Abstract
Patient-provider communication is modifiable and linked to diabetes outcomes. The association of communication quality with medical mistrust is unknown. We examined these factors within the context of a low-literacy/numeracy-focused intervention to improve diabetes care, using baseline data from diverse patients enrolled in a randomized trial of a health communication intervention. Demographics, measures of health communication (Communication Assessment Tool [CAT], Interpersonal Processes of Care [IPC-18]), health literacy (Short Test of Functional Health Literacy in Adults [s-TOFHLA]), depression, medical mistrust, and glycemic control were ascertained. Adjusted proportional odds models were used to test the association of mistrust with patient-reported communication quality. The interaction effect of health literacy on mistrust and communication quality was also assessed. A total of 410 patients were analyzed. High levels of mistrust were observed. In multivariable modeling, patients with higher mistrust had lower adjusted odds of reporting higher CAT score [adjusted odds ratio (AOR) 0.67 [95% CI: 0.52–0.86], p=0.003], and higher score for the Communication [AOR 0.69 [0.55–0.88], p=0.008], Decided Together [AOR 0.74 [0.59–0.93], p=0.02], and Interpersonal Style [AOR 0.69 [0.53–0.90], p=0.015] subscales of the IPC-18. We observed evidence for an interaction effect of health literacy for the association between mistrust and the Decided Together subscale of the IPC-18 such that patients with higher mistrust and lower literacy perceived worse communication relative to mistrustful patients with higher literacy. In conclusion, medical mistrust was associated with poorer communication with providers in this public health setting. Patients’ health literacy level may vary the effect of mistrust on the interactional aspects of communication. Providers should consider the impact of mistrust on communication with vulnerable diabetes populations and focus efforts on mitigating its influence.
Patient-provider communication is an important and potentially mutable component of high quality care for patients with diabetes. Sub-optimal communication is associated with less frequent self-care behaviors (e.g. medication adherence, diet/exercise), lower treatment satisfaction, and worse glycemic control (Bauer et al., 2014; Piette, Schilinger, Potter, & Heisler, 2003; White et al., 2015; White, Osborn, Gebretsadik, Kripilani, & Rothman, 2013). Several factors may influence both the perceived and actual quality of the patient-provider interaction including the skill level of the provider, the complexity and length of the encounter, the clinic environment, and patient-level factors such as health literacy and language proficiency (Nam, Chesla, Stotts, Kroon, & Janson, 2011; Pandit et al., 2014; Sarkar et al., 2008). Mistrust in the U.S. healthcare system is an under recognized factor known to influence health-related quality of life, self-care behaviors, and treatment adherence for vulnerable populations including those with diabetes (Bhattacharya, 2012; Egede & MIchel, 2006; Vest et al., 2013). Depression is also a common and often under-recognized comorbid condition among patients with diabetes (Naranjo, Fisher, Areán, Hessler, & Mullan, 2011; Naranjo, Hessler, Deol, & Chesla, 2012) that independently influences perceptions of provider cultural competency (Seligman et al., 2012), patients’ willingness to discuss self-care (Beverly et al., 2012), and glycemic control (Lustman et al., 2000). Providers who care for diabetes patients in safety net settings often face limitations of resources at the practice and patient level that may influence care processes and interpersonal interactions. Vulnerable patients in theses settings can experience communication gaps with providers as a result of the complex interplay of health literacy, resource limitations, and psychosocial factors such as mistrust (Duru et al., 2009; Egede & Michel, 2006; Varkey et al., 2009). Although provider communication and mistrust have shown independent associations with diabetes-related outcomes, the influence of mistrust on patient perceptions of provider communication in the context of diabetes has limited evidence (Cousin, Schmid, Mast, & Jaunin-Stalder, 2013; Peek et al., 2010).
We examined the association of medical mistrust with perceptions of communication quality with providers for diabetes patients enrolled in a clinical trial. We expected that patients reporting higher levels of medical mistrust would perceive lower quality of communication with their provider. As a secondary and pre-specified hypothesis we anticipated that the effect of mistrust on perceptions of communication quality would vary by health literacy level such that patients with mistrust and lower literacy would perceive poorer communication quality relative to patients with mistrust and higher literacy.
