Abstract
Objective:
To evaluate a digitally delivered, intensive behavioral counseling program for a workforce at risk for obesity-related chronic disease.
Methods:
Employees were offered a digital health program modeled after the diabetes prevention program (DPP). Annual workforce health assessments were used to examine changes in chronic disease risk factors between participants (n = 634) relative to a matched comparison group (n = 1268).
Results:
Overall, employees were gaining an average of 3.5 pounds annually before program inception. Program engagement was positive; 83% completed the majority of the curriculum and 31% lost at least 5% of their starting weight. Compared with non-participating peers, participants demonstrated reduced weight, improved fasting blood glucose, and improved nutritional intake after a year.
Conclusions:
The digital health program was effective for engaging employees in health behavior change. Digital options facilitate widespread implementation.
Overweight and obesity are highly prevalent conditions among working adults, with over 34% of the working population estimated to be overweight and close to 30% estimated to be obese.1 Excess body weight is associated with a host of chronic conditions, most notably Type 2 diabetes and cardiovascular disease.2 Obesity-related conditions among working adults are known to have significant economic impacts on employers through increased healthcare utilization costs, loss of worker productivity, and greater indemnity/worker's compensation claims.3–5 Weight loss through the adoption of healthful eating and physical activity patterns reliably reduces the risk of diabetes and improves intermediate risk factors for cardiovascular disease.6,7 Given the health and economic burden of excess weight on working adults and their employers, successful strategies to induce weight loss, prevent weight gain, and prevent the onset of chronic disease can attenuate the negative consequences on health, worker productivity, and increased health care spending.
The Diabetes Prevention Program (DPP) is an evidence-based program focused on sustainable behavior changes to reduce weight among adults at risk for Type 2 diabetes.6 The DPP program model was originally developed for face-to-face implementation with regular, on-site meetings for weight-tracking, delivery of educational curriculum, and supportive discussion with a health coach or peer group.8 The DPP has been translated for worksite settings; these translations are becoming more frequent in workplaces with promising results for promoting healthful weight loss and chronic disease risk reduction.9–15 However, many employers have employees who telecommute or are dispersed in around-the-clock shifts and/or multiple geographic locations; these logistical complications pose barriers to traditional DPP translational programs that are delivered through in-person, place-based formats.
To meet the need for more flexible program offerings, technology-enhanced methods and digital health programs have emerged to translate the DPP into alternative formats.16 Digital health programs integrate components of mobile technology, the internet, wireless devices, social networks, health information technology, etc., to deliver more personalized and precise health care and more efficiently support and serve users. Digital programs allow users to engage through multiple communication channels (eg, mobile apps, internet, connected devices), are accessible to users around the clock, and remove the barriers of scheduling conflicts and logistical difficulties that can arise with place-based and time-bound programs. Digital health programs have been shown to improve the physical activity and eating behaviors of participants.17–19 These programs can be an effective mechanism for supporting goal-setting, self-monitoring, and providing feedback on performance regarding physical activity and dietary changes,20,21 which are key components of DPP.
Concerns remain that digital programs may not succeed in engaging participants and achieving results in workplace settings, given the less familiar mode of program delivery and social environment relative to traditional, in-person worksite DPP programs. The purpose of this study was to examine the participation and diabetes risk factor-related outcomes of a digital diabetes prevention program during the first year it was offered to a workforce. It was hypothesized that employees and dependents who enrolled in the program would show substantial engagement in the online and digital content and achieve meaningful reduction in risk factors for Type 2 diabetes, as evidenced by reduced weight and fasting blood glucose. The secondary hypothesis was that the program participants would have corresponding improvements in health behaviors (nutrition and physical activity), and reductions in intermediate risk factors for cardiovascular disease.
METHODS
Design
The study employed a nonequivalent (ie, non-randomized) design with a matched control group. Data were collected annually for the workforce from 2013 through 2015, with 2014 to 2015 being the intervention year. Only those employees who had participated in the annual assessments in 2014 and 2015 were included in the primary analyses. The study was approved by Western Institutional Review Board.
