Abstract
Objective
We and others have previously shown that RhoA-dependent stimulation of myocardin related transcription factor (MRTF) nuclear localization promotes smooth muscle cell (SMC) marker gene expression. The goal of the present study was to provide direct in vivo evidence that actin polymerization by the diaphanous-related formins contributes to the regulation of SMC differentiation and/or phenotype.
Approach and Results
Conditional cre-based genetic approaches were used to over-express a well-characterized dominant negative variant of mDia1 (DNmDia) in SMC. DNmDia expression in SM22 expressing cells resulted in embryonic and perinatal lethality in ~20% of mice due to defects in myocardial development and SMC investment of peripheral vessels. While most DNmDia+/SM22Cre+ mice exhibited no overt phenotype, the re-expression of SMC differentiation marker gene expression that occurs following carotid artery ligation was delayed, and this effect was accompanied by a significant decrease in MRTF-A nuclear localization. Interestingly, neointima growth was inhibited by expression of DNmDia in SMC and this was likely due to a defect in directional SMC migration and not to defects in SMC proliferation or survival. Finally, by using the tamoxifen-inducible SM MHCCreERT2 line, we showed that SMC-specific induction of DNmDia in adult mice decreased SMC marker gene expression.
Conclusions
Our demonstration that diaphanous-related formin signaling plays a role in heart and vascular development and the maintenance of SMC phenotype provides important new evidence that Rho/actin/MRTF signaling plays a critical role in cardiovascular function.
Keywords: Smooth muscle, MRTF, RhoA, diaphanous, migration
INTRODUCTION
Smooth muscle cell (SMC) differentiation plays an important role in vascular development and maintenance. Although medial SMC express a repertoire of contractile-associated differentiation markers, they do not terminally differentiate even in adult animals. Phenotypically modulated SMC that exhibit decreased differentiation marker gene expression and increased growth, migration, and matrix production have been shown to contribute to the progression of several important cardiovascular diseases including atherosclerosis, hypertension, and restenosis (1, 2). Therefore, a better understanding of the signaling mechanisms that control these SMC functions will be critical.
Most SMC differentiation marker genes including SM myosin heavy chain (SM MHC), SM α-actin, calponin, and SM22 are regulated by serum response factor (SRF), a ubiquitously expressed MADS box transcription factor that binds to conserved CArG (CC(A/T)6GG) elements within the promoters of these genes (2). The cardiac and SMC selective SRF co-factor, myocardin, strongly transactivates SMC-specific transcription and is required for SMC differentiation in vivo (3). Two myocardin-related transcription factors, MRTF-A and MRTF-B, have also been identified. Although they are expressed more widely, their importance in the regulation of SMC differentiation marker gene expression is supported by the phenotypes of global and tissue-specific knockouts (4–7). We and others have demonstrated that RhoA-mediated actin polymerization stimulates MRTF nuclear localization and is critical for SMC marker gene expression in at least some SMC cell-types (8–11). However, direct in vivo evidence that this mechanism plays a significant role in the regulation of SMC differentiation or phenotypic modulation is lacking.
We have previously identified a number of signaling mechanisms that control RhoA activity in SMC (12, 13) and have characterized the pathways downstream of RhoA that regulate MRTF-dependent SMC-specific transcription (14, 15). The Rho-associated kinases (ROCK 1 and 2) increase actin polymerization indirectly through a kinase cascade that inhibits cofilin’s ability to sever F-actin, and they also promote stress fiber formation by enhancing actin fiber bundling and cell contractility (16, 17). However, we have shown that inhibition of ROCK signaling only partially inhibited SMC-specific promoter activity, suggesting that additional RhoA effectors were important. The diaphanous-related formins (DRFs), mDia1 and mDia2, are RhoA effectors that directly catalyze linear actin polymerization in cooperation with profilin (18–21). We have shown that both are highly expressed in SMC, and when activated, strongly up-regulate SMC marker gene expression (14). Importantly, siRNA-mediated knockdown of mDia1 and mDia2 in primary rat aortic SMC resulted in a significant reduction in SMC-specific promoter activity, endogenous SMC marker gene expression, and MRTF nuclear localization (14).