Methods
Design and Setting
Baseline data from the Partnership to Improve Diabetes Education (PRIDE) study was used to assess the aforementioned association. Patients seeking care at one of 10 state health department clinics across the Mid-Cumberland region of TN were enrolled into PRIDE, a cluster randomized controlled trial to evaluate the efficacy of a literacy-sensitive intervention on glycemic control. Providers at 5 intervention sites received training in effective health communication and on the use of the English and Spanish versions of the PRIDE Toolkit, a comprehensive, literacy-sensitive educational aid that covers a spectrum of self-care and management topics for diabetes patients (Wolff et al., 2016). Providers at control sites received exposure to updated National Diabetes Educational Program (NDEP) materials without the provision of additional education in effective health communication including how to interact with low literacy populations. Study approval was obtained from the institutional review boards of the Tennessee Department of Health (TDOH) and Vanderbilt University, with CME/CMU credits provided for participating TDOH providers and staff during trainings.
Participants
Participants were recruited during regular hours at health department clinics and from communications/referrals from staff. English and/or Spanish-speaking adults aged 18–85 years, with diagnosed Type 2 diabetes, and A1C ≥ 7.5% (i.e. an indicator of poor glycemic control) were eligible for participation. Patients were excluded for poor visual acuity (>20/50 on Rosenbaum Pocket Screen), clinically significant dementia or psychosis, or life expectancy less than the 2 year study duration. Participants were given $20 following baseline data collection for remuneration of their time and participation.
Data collection
Following the collection of verbal and written consent by bilingual research staff and in the participant’s preferred language, each participant provided socio-demographic data including age, gender, race/ethnicity, education, income, and insurance status. Participants also completed surveys to assess their health literacy status (Short Test of Functional Health Literacy in Adults (s-TOFHLA)), level of medical mistrust (Medical Mistrust Index (MMI)), symptoms of depression (Center for Epidemiologic Studies-Depression Scale (CES-D)), and perceptions of provider communication (Communication Assessment Tool (CAT), Interpersonal Processes of Care (IPC-18)) in general and after their baseline visit. Pertinent clinical data were extracted from the medical record and recorded (A1C, insulin use, years since diagnosis, body mass index (BMI), low density lipoprotein (LDL), and blood pressure (BP))
Measures
Exposure variables
Health literacy level was measured using the Short Test of Functional Health Literacy in Adults (s-TOFHLA). The s-TOFHLA ranges from scores of 0–36 and identifies individuals with adequate (≥23), marginal (Baker, Williams, Parker, Gazmararian, & Nurss, 1999; Cousin et al., 2013; Duru et al., 2009; Hann, Winter, & Jacobsen, 1999; Peek et al., 2010; Wolff et al., 2016), and inadequate (≤16) health literacy skills (Baker et al., 1999). Depression was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D). Scores range from 0–60 with higher scores indicating greater symptoms of depression. Scores ≥ 16 are often used as a clinically significant cut-point for a positive screen (Hann et al., 1999). Medical mistrust was measured using the Medical Mistrust Index, a validated instrument that measures general mistrust of the medical care system and has been tested in multi-ethnic populations. Average scores range from 0–4 with scores ≥2 indicating greater levels of mistrust (LaVeist, Isaac, & Williams, 2009). Glycemic control was ascertained via chart review and documentation of the participant’s glycated hemoglobin level (A1C) at enrollment which reflects average blood glucose levels over the preceding 3 months.
Outcome variables
The Communication Assessment Tool (CAT) and Interpersonal Processes of Care (IPC-18) measure are patient-reported assessments of provider communication quality that have been validated in multi-ethnic samples. Details of psychometric testing demonstrating adequate reliability and validity for each scale have been published previously (Makoul, Krupat, & Chang, 2007; Stewart, Nápoles-Springer, & Pérez-Stable, 1999). Each instrument evaluates the interpersonal and technical aspects of provider communication. The IPC-18 was administered before the baseline encounter and was summarized using three broad subscales as suggested by Stewart et al.: Communication (7 questions) includes the subscales “lack of clarity” (reverse scored), “elicitation of concerns,” and “explanation of results;” Decided Together (2 questions) reflects the subscale “working together;” and Interpersonal Style (5 questions) comprises the subscales “discriminated due to race/ethnicity” (reverse scored) and “compassionate” (Stewart et al., 1999; Stewart, Nápoles-Springer, Gregorich, & Santoyo-Olsson, 2007). The CAT (14 questions) was administered after the baseline encounter (Makoul et al., 2007). Average scores ranging from 0–5 were reported for each measure, and questions pertaining to office staff (2 questions) were excluded so as to isolate patient perceptions of provider communication only.