Setting and Participants
Iron Mountain, Incorporated is a global storage and information management services company with headquarters in Boston, MA. The company offers services for records and document management, data management, data centers, art storage and logistics, and secure document destruction services through a network of 1,400 locations in 46 countries. The company employs 25,000 people worldwide, with 8800 employees in the United States spread across 44 states. The average United States employee is 43 years old, with an average company tenure of 8.5 years. However, the proportion of long-service employees continues to grow, resulting in the need for long-term health and wellness solutions. In 2013, 29% of the workforce had been employees for 10 years or longer. By January, 2017, 40% of the workforce had 10 years or longer tenure. The majority of employees (74%) are men. The workforce spans several job categories and includes truck drivers, record center specialists, consultants, data center technicians, sales and customer service representatives, and corporate service providers.
All US-based employees and their spouses/domestic partners (if covered on Iron Mountain's health plans) were offered the opportunity to participate in the digital diabetes prevention program if they were determined to be eligible. Program eligibility requirements included: 18 years of age or older; body mass index (BMI) greater than or equal to 24 kg/m2 (or 22 kg/m2 if the person endorses Asian racial identity); able to engage in light physical activity; and at risk for diabetes as evidenced by (a) a blood-based laboratory test in the prediabetic range (fasting blood glucose 100 to 125 mg/dL, hemoglobin A1c 5.7% to 6.4%, or oral glucose tolerance test 140 to 199 mg/dL), or (b) self-reported diagnosis of prediabetes or previous diagnosis of gestational diabetes, or (c) elevated score on a diabetes risk screener.22 Participants were excluded based on the following criteria: already diagnosed with Type 1 or 2 diabetes; on a medically prescribed diet; under treatment for an acute medical/psychiatric condition that would prohibit full participation; currently pregnant or planning to become pregnant; and scheduled for bariatric surgery or recently had bariatric surgery.
INTERVENTION
The Omada Health Program® is a digital adaptation of the DPP lifestyle intervention using its own proprietary curriculum.23 The program consisted of small group support, personalized health coaching, a weekly behavior change curriculum approved by the diabetes prevention recognition program,24 and various online tools, mobile tools, and wireless devices to track eating patterns, physical activity, and body weight. Participants were matched into geographically similar small groups and connected through a private online social network where they could discuss goal progress and provide social support to one another. Trained health coaches were assigned to each group for the duration of the program. The coaches were responsible for monitoring participant progress and lesson completion, and facilitating group discussions. The program allowed participants to asynchronously complete weekly lessons through an online or mobile platform, privately communicate via phone, text, email, or private message with their health coach for individual counseling, track weight loss and physical activity using a wireless weight scale and activity tracker, and view their weight loss progress on any laptop, tablet, or smartphone. The program started with a 16-week curriculum similar to the original DPP, with one new lesson released each week. After the first 16 weeks, the program continued with 36 weeks of additional weekly curriculum lessons focused on the reinforcement of healthful habits, weight maintenance, and relapse prevention.
MEASURES
As part of the organization's employee health and wellness program LiveWell, employees voluntarily completed annual biometric and health risk assessments. These annual measurements served as the basis for the outcome measures.
Annual Biometric Assessments
A corporate wellness provider (ADURO, Inc., Redmond, WA) provided annual biometric assessments and health risk appraisals for the workforce to evaluate clinically valid risk factors for Type 2 diabetes and cardiovascular disease. All tests were administered either at the worksite by an ADURO health professional, by a health care provider at a physician's office, or at a contracted medical laboratory facility. The ADURO staff are required to receive internal certification, complete best-practice guideline training and annual competency reviews, and have a current license as either a phlebotomist, medical assistant, licensed practical nurse, vocational nurse, registered nurse, or nurse practitioner.
Anthropometric Measurements
Body weight was measured under fasting conditions, stocking feet, and light clothing using a Health o Meter Professional Digital Floor Scale (model 800KL, Pelstar LLC, McCook, IL), which measures weight up to 400 pounds. Participants weighing more than 400 lbs were recorded as 400 lbs and noted in the system as weight more than 400 lbs. Height was measured using a calibrated stadiometer with the subject in stocking feet. Waist circumference was measured using a 72-inch cloth measuring tape at the umbilical line over clothing.
Blood Pressure
Blood pressure was measured with a manual sphygmomanometer and stethoscope and with the participant seated in a resting state.
Blood Glucose and Lipids
Participants provided a blood sample via fingerstick to obtain fasting blood glucose and blood lipid measurements. Participants were instructed to fast for 8 hours prior to giving the sample. The fingerstick glucose measure was analyzed using the Cholestech LDX® System (Alere Inc., Waltham, MA). The lipid panel quantified total cholesterol, high-density lipoprotein (HDL), estimated low-density lipoprotein (LDL), and triglycerides, also using the Cholestech LDX®.