RhoA signaling also regulates the actin and adhesion dynamics that control cell migration and division. Using fluorescent biosensors, Pertz et al. detected RhoA activity near the leading edge of migrating cells where it is thought to promote actin-based cell protrusion (22). RhoA activity is also high at the rear of migrating cells where it induces the contractile forces necessary for trailing edge retraction Several studies have shown that mDia1 and mDia2 catalyze the linear actin polymerization that is required for filopodia formation (23). RhoA signaling also controls cell adhesion by modulating focal adhesion formation and RhoA dependent activation of mDia2 at the cleavage furrow may be important for cytokinesis (24, 25). Given its pleiotropic effects on nearly all of the parameters that define SMC phenotype it will be critical to further study RhoA/mDia/actin signaling in SMC.
The goal of the present study was to define the contributions of mDia-mediated actin polymerization to SMC phenotype in vivo. We used genetic models to inhibit DRF signaling in SMCs during cardiovascular development, and we studied the effects of mDia inhibition on SMC phenotypic modulation in adult mice subjected to carotid artery ligation. Our results indicate that mDia signaling was required for normal cardiovascular development, and that inhibition of mDia signaling attenuated SMC differentiation marker gene expression but reduced neointima formation by inhibiting SMC migration.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Inhibition of mDia signaling in SM22-expressing cells led to some embryonic/perinatal lethality
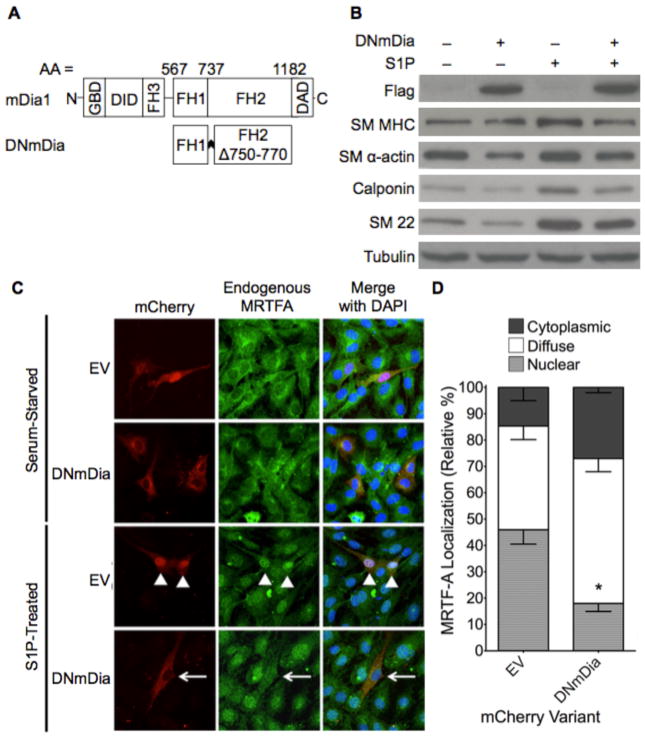
To determine the contributions of actin polymerization to the regulation of SMC differentiation and/or phenotype in vivo, we used a genetic approach to over-express a dominant negative variant of mDia1 (F1FHΔ1) specifically in SMC (Figure 1a). Because mDia1 and mDia2 are RhoA effectors with considerable functional overlap (26, 27), it is important to note that this variant inhibits the function of all DRFs by dimerizing with endogenous proteins and/or binding nonproductively with barbed actin filaments (18, 28). Another consideration for our approach was that DNmDia inhibits actin polymerization more specifically than alternative interventions that target Rho and its many effectors or ROCK and its many substrates. In excellent agreement with our previous demonstration that DNmDia inhibited the activities of exogenous SMC-specific promoters (14), over-expression of DNmDia inhibited the endogenous expression of multiple SMC-specific marker genes and the nuclear localization of MRTF-A in S1P-treated 10T1/2 cells (Figure 1b–d).
Figure 1. DNmDia expression inhibited SM-specific marker expression.
A) Schematic of full-length and dominant negative mDia1. FH, formin homology domain; GBD, GTPase-binding domain; DID, diaphanous inhibitory domain; DAD, diaphanous autoinhibitory domain. B) 10T1/2 cells transfected with DNmDia or empty expression vector were serum-starved, and treated with sphingosine-1-phosphate (S1P) for 16 hr. Endogenous SMC marker expression was detected by immunoblotting. C) 10T1/2 cells expressing mCherry empty vector (EV) or mCherry-DNmDia were serum-starved, treated with S1P for 4 min, and then fixed. Localization of endogenous MRTF-A was determined by immunohistochemistry. D) Quantification of MRTF-A nuclear localization from three separate S1P-treated experiments with over 100 cells counted per condition. * p < 0.05.