Statistical analysis
Demographic, survey, and clinical data are summarized with the median and interquartile range for continuous variables and with percentages for categorical variables. To account for the skewed distribution of responses to the surveys assessing communication quality (CAT, IPC-18), we conceptualized responses for these measures such that scores of 0–4 were considered to represent poorer communication and scores of 5 represented higher communication quality. This was done for uniformity across measures and to be consistent with how other studies have reported these outcomes (Fernandez et al., 2004; Schenker, Stewart, Na, & Whooley, 2009; Schillinger, Bindman, Wang, Stewart, & Piette, 2004). We also assessed the internal consistency of the CAT and each of the aforementioned subscales of the IPC-18 using Cronbach’s alpha for our sample.
We used proportional odds to model simultaneously the association between our main exposure of interest (i.e. medical mistrust) with the CAT total score and with each of the IPC-18 subscales (Communication, Decided Together, and Interpersonal Style). The referent group for each communication outcome was set at 5 representing higher communication quality, and to quantify the association between mistrust and communication quality we used odds ratios for better communication associated with an interquartile range change in medical mistrust score. To control for the potential for confounding, all 4 models were adjusted for age, race/ethnicity, gender, income, health literacy level, diabetes duration, study status, glycemic control (A1C), and depressive symptoms (CES-D). Diabetes duration was selected as a confounder based upon the idea that over time patient’s skills with self-management may improve and thus influence their attitudes towards their provider and/or care received. Study status was chosen as a potential confounder due to the fact that some intervention sites for the main study had begun to receive training in health communication prior to the final collection of all baseline data. Depressive symptoms and glycemic control were included in each model as additional confounders due to the significant correlation between depression and mistrust in the sample and the potential impact of glycemic control on perceptions of provider communication. Finally, for each model an additional health literacy x mistrust variable was added to test whether the association of mistrust and communication quality varied by health literacy status. We report the adjusted odds ratio (AOR) for each model with 95% confidence intervals with p-values ≤0.05 being considered statistically significant for all tested associations and interactions. All analyses were performed using the R version 3.2.1 (R Foundation for Statistical Computing, 2015)
Results
From July 2011 through April 2013, 410 patients were eligible and agreed to enrollment out of 573 patients approached (71.5%). Participants were middle aged (49.6 ± 9.4 years), predominantly female (60%), and represented White (63%), Black (18%), and Hispanic (24%) racial/ethnic groups. Most had modest educational attainment and low income with 36% not completing high school, and only 18% earning more than $20k annually. The vast majority of participants were uninsured (96%). Despite evidence of modest educational attainment across the sample, only 17% had scores suggestive of marginal or inadequate health literacy based on the s-TOFHLA. High levels of medical mistrust were observed in 87% of participants, and the median depression score was 16 [8, 26] suggesting that symptoms of depression were common in the sample. Mistrust and depression scores were modestly correlated (r=0.17; p=0.001) but neither correlated significantly with glycemic control. Median scores on each of the communication outcome variables were 4.5 or higher with patients reporting the lowest scores for the Decided Together subscale of the IPC-18. Median A1C was 9.1 [7.5, 12.5] indicative of poor glycemic control, and BMI was 34.4 [26.3, 46.7] with median blood pressure and LDL near goal based on recently modified treatment guidelines (American Diabetes Association, 2015) (Table 1). Cronbach’s alphas for the communication outcome variables were as follows: CAT (0.98), IPC-18 Communication (0.71), IPC-18 Decided Together (0.65), and IPC-18 Interpersonal Style (0.35).
Table 1.