Self-report Health Risk Appraisal
Health risk appraisals (HRA) were completed by employees using a validated and reliable survey system (Limeade®, Bellevue, WA) that assesses psychological health, well-being, health behaviors or habits, and perceived workplace productivity. The 107 items on the instrument are rated on a scale of 1 to 5, with higher scores indicating better health or health behavior patterns. A summary score and subscores are calculated by taking the combined average of relevant items clustered in different well-being dimensions, including physical activity level, healthful nutritional intake, overall health, and well-being. Cronbach α for subscales range from 0.56 to 0.97 for the various dimensions.25 The assessment was administered via the online survey tool through the employer's organizational wellness platform.
Program Participation
The digital health program software platform captured completion of curriculum lessons (paced at a weekly frequency) and weigh-ins on the wirelessly connected scale, which recorded weights every time the participant stepped on the scale. Each curriculum lesson was given a score of 0 (zero) if it was not completed during a week, or 1 if the lesson was completed.
ANALYSIS PLAN
Data Preparation
The data were merged from three sources: biometric assessments, the self-reported HRA, and program weight and lesson completion data. Of the 829 participants who began the Omada program, 764 could be linked to HRA/biometric data from 2013, 2014, or 2015. Of these 764 IDs, 634 had records in either or both of the HRA and biometric datasets for both 2014 and 2015. Because changes in risk factors from 2014 to 2015 were of primary interest, 634 Omada participants were retained for the primary analyses.
For the 7,026 people with data from 2014 to 2015 in one or both of the Aduro datasets, approximately 9.7% of data were missing across 42 variables, with the degree of missingness per variable ranging from 0% to 33%. We carried out 10 imputations of missing data using the R package Amelia II.26 Given a missing datum for person i, variable j, and year k, Amelia makes imputations on the basis of (a) data from person i and year k, for variables other than j, as well as (b) data from person i and variable j for years other than k. For the across-time imputations (b), we set Amelia to use data from 2013 where available and to fit polynomials of order 2 for making predictions. We set Amelia's empirical ridge parameter to 4% of the number of rows in the dataset, and we set variables with discrete data to be ordinal.
Identifying Matched Comparison Participants
The R package Matchit27,28 was used to identify a matched sample for comparison with program participants. Though employees selected for the matching did not participate in Omada, they may have been eligible for other health and wellness benefits from the employer. The sample was matched on age, sex, and employee/dependent status, as well as on pretreatment (data from 2014) body mass index, total cholesterol, LDL cholesterol, systolic and diastolic blood pressure, fasting blood glucose, triglycerides, and waist circumference. We identified a comparison sample matched on these covariates that was twice as large as the Omada sample (n = 634 × 2 = 1268). The matching was performed in one of the 10 imputed datasets, but the identified sample matched the Omada participants well in all 10 datasets. Across all 10 imputed datasets, the two groups did not differ on any of the 11 covariates (Wilcoxon Ps > 0.19). The largest standardized difference between Omada and comparison cases across the 10 imputed datasets (where “standardized difference” indicates mean difference divided by standard deviation in the control group) was 0.074.
Analytic Strategy
Primary outcomes included changes in weight, BMI, and fasting blood glucose from the year prior to commencement of the program to the year following the program. Secondary outcomes included changes in blood pressure, lipids, waist circumference, health behaviors, and perceived health over the same period of time. For our main analyses, we used generalized estimating equations (GEEs) as implemented in the R package gee, which can account for correlations among observations from the same person at different times. We used an exchangeable covariance matrix and robust Huber-White standard errors. For primary analyses (BMI, fasting blood glucose, and weight), we compared GEE results with those of linear mixed models. The results were found to be entirely parallel, so we report only the GEE results. Analyses were completed in all 10 imputed datasets and pooled using Rubin's rules.29,30
Results
A total of 963 people completed the enrollment process, of which 829 (86.0%) began the program. Of the participants who enrolled in the digital health program, 58.4% were women and 68% were white, 14% were black/African American, and 9% were Latino. Participants ranged in age from 23 to 68 years old, with a median age of 46 years. The average initial BMI of the workforce was 34.5 kg/m2, which was in the obese category (BMI is more than 30). Average waist circumference was in the high risk range (waist circumference is more than 105 cm for men and more than 88 cm for women31). The average resting blood pressure, fasting blood glucose, and lipids levels were within normal limits (see preintervention values on Tables 1 and 2). When the sample was examined for those with risk factor elevations consistent with prediabetes and metabolic syndrome,32,33 approximately 22% had fasting blood glucose more than 100 mg/dL; 31% of the sample had systolic blood pressure more than 130 mmHg; 25% had diastolic blood pressure more than 85 mmHg; 37% of the sample had total cholesterol more than 200 mg/dL; 38% had triglycerides more than 150 mg/dL; 48% of men and 44% of women had low HDL cholesterol (less than 40 mg/dL for men, less than 50 mg/dL for women).