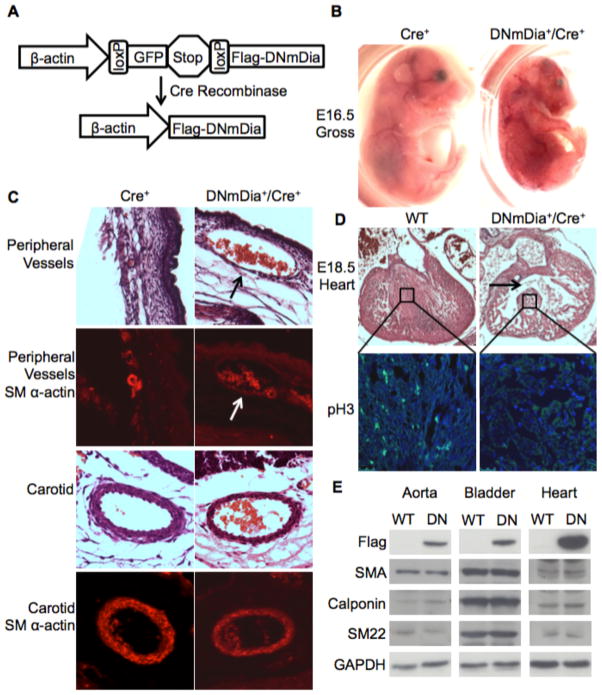
Flag-tagged DNmDia was cloned into a previously described transgene construct in which the constitutive expression of DNmDia (driven by a fragment of the β-actin promoter) is inhibited by the upstream insertion of a floxed EGFP-Stop cassette (Figure 2a and (29, 30)). DNmDia transgenic mice were generated on a C57BL/6 background and were crossed to a well-characterized SM22Cre line (31) that expresses Cre in most SMC subsets and in the developing myocardium (Supp. Figure Ia and (32)). Although many DNmDia+/SM22Cre+ mice were viable, the number that reached adulthood was approximately 20% less than that expected by Mendelian ratios (Table 1). A number of DNmDia+/SM22Cre+ mice died in utero with non-viable embryos beginning to appear at around E15.5. Hemorrhage was detected in 6 out of 17 DNmDia+/SM22Cre+ embryos between E15.5 – E18.5 and was most visible near the smaller, more peripheral blood vessels of the head, limbs, and body wall (Figure 2b). Immunohistologic examination of these mice revealed severely dilated blood vessels that were poorly invested with SM αactin expressing cells (Figure 2c). Importantly, SMC coverage of larger blood vessels like the aorta and carotid arteries was not significantly affected in hemorrhagic DNmDia+/SM22Cre+ embryos (Figure 2c).
Figure 2. DNmDia expression in SM22-expressing cells resulted in some embryonic/perinatal death.
A) Schematic of the Cre-dependent transgene construct used to drive DNmDia. B) Gross images of littermate control and hemorrhagic DNmDia+/SM22Cre+ embryos. C) H&E and SM α-actin staining of the indicated blood vessels from DNmDia+/SM22Cre+ and littermate control mice. Arrows point to a severely dilated vessel with incomplete SMC investment. D) H&E and phospho-histone H3 staining of hearts from DNmDia+/SM22Cre+ and control mice at E18.5. Arrow points to ventricular septal defect. E) Western blot for SMC differentiation marker gene expression in the aorta, bladder, and heart from littermate control (WT) and phenotypically normal 4-week-old DNmDia+/SM22Cre+ (DN) mice.
Table 1.
Genotype ratios from the DNmDia+ x SM22Cre+ cross.