Patient Characteristics and diabetes-related factors
| Variable | Median [IQR] or n (%) |
|---|---|
|
| |
| N=410 | |
|
| |
| Demographics | |
|
| |
| Age | 51 [36.0, 61.0] |
|
| |
| Gender | |
| Female | 248 (60) |
|
| |
| Race | |
| White | 259 (63) |
| Black | 72 (18) |
| Other | 79 (19) |
|
| |
| Hispanic | |
| Yes | 98 (24) |
|
| |
| Education | |
| Less than HS | 148 (36) |
| HS grad/equivalent | 144 (35) |
| Some college or beyond | 116 (28) |
|
| |
| Health Literacy (s-TOFHLA) | |
| Adequate | 334 (83) |
| Inadequate + Marginal (Limited) | 68 (17) |
|
| |
| Household Income | |
| <$10,000 | 218 (54) |
| $10,000–19,999 | 116 (29) |
| >$20,000 | 72 (18) |
|
| |
| Insurance Status | |
| Uninsured | 390 (96) |
| Medicaid (Tenncare) | 10 (2.5) |
| Medicare | 7 (1.7) |
|
| |
| Measures of Patient-Provider Interaction & Psychosocial factors | |
|
| |
| Medical Mistrust (0–4) | 2.6 [2.0, 3.1] |
| High (≥2) | 344 (87) |
|
| |
| Depressive Symptoms (CESD) [0–36] | 16 [4.0, 37.0] |
|
| |
| CAT Median Score (1–5) | 4.9 [1.0, 5.0] |
| <5 | 209 (51) |
|
| |
| IPC-18 (1–5) | |
| Communication Median Score (1–5) | 4.7 [3.6, 5.0] |
| <5 | 263 (64) |
| Decision Together Median Score (1–5) | 4.5 [3.0, 5.0] |
| <5 | 231 (56) |
| Interpersonal Style Median Score (1–5) | 5.0 [4.0, 5.0] |
| <5 | 166 (41) |
|
| |
| Diabetes Characteristics | |
|
| |
| A1C | 9.1 [7.5, 12.5] |
|
| |
| Insulin use | |
| Yes | 243 (59) |
|
| |
| Time since diagnosis, years | 7.5 [1.0, 19.5] |
|
| |
| BMI (kg/m2) | 34.4 [26.3, 46.7] |
|
| |
| LDL (mg/dl) | 100 [55.0, 148.0] |
|
| |
| Blood Pressure | |
| Systolic | 132 [110.0, 158.0] |
| Diastolic | 80 [69.0, 92.0] |
Using proportional odds models (Table 2), Model 1 assessed the association of mistrust with scores on the Communication Assessment Tool (CAT). After adjustment for potential confounders, higher mistrust was associated with lower CAT scores. The adjusted odds ratio (AOR) for reporting a higher CAT score per IQR range change in medical mistrust was 0.67 ([95% CI: 0.52–0.86]; p=0.003). This relationship was observed to be non-linear in that the magnitude of the effect of mistrust on perceptions of communication quality was most pronounced at lower levels of mistrust (Figure 1). In this model race (Black vs. White) emerged as an independent predictor of poorer communication as well (AOR 0.44 [0.25–0.75]; p=0.006).
Table 2.
Adjusted Odds of Higher Communication Quality for Patients with Increasing Medical Mistrust, Black race, and greater duration of diabetes.
| Exposure Variables | Outcome Variables | |||
|---|---|---|---|---|
| Model 1: CAT | Model 2: Communication Subscale (IPC-18) | Model 3: Decided Together Subscale (IPC-18) | Model 4: Interpersonal Style Subscale (IPC-18) | |
| Medical Mistrust Index (MMI) | 0.67 [0.52–0.86]** | 0.69 [0.55–0.88]** | 0.74 [0.59–0.93]* | 0.69 [0.53–0.90]* |
| Race (Black) | 0.44 [0.25–0.75]** | 0.93 [0.55–1.56]ns | 1.01 [0.59–1.71]ns | 0.92 [0.53–1.62]ns |
| Diabetes Duration | 0.87 [0.58–1.31]ns | 0.66 [0.45–0.97]ns | 0.51 [0.34–0.76]** | 0.68 [0.44–1.04]ns |
p≤0.05*, p<0.01**, ns=non-significant; proportional odds models adjusted for age, race, gender, income, health literacy level, diabetes duration, study status, glycemic control, and depressive symptoms. CAT=Communication Assessment Tool, MMI=Medical Mistrust Index, IPC-18=Interpersonal Processes of Care Survey.
Figure 1. Main Effects of Medical Mistrust on Communication Outcomes.