TABLE 1.
Changes in the Primary Outcomes Before and After the Intervention
| Variable | Preintervention Estimate (SE) | Postintervention Estimate (SE) | Intervention-Control Difference in Trajectory Estimate (SE) |
| Weight (pounds) | |||
| Intervention group | 215.7 (1.9) | 213.7 (1.9) | −3.4 (1.4)* |
| Control group | 214.8 (1.6) | 216.1 (1.6) | |
| BMI (kg/m2) | |||
| Intervention group | 33.9 (0.3) | 33.4 (0.3) | −0.60 (0.18)** |
| Control group | 33.7 (0.2) | 33.9 (0.2) | |
| Fasting blood glucose (mg/dL) | |||
| Intervention group | 95.2 (1.0) | 93.7 (0.9) | −2.6 (1.1)* |
| Control group | 96.4 (0.8) | 97.5 (0.8) | |
| Reduction in BMI category (%) | |||
| Intervention group | 22%** | ||
| Control group | 15% | ||
Estimates combined from 10 generalized estimating equations (GEE) fitted to multiple imputed datasets. Models adjusted for age and sex, which were mean-centered.
BMI, body mass index; SE, standard error.
*P = 0.05.
**P = 0.001.
TABLE 2.
Changes in the Secondary Outcomes Before and After the Intervention
| Variable | Intervention Group Mean (SE) | Control Group Mean (SE) | |
| Systolic BP (mmHg) | Pretest | 122.9 (0.6) | 123.2 (0.4) |
| Post-test | 124.2 (0.6) | 124.3 (0.4) | |
| Diastolic BP (mmHg) | Pretest | 79.3 (0.4) | 79.3 (0.3) |
| Post-test | 79.4 (0.4) | 79.2 (0.3) | |
| Total cholesterol (mg/dL) | Pretest | 191.0 (1.5) | 191.0 (1.1) |
| Post-test | 186.4 (1.5) | 188.7 (1.1) | |
| HDL cholesterol (mg/dL) | Pretest | 48.2 (0.5) | 49.4 (0.4) |
| Post-test | 48.2 (0.5) | 49.0 (0.4) | |
| Triglycerides (mg/dL) | Pretest | 145.8 (3.6) | 147.5 (3.0) |
| Post-test | 139.9 (4.0) | 143.0 (2.5) | |
| Waist circumference** (cm) | |||
| Women | Pretest | 100.3 (0.8) | 99.6 (0.8) |
| Post-test | 99.3 (1.0) | 99.8 (0.8) | |
| Men | Pretest | 105.7 (1.0) | 106.7 (0.8) |
| Post-test | 104.6 (1.0) | 106.9 (0.8) | |
| Overall health | Pretest | 2.89 (0.03) | 2.74 (0.03) |
| Post-test | 2.86 (0.03) | 2.75 (0.03) | |
| Overall physical condition | Pretest | 3.76 (0.01) | 3.76 (0.01) |
| Post-test | 3.77 (0.01) | 3.76 (0.01) | |
| Nutrition* | Pretest | 3.72 (0.03) | 3.80 (0.02) |
| Post-test | 3.88 (0.03) | 3.83 (0.02) | |
| Exercise and fitness | Pretest | 3.42 (0.03) | 3.48 (0.02) |
| Post-test | 3.47 (0.03) | 3.47 (0.02) | |
Estimates combined from 10 generalized estimating equations (GEE) fitted to multiple imputed datasets. Models adjusted for age and sex, which were mean-centered.