| DN+/Cre+ | DN−/Cre+ | DN+/Cre− | DN−/Cre− | Total | X2 | |
|---|---|---|---|---|---|---|
| 4 week old | 84 | 117 | 132 | 121 | 454 | P = 0.0103 |
| Dead pups (P1 – P9) | 17 | 4 | 1 | 3 | 25 | P = < 0.0001 |
A subset of DNmDia+/SM22Cre+ mice also exhibited cardiac abnormalities, including ventricular septal defect and hypoplasticity of the ventricular wall and septum at E18.5 (Figure 2d). Since this SM22Cre line can drive Cre expression in the embryonic heart, it was likely that DRF signaling was important for myocardial cell proliferation/survival during this developmental window. Indeed, phospho-histone H3 staining revealed a dramatic reduction in myocardial cell proliferation in DNmDia+/SM22Cre+ mice. A significant number of DNmDia+/SM22Cre+ mice died perinatally and while these mice appeared fairly normal at birth they quickly became runted and did not survive past postnatal day 9. All dead pups had milk in their stomach, suggesting that the runted phenotype was not due to a nursing defect. Histological analysis revealed lung congestion and hearts with thin ventricular walls and low phospho-histone H3 expression, again suggestive of heart function defects in these mice (Supp. Figure II).
Importantly, the DNmDia+/SM22Cre+ mice that survived to adulthood had no overt phenotype and a normal lifespan. Somewhat surprisingly, heart and vessel morphology and SMC marker gene expression were unaffected even though DNmDia was strongly expressed in these tissues (Figure 2e).
DNmDia expression altered SMC phenotype following arterial injury
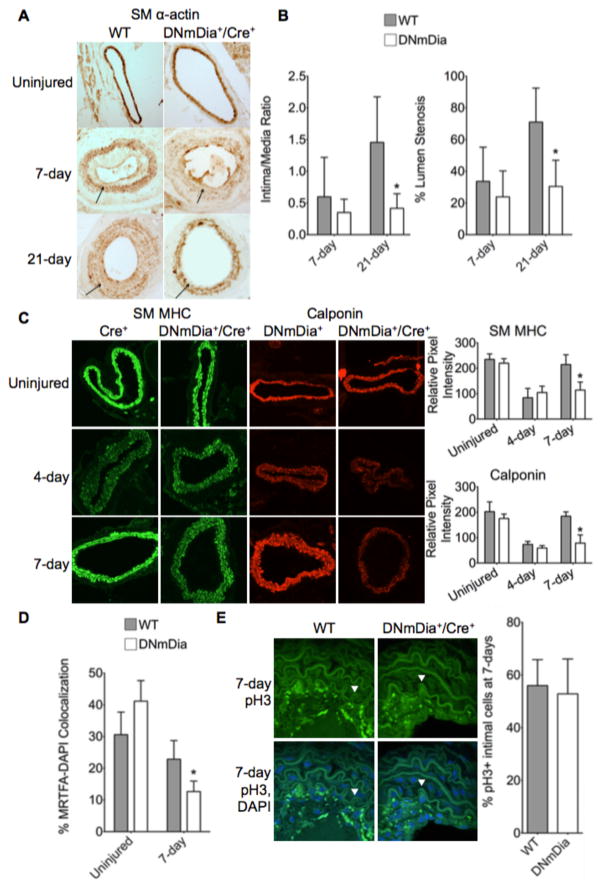
It is clear that the activation of compensatory pathways during development can modify the phenotype of genetically modified mice and that the application of acute stresses can unmask previously undetected effects of genetic modifications. The lack of phenotype in adult DNmDia+/SM22Cre+ provided us with an excellent model for examining the effects of mDia signaling on SMC phenotypic modulation following vascular injury. To this end, we subjected DNmDia+/SM22Cre+ and control littermates to a carotid artery ligation procedure that results in a significant neointima by 21 days and a down-regulation of SMC differentiation marker gene expression in medial SMC that peaks at ~4–5 days post-injury and eventually returns to normal (Figure 3 and (33)). Notably we observed no statistical significant difference in vessel diameter between uninjured and ligated arteries in either control or DNmDia+/SM22Cre+ animals.
Figure 3. DNmDia delayed SM marker gene re-expression and reduced neointima formation after carotid artery ligation.