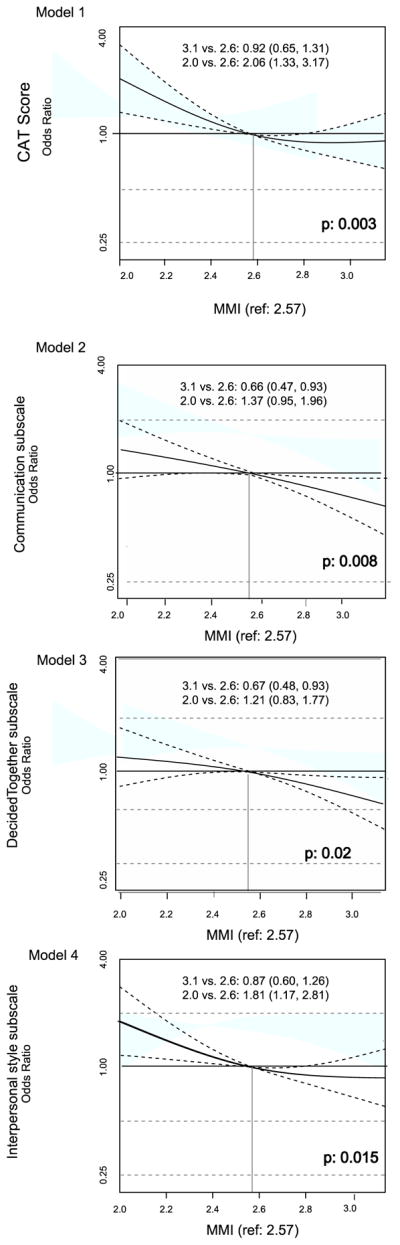
MMI=Medical Mistrust Index, CAT=Communication Assessment Tool
In Model 2, the association of mistrust with scores on the Communication subscale of the IPC-18 was examined. Here, higher medical mistrust was associated with lower Communication score. The adjusted odds ratio for reporting a higher Communication score per IQR range change in medical mistrust was 0.69 ([95% CI: 0.55–0.88]; p=0.008). Mistrust in this model had more of a linear relationship with communication quality across the spectrum of responses.
Model 3 examined the association of mistrust with the Decided Together subscale of the IPC-18. After adjustment, higher mistrust again was nominally associated with lower Decided Together scores. The AOR for reporting higher Decided Together score per IQR range change in medical mistrust was 0.74 ([95% CI: 0.59–0.93]; p=0.02). Interestingly, in this model increasing years of diabetes diagnosis was independently associated with lower Decided Together score (AOR 0.51 [95% CI: 0.34–0.76]; p=0.004).
In Model 4, we examined the association of mistrust with the Interpersonal Style subscale of the IPC-18 and found that higher mistrust was associated with lower Interpersonal Style score. The AOR for reporting higher Interpersonal Style score per IQR range change in medical mistrust was 0.69 ([95% CI: 0.53–0.90]; p=0.015). Table 2 and Figure 1 provide the descriptive and graphical versions respectively of the main effects from our multivariable modeling.
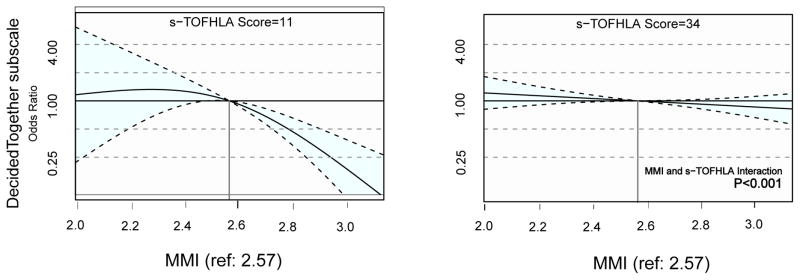
In the models that assessed the interaction effect (Figure 2) of health literacy on the above associations, we observed evidence that the association of mistrust score with the Decided Together subscale of the IPC-18 varied by health literacy level (p<0.001 for interaction). Specifically, it was evident that the negative impact of higher medical mistrust on perceptions of adequate involvement in the decision making process during encounters was more pronounced for patients with low health literacy than for those with higher health literacy levels in this sample. Figures 1 and 2 respectively provide graphical illustrations of the non-linear main effects of each association and the interaction effects of health literacy where significance was reached.
Figure 2. Interaction effect of Health Literacy with Mistrust and the Decided Together domain of the IPC-18.
MMI=Medical Mistrust Index, s-TOFHLA=Short Test of Functional Health Literacy in Adults, IPC-18=Interpersonal Processes of Care Survey.
Discussion
We observed high levels of medical mistrust among low income diabetes patients seeking care in a public health setting. Higher medical mistrust was significantly associated with lower odds of reporting better communication quality with providers. These findings support Swenson et al. who posited that patient cognitive factors may differentially impede perceptions of communication quality (Swenson, Rose, Vittinghoff, Stewart, & Schilinger, 2008). Patient-reported communication deficits in our study were related to a provider’s ability to speak slowly and in a manner that was understood, appropriately gather information from the patient, explain the results of tests and exams, and check for patient understanding. Similarly, our results showed that patients with higher mistrust did not feel as welcomed by their providers into the decision making process during encounters and tended to grade their providers’ communication style as less interpersonal. Additionally, for the more interactional aspects of communication it appears that the negative association with mistrust was more pronounced for patients with lower health literacy such that mistrustful patients with low literacy perceive worse communication with their provider relative to mistrustful patients with higher literacy skills.