BMI, body mass index; SE, standard error.
*P = .0.001.
**P = 0.09.
Program Participation
Across all program enrollees, a total of 775 participants (94.2%) completed at least four curriculum lessons during the intensive phase of the program (ie, the first 16 weeks), and 685 participants (82.6%) completed at least nine lessons. On average, participants completed 19.7 lessons over the course of the year.
Biometric and Self-report Health Risk Appraisal
Of the 829 participants in the program, 634 had a sufficient biometric or self-report data for analysis. The matched comparison group was constructed from 1,268 employees and dependents who did not participate in the intervention. As illustrated in Fig. 1, the average weight in the workforce increased by approximately 3.5 pounds from 2013 to 2014. From 2014 to 2015 when the program was underway, those who participated in the intervention did not continue to gain weight. The average weight in the non-treated comparison group increased by approximately a pound. Adjusting for age and sex, the pre-post difference in weight was significantly different by group (β = −3.4 lbs, standard error [SE] = 1.4 lbs, Wald Z = 2.5, P = 0.01), with comparison group gaining more weight relative to the intervention group's trajectory. A total of 31% of Omada participants lost at least 5% of their starting body weight, compared with 20% of controls (adjusting for age, sex, and baseline weight, logistic β = 0.59, SE = 0.13, t = 4.6, P = 0.001, see Fig. 2). Approximately, 22% of intervention participants dropped one or more BMI category (ie, from overweight to normal weight, or from obese to overweight, etc.), compared with 15% of matched controls, which was significant after adjusting for age, sex, and baseline BMI (logistic β = 0.48, SE = 0.14, t = 3.4, P = 0.001). Program participants experienced an average reduction of 1.49 mg/dL in fasting blood glucose, whereas controls had an increase of 1.15 mg/dL (adjusting for age and sex, β = −2.6, SE = 1.1, Wald Z = −2.4, P = 0.02).
FIGURE 1.
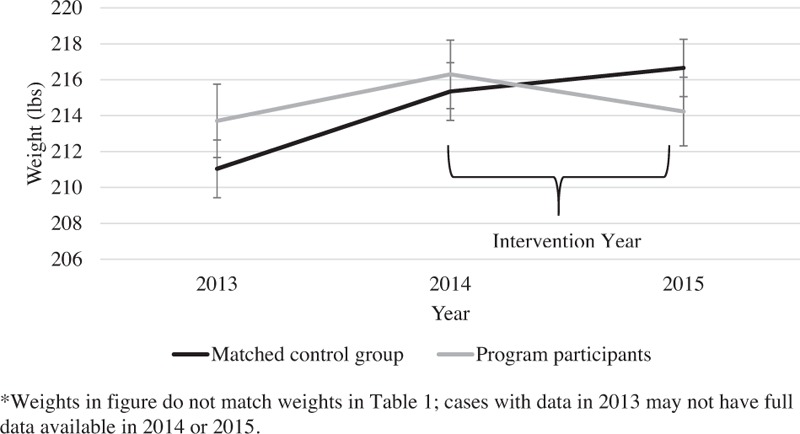
Trends in weight gain and loss year by year.
FIGURE 2.
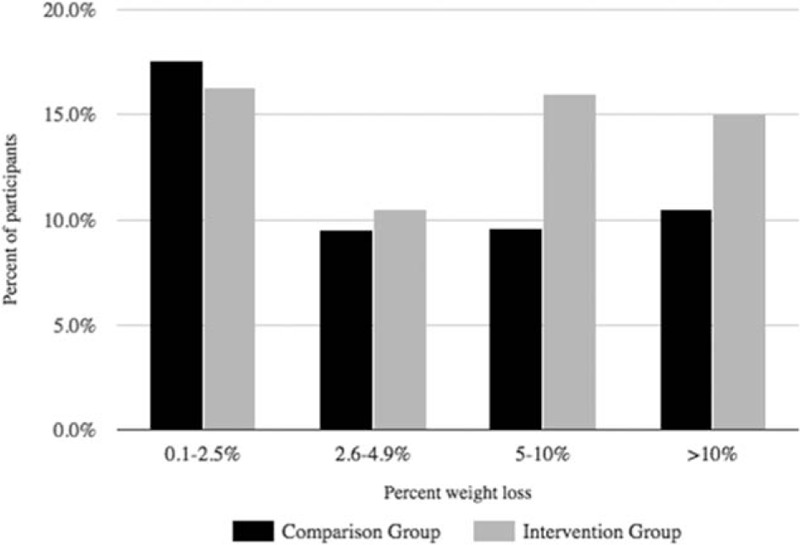
Percent weight change from 2014 to 2015.
