Exp Dermatol. 2010;19(8):e80–e88. doi: 10.1111/j.1600-0625.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- Schwarz G, Boehncke WH, Braun M, Schroter CJ, Burster T, Flad T, Dressel D, Weber E, Schmid H, Kalbacher H. Cathepsin S activity is detectable in human keratinocytes and is selectively upregulated upon stimulation with interferon-gamma. J Invest Dermatol. 2002;119(1):44–49. doi: 10.1046/j.1523-1747.2002.01800.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Prasad V, McCarthy ET, Savin VJ, Dileepan KN, Stechschulte DJ, Lianos E, Wiegmann T, Sharma M. Chymase increases glomerular albumin permeability via protease-activated receptor-2. Mol Cell Biochem. 2007;297(1–2):161–169. doi: 10.1007/s11010-006-9342-0. [DOI] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpacovitch VM, Brzoska T, Buddenkotte J, Stroh C, Sommerhoff CP, Ansel JC, Schulze-Osthoff K, Bunnett NW, Luger TA, Steinhoff M. Agonists of proteinase-activated receptor 2 induce cytokine release and activation of nuclear transcription factor kappaB in human dermal microvascular endothelial cells. J Invest Dermatol. 2002;118(2):380–385. doi: 10.1046/j.0022-202x.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- Shpacovitch V, Feld M, Bunnett NW, Steinhoff M. Protease-activated receptors: novel PARtners in innate immunity. Trends Immunol. 2007;28(12):541–550. doi: 10.1016/j.it.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144(1–2):66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, Egelrud T. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128(1):18–25. doi: 10.1038/sj.jid.5700965. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23(15):6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen JY, Barry GD, Lohman RJ, Halili MA, Cotterell AJ, Le GT, Fairlie DP. Modulating human proteinase activated receptor 2 with a novel antagonist (GB88) and agonist (GB110) Br J Pharmacol. 2012;165(5):1413–1423. doi: 10.1111/j.1476-5381.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakawa M, Fujieda Y, Tomimori Y, Muto T, Tanaka T, Maruoka H, Nagahira K, Ogata A, Nakatsuka T, Fukuda Y. Oral chymase inhibitor SUN13834 ameliorates skin inflammation as well as pruritus in mouse model for atopic dermatitis. Eur J Pharmacol. 2008;601(1–3):186–191. doi: 10.1016/j.ejphar.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Ozawa S, Tengara S, Ogawa H, Takamori K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. J Dermatol Sci. 2007;48(2):103–111. doi: 10.1016/j.jdermsci.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Lee JB, Kuraishi Y. Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J Pharmacol Sci. 2008;108(3):385–388. doi: 10.1254/jphs.08200sc. [DOI] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Ui H, Lee JB, Kuraishi Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J Pharmacol Sci. 2009;109(3):388–395. doi: 10.1254/jphs.08332fp. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530(1–2):172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Vellani V, Kinsey AM, Prandini M, Hechtfischer SC, Reeh P, Magherini PC, Giacomoni C, McNaughton PA. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol Pain. 2010;6:61. doi: 10.1186/1744-8069-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dai Y, Kobayashi K, Zhu W, Kogure Y, Yamanaka H, Wan Y, Zhang W, Noguchi K. Potentiation of the P2X3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain. Eur J Neurosci. 2012;36(3):2293–2301. doi: 10.1111/j.1460-9568.2012.08142.x. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14(5):595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Yamanaka H, Obata K, Dai Y, Kobayashi K, Kozai T, Tokunaga A, Noguchi K. Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Res. 2005;1041(2):205–211. doi: 10.1016/j.brainres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Peng C, Xu JG, Liu YX, Zhu QG, Liu JY, Li FQ, Wu JH, Hu JH. Participation of proteinase-activated receptor-2 in passive cutaneous anaphylaxis-induced scratching behavior and the inhibitory effect of tacrolimus. Biol Pharm Bull. 2009a;32(7):1173–1176. doi: 10.1248/bpb.32.1173. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang XR, Peng C, Xu JG, Liu YX, Wu L, Zhu QG, Liu JY, Li FQ, Pan YH, You BM, Hu JH. Induction of leukotriene B(4) and prostaglandin E(2) release from keratinocytes by protease-activated receptor-2-activating peptide in ICR mice. Int Immunopharmacol. 2009b;9(11):1332–1336. doi: 10.1016/j.intimp.2009.08.006. [DOI] [PubMed] [Google Scholar]