Patients in this sample were representative of individuals who often face multiple socio-demographic challenges to the optimal receipt of diabetes care. Care settings such as the ones in which the parent trial occurred are often characterized by multiple factors that function as barriers to more favorable patient-provider and patient-health system interactions. Several studies have highlighted the importance of these barriers which may include transient populations, competing and often complex social needs (e.g. housing, transportation), financial challenges, and lack of family and other social network support ( Egede & Michel, 2006; Kahn et al., 2009; Nam et al., 2011; Vest et al., 2013). The multiplicative nature of these barriers and their impact on the clinical encounter may be magnified if patients arrive for care with pre-established beliefs and psychological barriers such as high levels of medical mistrust. We surprisingly observed that patients with longer diabetes disease duration also had significantly lower odds of perceiving adequate involvement in their care. We believe that this may be reflective of a relative lack of continuity of care experienced by patients in the safety-net setting, and it is possible that, under these circumstances, time may serve to strengthen rather than mitigate mistrust. Thus, providers may find themselves at a pre-ordained disadvantage if patients fundamentally believe that health systems do not genuinely have their best interests at heart as they attempt to engage patients in their care. Another plausible explanation is that longer disease duration may reflect increasing comorbidity and/or disease complexity which in turn could be associated with perceptions of worse provider communication.
Schenker et al.(2009) evaluated the relationship between depression and communication quality for patients with coronary heart disease and suggested that patients with depression may view their encounters through a different lens, irrespective of disease severity, and the same is arguably true for patients with high levels of mistrust. The challenge however is that if and when discovered, depression may respond to medical and psychological therapy whereas mistrust may be more difficult to ascertain and influence particularly at the point of care. Regardless, providers should be aware of the potential impact of underlying mistrust among their patients and especially for those who represent groups that have historically experienced discrimination, marginalization, and stigma. Greater attention and effort should be exerted to establish a therapeutic alliance with mistrustful patients with special attention given to the development of those skills that have been postulated to improve patient-provider interactions such as teaching to goal, use of decision aids and literacy-matched educational materials, teach-back techniques, and cultural sensitivity (AADE, 2007; Baker et al., 2011; Schillinger et al., 2003; White, Beech, & Miller, 2009).
Our findings add to the literature on the impact of mistrust on health outcomes for vulnerable populations and are the first to specifically assess its impact on perceptions of communication quality in the context of diabetes care. We must consider, however, several limitations to our findings. It is important to point out that we observed ceiling effects with our self-reported communication measures as reflected by the high median scores on the CAT and IPC-18 subscales. This in fact is quite common when assessments of communication quality are derived subjectively from respondents (Fernandez et al., 2004; Swenson et al., 2008). Additionally two of the four IPC-18 subscales had low internal consistency (Decided Together and Interpersonal style). Nonetheless, we found among a linguistically and ethnically diverse sample, significant associations between levels of mistrust and reports of two separate measures of communication quality lending support to the validity of our findings. Additionally, the temporal administration of the IPC-18 and CAT, before and after the baseline encounter respectively, arguably captured both general and immediate care experiences of enrolled participants. Another limitation is that we were underpowered to make additional inferences regarding potential evidence for disparities in care experiences with provider communication across racial and ethnic groups in our sample. Future work could specifically assess for these differences and explore potential targets for minimizing mistrust and the subsequent impact on care perceptions for vulnerable populations. Finally, due to the cross sectional nature of our design we are not able to make definitive assessments of causality nor do we presuppose that our findings will necessarily generalize to populations with comparable demographics.
Conclusion
In summary, diabetes patients with self-reported mistrust in the health system experience lower quality of communication with providers, perceive less involvement in their diabetes care, and view their providers as less interpersonal in their style. Patient health literacy level may be an important influential factor that varies the effect of mistrust on perceptions of communication quality; we continue to support a “universal precautions” approach to care that incorporates consistent use of effective communication strategies for all patient encounters irrespective of measured literacy level (38). Overall, the implications of our findings are that health systems that are charged with the task of providing high value care for vulnerable populations should be aware of the potential impact of mistrust on current care experiences. Practice-based interventions could seek to minimize the impact of mistrust in their design so that both patients and providers derive maximal benefit from collaborative efforts to improve the health and health outcomes for safety-net populations with diabetes.