There were no significant differences between controls and intervention participants in the change from pre- to post-treatment measures for systolic and diastolic blood pressure, total cholesterol, LDL, HDL, or triglycerides. Though not statistically significant, waist circumference decreased by 1 cm in participants and increased by 0.2 cm in controls (adjusting for age and sex, β = −0.49, SE = 0.26, Wald Z = −1.85, P = 0.06). Participants improved their nutritional intake score by 0.16 points, compared with a smaller increase of 0.03 points in controls (adjusting for age and sex, β = 0.13, SE = 0.03, Wald Z = 4.0, P = 0.001).
Post Hoc Analyses
Among Omada program participants, we evaluated whether weight loss (as assessed by the difference between participants’ first and last weigh-in on the Omada scale) was associated with changes in other biometric or HRA measures. As measured by the Omada scale, program participants lost on average 4.6% (SD = 5.0%) of initial body weight at 16 weeks. After adjusting for age and sex, weight loss was associated with decreases in total cholesterol (β = 0.41, SE = 0.11, Wald Z = 3.6, P = 0.001), LDL (β = 0.27, SE = 0.10, Wald Z = 2.7, P = 0.001), and triglycerides (β = 0.75, SE = 0.32, Wald Z = 2.4, P = 0.02); no associations were detected with other risk factors.
The Diabetes Prevention Recognition Program emphasizes a goal for participants to complete at least 9 of the weekly curriculum lessons during the intensive phase of the program.34 With this in mind, we conducted post-hoc analyses of those who completed at least 9 of 16 lessons (completers) compared with those who completed fewer than 9 lessons (non-completers). In general, program completers (n = 540) had better outcomes than non-completers (n = 94). Completers lost more weight (β = 8.2, SE = 2.4, Wald Z = 3.4, P = 0.001) and reduced waist circumference (β = 1.5, SE = 0.5, Wald Z = 2.9, P = 0.004) more than non-completers, and experienced better improvements in nutrition (β = 0.32, SE = 0.08, Wald Z = 3.8, P = 0.001) and exercise (β = 0.27, SE = 0.08, Wald Z = 3.2, P = 0.001).
DISCUSSION
Overall, the digital health program was effective at reducing the risk factors for diabetes and cardiovascular disease by reducing weight and blood glucose in this workforce sample. Participants in the intervention program lost a significant amount of body weight, while non-participants continued to gain weight. Though the observed decreases in fasting blood glucose levels and waist circumference were modest among program participants, all measures moved in the desired direction and were indicative of progress towards better health and reduced risk. Additionally, a significant percentage (30%) of program participants lost a meaningful amount of weight (>5%, according to the Diabetes Prevention Recognition Program Standards).34 The comparison group's year after year weight differences indicated more weight gain relative to the intervention group. Both weight loss and prevention of weight gain are important objectives for diabetes risk reduction,35 and thus these findings help to validate the effectiveness of the program in inducing weight loss and preventing weight gain.
The majority of program participants (85%) completed the bulk of the program curriculum lessons. This level of engagement suggests that digital and mobile platforms are a feasible and accessible method for receipt of intensive behavioral counseling services among a diverse and dispersed workforce. The finding that greater program engagement was related to greater changes in the targeted health behaviors (diet and exercise) lends further credibility, as people transformed their learnings into expected behavior changes. Taken together, these results provide further support for the application of digital behavior change programs for workforce chronic disease prevention.
Even the most committed organizations have limitations on the number of health professionals that can be employed to drive wellness efforts. The problem is magnified in large organizations with multiple worksites, telecommuting employees, traveling employees, and off-site employees. Organizations are looking for the best way to maximize their resources while reaching the largest number of employees possible. Digital health programs provide the needed flexibility to simultaneously enroll a large number of employees without encountering scheduling issues or other logistical obstacles, making the digital format a promising solution to increase the reach of health promotion programs. Digital programs also have the benefit of being easy to implement. These factors are critical to the adoption and continued use of the programs.