Adult DNmDia+/SM22Cre+ mice and littermate controls were subjected to ligation of the right carotid artery just below the bifurcation. Left carotid arteries served as uninjured controls. A) Carotid arteries were harvested at 7- or 21-days post-ligation, fixed, and embedded in paraffin. DAB staining for SM α-actin was performed on 12 μm sections. Arrows indicate the internal elastic lamina (IEL). B) Neointimal area 500 μm downstream of the ligation was quantified from 12 μm H&E-stained paraffin sections at 7- and 21-days post-ligation using ImageJ and is expressed relative to medial area. Percent lumen stenosis was calculated as intimal area/total area encompassed by the IEL. n=at least 7 per genotype, per time-point. C) Carotid arteries were harvested from mice at 4 days or 7 days post-ligation and frozen in OCT medium. Immunofluorescence staining for SM MHC and Calponin was performed on 8 μm sections. ImageJ was used to quantify fluorescence intensity. n=at least 3 per genotype, per time-point. D) MRTFA localization was imaged by con-focal immunofluorescence in frozen sections taken from control and injured carotid arteries 7-days after ligation. MRTF-A nuclear localization was determined using ImageJ and is expressed relative to DAPI co-staining. n=at least 3 per genotype. E) Phospho-histone H3 staining of paraffin sections from 7-day ligated WT and DNmDia carotid arteries. pH3-positive cells were counted and expressed as a percentage of DAPI-stained cells in the intima. Arrowheads point to IEL. n=at least 3 per genotype. For all measurements * p < 0.05.
In excellent agreement with the Western blot data shown in Figure 2e, immunohistochemical analyses revealed no differences in SM α-actin, SM MHC, or calponin expression between uninjured arteries from control and DNmDia+/SM22Cre+ mice (Figure 3 and Suppl. Figure III). As expected, differentiation marker expression in medial SMC was reduced at 4 days post-ligation in control mice, and a similar reduction was observed in DNmDia+/SM22Cre+ animals (Figure 3c). However, while SMC differentiation marker gene expression was already returning to baseline by day 7 post-injury in control mice, it remained down-regulated in DNmDia+/SM22Cre+ mice (Figure 3a, c). This difference was not apparent at the 21-day time-point (Figure 3a), suggesting that the recovery of SMC marker gene expression was delayed but not completely inhibited in this model. Importantly, the differences in SMC marker gene expression at the 7-day time point were accompanied by reductions in MRTF-A nuclear localization (Figure 3d and Suppl. Figure IVa), an effect likely due to decreased actin polymerization in DNmDia-expressing SMC.
The ability of DNmDia to inhibit SMC marker gene expression in this model suggested that these cells were more phenotypically modulated. However, injured vessels from DNmDia+/SM22Cre+ mice had significantly less neointima formation and overall stenosis than controls (Figure 3a, b) and reduced collagen deposition (Supp. Figure IVb). Taken together, these data suggest that mDia signaling has complex effects on SMC phenotype and provide strong evidence that SMC differentiation and growth/migration are not mutually exclusive and are independently regulated.
Inhibition of mDia signaling reduced directional SMC migration
Extensive evidence including the results from elegant lineage tracing studies indicates that the majority of neointima SMC originate from the medial SMC layer and that injury-induced increases in SMC migration and proliferation are critical for neointima formation (34). Although it is well known that RhoA signaling has complex and sometimes biphasic effects on these processes (35), the role of mDia signaling in SMC is less clear. Thus, we performed additional analyses to identify the mechanism for decreased neointima formation in DNmDia+/SM22Cre+ mice.
Although control mice exhibited larger neointimas than DNmDia mice at 21 days post-ligation, we observed similar percentages of proliferating cells within the neointima at day 7 (Figure 3e). In agreement with the lack of effect on neointimal cell proliferation we observed in vivo, knockdown of mDia1, mDia2, or both in mouse aortic SMC or 10T1/2 cells had no significant effects on cell proliferation as measured by phospho-histone H3 staining or MTT assays (Supp. Figure Va–c). In addition, mDia knockdown or DNmDia overexpression had no effect on cell cycle progression as measured by flow cytometry of DAPI-stained cells, and results from TUNEL and lactate dehydrogenase release assays revealed no effects on apoptosis or cytotoxicity, respectively (Supp. Figure Vd–g). Furthermore, DNmDia+/Rosa26+/SM22Cre+ mice showed uniform expression in the aorta, bladder, lungs, and heart, indicating no significant cell death in DNmDia+ cells in vivo (Supp. Figure Ia).