Acknowledgments
The PRIDE Study Team members (not listed as co-authors): David G. Schlundt, PHD, Department of Psychology, Vanderbilt University, Nashville, TN; Anne Sizemore, MA, CMI, Institute for Medicine and Public Health, Vanderbilt University, Nashville, TN; Karen Trochez, AA, BA, Institute for Medicine and Public Health, Vanderbilt University, Nashville, TN. We are thankful for the collaboration of the physicians and staff at the Mid-Cumberland Region Tennessee Department of Health and for each patient who voluntarily gave of their time and energy to make the PRIDE project possible.
Funding
This research study, The Public-Private Partnership Addressing Literacy-Numeracy to Improve Diabetes Care is funded by a grant (5R18 DK083264) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). Dr. White is additionally funded by (5K23 DK092470) from NIDDK. Data capture and management was supported by the Vanderbilt University CTSA 5UL1TR000445. Content of this manuscript was presented in oral abstract format at the International Conference for Communication in Healthcare, Montreal, Canada in October 2013.
References
- AADE. AADE position statement. Cultural sensitivity and diabetes education: recommendations for diabetes educators. Diabetes Educ. 2007;33(1):41–4. doi: 10.1177/0145721706298202. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DW, DeWalt DA, Schillinger D, Hawk V, Ruo B, Bibbins-Domingo K, et al. “Teach to goal”: theory and design principles of an intervention to improve heart failure self-management skills of patients with low health literacy. J Health Commun. 2011;16(Suppl 3):73–88. doi: 10.1080/10810730.2011.604379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38(1):33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Parker MM, Schillinger D, Katon W, Adler N, Adams AS, et al. Associations between antidepressant adherence and shared decision-making, patient-provider trust, and communication among adults with diabetes: diabetes study of Northern California (DISTANCE) J Gen Intern Med. 2014;29(8):1139–47. doi: 10.1007/s11606-014-2845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA, Ganda OP, Ritholz MD, Lee Y, Brooks KM, Lewis-Schroeder NF, et al. Look who’s (not) talking: diabetic patients’ willingness to discuss self-care with physicians. Diabetes Care. 2012;35(7):1466–72. doi: 10.2337/dc11-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya G. Self-management of type 2 diabetes among African Americans in the Arkansas Delta: a strengths perspective in social-cultural context. J Health Care Poor Underserved. 2012;23(1):161–78. doi: 10.1353/hpu.2012.0035. [DOI] [PubMed] [Google Scholar]
- Cousin G, Schmid Mast M, Jaunin-Stalder N. Finding the right interactional temperature: do colder patients need more warmth in physician communication style? Soc Sci Med. 2013;98:18–23. doi: 10.1016/j.socscimed.2013.08.034. [DOI] [PubMed] [Google Scholar]
- DeWalt DA, Broucksou KA, Hawk V, Brach C, Hink A, Rudd R, et al. Developing and testing the health literacy universal precautions toolkit. Nurs Outlook. 2011;59(2):85–94. doi: 10.1016/j.outlook.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru OK, Gerzoff RB, Selby JV, Brown AF, Ackermann RT, Karter AJ, et al. Identifying risk factors for racial disparities in diabetes outcomes: the translating research into action for diabetes study. Med Care. 2009;47(6):700–6. doi: 10.1097/mlr.0b013e318192609d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede LE, Michel Y. Medical mistrust, diabetes self-management, and glycemic control in an indigent population with type 2 diabetes. Diabetes Care. 2006;29(1):131–2. doi: 10.2337/diacare.29.1.131. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Schillinger D, Grumbach K, Rosenthal A, Stewart AL, Wang F, et al. Physician language ability and cultural competence. An exploratory study of communication with Spanish-speaking patients. J Gen Intern Med. 2004;19(2):167–74. doi: 10.1111/j.1525-1497.2004.30266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46(5):437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Kahn LS, Fox CH, Carrington J, Desai U, Bartlett DP, Lyle H, et al. Telephonic nurse case management for patients with diabetes and mental illnesses: a qualitative perspective. Chronic Illn. 2009;5(4):257–67. doi: 10.1177/1742395309350229. [DOI] [PubMed] [Google Scholar]
- LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. 2009;44(6):2093–105. doi: 10.1111/j.1475-6773.2009.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Makoul G, Krupat E, Chang CH. Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67(3):333–42. doi: 10.1016/j.pec.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1–9. doi: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Naranjo DM, Fisher L, Areán PA, Hessler D, Mullan J. Patients with type 2 diabetes at risk for major depressive disorder over time. Ann Fam Med. 2011;9(2):115–20. doi: 10.1370/afm.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo D, Hessler DM, Deol R, Chesla CA. Health and psychosocial outcomes in U.S. adult patients with diabetes from diverse ethnicities. Curr Diab Rep. 2012;12(6):729–38. doi: 10.1007/s11892-012-0319-y. [DOI] [PubMed] [Google Scholar]
- Pandit AU, Bailey SC, Curtis LM, Seligman HK, Davis TC, Parker RM, et al. Disease-related distress, self-care and clinical outcomes among low-income patients with diabetes. J Epidemiol Community Health. 2014;68(6):557–64. doi: 10.1136/jech-2013-203063. [DOI] [PubMed] [Google Scholar]
- Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71(1):1–9. doi: 10.1016/j.socscimed.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18(8):624–33. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Sarkar U, Piette JD, Gonzales R, Lessler D, Chew LD, Reilly B, et al. Preferences for self-management support: findings from a survey of diabetes patients in safety-net health systems. Patient Educ Couns. 2008;70(1):102–10. doi: 10.1016/j.pec.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker Y, Stewart A, Na B, Whooley MA. Depressive symptoms and perceived doctor-patient communication in the Heart and Soul study. J Gen Intern Med. 2009;24(5):550–6. doi: 10.1007/s11606-009-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger D, Bindman A, Wang F, Stewart A, Piette J. Functional health literacy and the quality of physician-patient communication among diabetes patients. Patient Educ Couns. 2004;52(3):315–23. doi: 10.1016/S0738-3991(03)00107-1. [DOI] [PubMed] [Google Scholar]
- Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- Seligman HK, Fernandez A, Stern RJ, Weech-Maldonado R, Quan J, Jacobs EA. Risk factors for reporting poor cultural competency among patients with diabetes in safety net clinics. Med Care. 2012;50(9 Suppl 2):S56–61. doi: 10.1097/MLR.0b013e3182640adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Nápoles-Springer AM, Gregorich SE, Santoyo-Olsson J. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res. 2007;42(3 Pt 1):1235–56. doi: 10.1111/j.1475-6773.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Nápoles-Springer A, Pérez-Stable EJ. Interpersonal processes of care in diverse populations. Milbank Q. 1999;77(3):305–39. 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson SL, Rose M, Vittinghoff E, Stewart A, Schillinger D. The influence of depressive symptoms on clinician-patient communication among patients with type 2 diabetes. Med Care. 2008;46(3):257–65. doi: 10.1097/MLR.0b013e31816080e9. [DOI] [PubMed] [Google Scholar]
- Varkey AB, Manwell LB, Williams ES, Ibrahim SA, Brown RL, Bobula JA, et al. Separate and unequal: clinics where minority and nonminority patients receive primary care. Arch Intern Med. 2009;169(3):243–50. doi: 10.1001/archinternmed.2008.559. [DOI] [PubMed] [Google Scholar]
- Vest BM, Kahn LS, Danzo A, Tumiel-Berhalter L, Schuster RC, Karl R, et al. Diabetes self-management in a low-income population: impacts of social support and relationships with the health care system. Chronic Illn. 2013;9(2):145–55. doi: 10.1177/1742395313475674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RO, Beech BM, Miller S. Health Care Disparities and Diabetes Care: Practical Considerations for Primary Care Providers. Clin Diabetes. 2009;27(3):105–12. doi: 10.2337/diaclin.27.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RO, Eden S, Wallston KA, Kripalani S, Barto S, Shintani A, et al. Health communication, self-care, and treatment satisfaction among low-income diabetes patients in a public health setting. Patient Educ Couns. 2015;98(2):144–9. doi: 10.1016/j.pec.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RO, Osborn CY, Gebretsadik T, Kripalani S, Rothman RL. Health literacy, physician trust, and diabetes-related self-care activities in Hispanics with limited resources. J Health Care Poor Underserved. 2013;24(4):1756–68. doi: 10.1353/hpu.2013.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K, Chambers L, Bumol S, White RO, Gregory BP, Davis D, et al. The PRIDE (Partnership to Improve Diabetes Education) Toolkit: Development and Evaluation of Novel Literacy and Culturally Sensitive Diabetes Education Materials. Diabetes Educ. 2016;42(1):23–33. doi: 10.1177/0145721715620019. [DOI] [PMC free article] [PubMed] [Google Scholar]