Though the study findings are encouraging, the results should be interpreted with caution. All employees throughout the workforce had access to additional corporate-sponsored wellness programs during the 2014 to 2015 time frame. This may have affected the magnitude of the studied program's effects. Participation in other programs by the comparison group members may have concealed some between-group differences. The study sample consisted of individuals who self-selected into the digital health program, which could bias the sample towards better outcomes. However, this was an observational study of how corporate wellness and risk reduction programs operate under ecologically valid conditions, with employees exercising freedom to opt into programs that may benefit them. Whereas the non-randomized, non-controlled setting in which this study took place limits causal inference, it may reflect the real-world implications and outcomes of offering an online diabetes prevention program in a workplace. The use of a matched control group also provided an indicator of natural trends in the workforce over time. Further research is needed to determine the effects of these programs on long-term outcomes, such as health care utilization and organizational costs. Despite these limitations, the program participants successfully made meaningful lifestyle changes to reduce the risk of chronic disease through weight loss, prevention of weight gain, improved glucose control, and better nutritional intake.
In conclusion, this study provides encouraging evidence that digital lifestyle intervention programs can be successfully delivered in worksite settings, and can achieve results in chronic disease risk factor reduction via weight loss, prevention of weight gain, and improved biometric indicators. These findings support the feasibility of utilizing digital health programs in the workplace, and should encourage expanded use of digital health formats in workplaces with dispersed and mobile members. Effective options for scalable and flexible chronic disease prevention programs will give greater choice and access to workers, and help to improve the health of workforces. Future research will be able to examine the long-term impact of programs on subsequent delay of disease onset or progression, and eventual changes in health care utilization, workplace productivity, and related long-term outcomes.
Acknowledgments
The authors thank Christian Potts at Iron Mountain, Crystal Hammond at Mercer Health and Benefits, LLC, Scott Durbin and Krista Fink at Aduro, Inc., Matt Cook, Kristen Sherman, and Alicia Anderson at Omada Health, Inc. and the Iron Mountain workforce for their contributions to this work.
Footnotes
Sources of Support: Omada Health, Inc. sponsored the work and is the maker of the Omada Health Program®.
Conflicts of Interest: Dr. Castro Sweet and Mr. Carpenter are employed by Omada Health and receive salary and stock options. Ms. Madero is employed by Omada Health and receives a salary. Drs. Wilson and Edge received consultation fees from Omada Health for their contributions. Iron Mountain, Inc. is a business partner of Omada Health. Mr. Kirschner is employed by Iron Mountain and receives salary, bonus and restricted stock units. Ms. Pilsmaker is employed by Iron Mountain and receives salary and bonus. Mercer, LLC is a business associate of Omada Health; Ms. McGuire is employed by Mercer and receives salary and bonus.
REFERENCES
- 1.Hertz RP, Unger AN, McDonald M, Lustik MB, Biddulph-Krentar J. The impact of obesity on work limitations and cardiovascular risk factors in the U.S. workforce. J Occup Environ Med 2004; 46:1196–1203. [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999; 282:1523–1529. [DOI] [PubMed] [Google Scholar]
- 3.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes 2010; 3:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Østbye T, Dement JM, Krause KM. Obesity and workers compensation: Results from the Duke Health and Safety Surveillance System. Arch Intern Med 2007; 167:766–773. [DOI] [PubMed] [Google Scholar]
- 5.Goetzel RZ, Gibson TB, Short ME, et al. A multi-worksite analysis of the relationships among body mass index, medical utilization, and worker productivity. J Occup Environ Med 2010; 52 Suppl:S52–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JS, O’Connor EA, Evans CV, et al. Behavioral Counseling to Promote a Healthy Lifestyle for Cardiovascular Disease Prevention in Persons With Cardiovascular Risk Factors: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 113. AHRQ Publication No. 13–05179-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 8.Kriska AM, Delahanty LM, Pettee KK. Lifestyle intervention for the prevention of type 2 diabetes: translation and future recommendations. Curr Diab Rep 2004; 4:113–118. [DOI] [PubMed] [Google Scholar]
- 9.Aldana S, Barlow M, Smith R, et al. A worksite diabetes prevention program: two-year impact on employee health. AAOHN J 2006; 54:389–395. [DOI] [PubMed] [Google Scholar]
- 10.Barham K, West S, Trief P, Morrow C, Wade M, Weinstock RS. Diabetes prevention and control in the workplace: a pilot project for county employees. J Public Health Manag Pract 2011; 17:233–241. [DOI] [PubMed] [Google Scholar]
- 11.Dallam GM, Foust CP. A comparative approach to using the diabetes prevention program to reduce diabetes risk in a worksite setting. Health Promot Pract 2013; 14:199–204. [DOI] [PubMed] [Google Scholar]
- 12.Giese KK, Cook PF. Reducing obesity among employees of a manufacturing plant: translating the Diabetes Prevention Program to the workplace. Workplace Health Saf 2014; 62:136–141. [DOI] [PubMed] [Google Scholar]
- 13.Kramer MK, Molenaar DM, Arena VC, et al. Improving employee health: evaluation of a worksite lifestyle change program to decrease risk factors for diabetes and cardiovascular disease. J Occup Environ Med 2015; 57:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinhold KR, Miller CK, Marrero DG, Nagaraja HN, Focht BC, Gascon GM. A randomized controlled trial translating the Diabetes Prevention Program to a university worksite, Ohio, 2012-2014. Prev Chronic Dis 2015; 12:E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson MG, DeJoy DM, Vandenberg R, Padilla H, Davis M. FUEL Your Life: A translation of the diabetes prevention program to worksites. Am J Health Promot 2016; 30:188–197. [DOI] [PubMed] [Google Scholar]
- 16.Sepah CS, Jiang L, Peters AL. Translating the diabetes prevention program into an online social network: validation against CDC standards. Diabetes Educ 2014; 40:435–443. [DOI] [PubMed] [Google Scholar]
- 17.Silberman J, Schwartz S, Giuseffi DL, Wang C, Nevedal D, Bedrosian R. Reductions in employee productivity impairment observed after implementation of web-based worksite health promotion programs. J Occup Environ Med 2011; 53:1404–1412. [DOI] [PubMed] [Google Scholar]
- 18.Hunter CM, Peterson AL, Alvarez LM, et al. Weight management using the internet. A randomized controlled trial. Am J Prev Med 2008; 34:119–126. [DOI] [PubMed] [Google Scholar]
- 19.Petersen R, Sill S, Lu C, Young J, Edington DW. Effectiveness of employee internet-based weight management program. J Occup Environ Med 2008; 50:163–171. [DOI] [PubMed] [Google Scholar]
- 20.Buis L. The potential for web-based social network sites and self-regulation for health promotion. Am J Health Promot 2011; 26:73–76. [DOI] [PubMed] [Google Scholar]
- 21.Kwon BC, Hur I, Yi JS. A review of web-based dietary interventions: From the human-computer interaction practitioners’ perspective. Hum Fact Ergon Manuf Serv Ind 2014; 24:241–261. [Google Scholar]
- 22.Bullard KM, Williamson DF, Imperatore G, Gregg EW. Identification of US adults with pre-diabetes or undiagnosed diabetes using a simple risk score. Diabetes 2011; 60 Suppl:A357. [Google Scholar]
- 23.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999; 22:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the US: the National Diabetes Prevention Program. Am J Prev Med 2013; 44:S346–S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limeade Well-Being Assessment Technical Report. Technical Report. Bellevue, WA: Limeade Institute, March; 2015. [Google Scholar]
- 26.Honaker J, King G, Blackwell M. Amelia II: A program for missing data. J Stat Softw 2011; 45:1–47. [Google Scholar]
- 27.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007; 15:199–236. [Google Scholar]
- 28.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42: doi: 10.18637/jss.v042.i08. [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 30.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002; 7:147–177. [PubMed] [Google Scholar]
- 31.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. NIH Publication No. 98-4083. Bethesda, MD: National Institutes of Health; 1998. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf Accessed August 12, 2016. [Google Scholar]
- 32.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109:433–438. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2016; 39 suppl:S13–S22. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Diabetes Prevention Program Standards and Operating Procedures. 2015; Atlanta, GA: Centers for Disease Control and Prevention, Retrieved from http://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf. Accessed July 8, 2016. [Google Scholar]
- 35.Feldman AL, Griffin SJ, Ahern AL, et al. Impact of weight maintenance and loss on diabetes risk and burden: a population-based study in 33,184 participants. BMC Public Health 2017; 17:170. [DOI] [PMC free article] [PubMed] [Google Scholar]