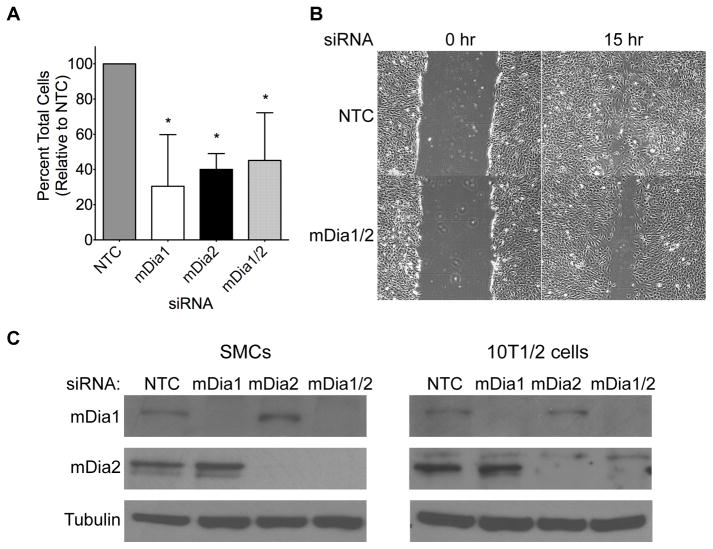
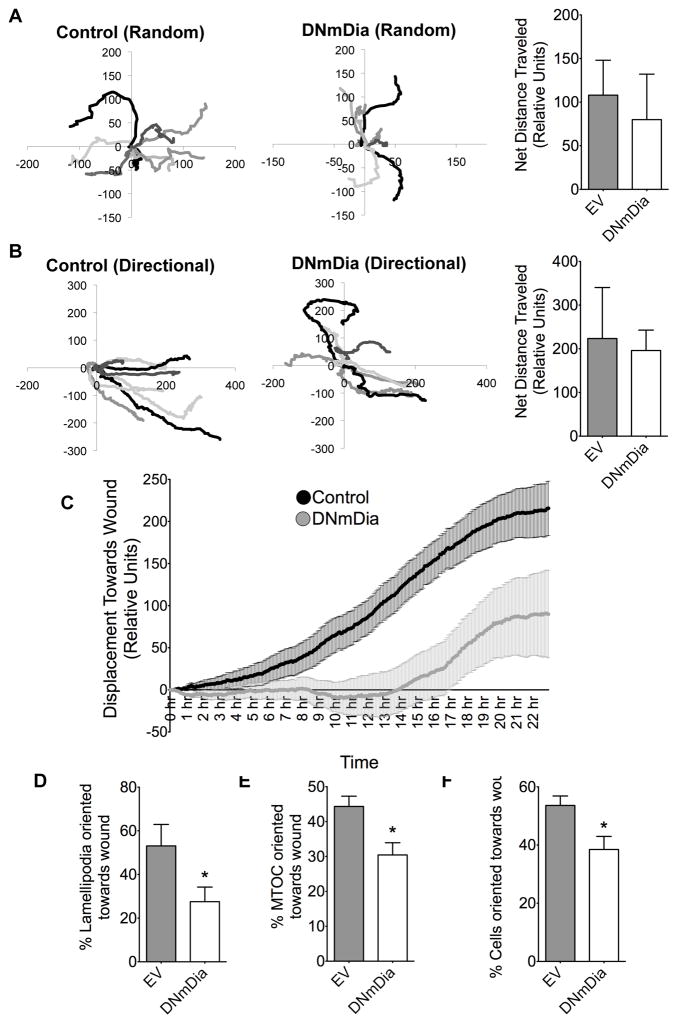
Based upon these results and previous studies demonstrating that mDia-mediated actin polymerization was required for filopodia formation and cell migration (23, 36), we hypothesized that the decrease in neointima size in DNmDia+/SM22Cre+ mice resulted from a decrease in migration from the medial SMC layer. In strong support of this, siRNA-mediated knockdown of mDia1, mDia2, or both (Figure 4c) resulted in a 50–70% decrease in SMC migration in transwell assays (Figure 4a) and a significant decrease in scratch wound closure in 10T1/2 cells (Figure 4b). Interestingly, live cell tracking studies demonstrated that sub-confluent cells expressing DNmDia-mCherry were capable of random migration and that these cells migrated similar distances to control cells (Figure 5a). Although DNmDia-expressing and control cells also traversed similar distances in scratch wound assays, directional migration toward the wound was significantly inhibited in DNmDia expressing cells (Figure 5b–c, Supp. Videos I–II), strongly suggesting that inhibition of mDia signaling in SMC leads to defects in directional migration and/or cell polarity. Accordingly, DNmDia-expressing cells exhibited significant polarity defects as evidenced by lamellipodia formation in multiple directions (Figure 5d, Supp. Figure VIa) and failure of the microtubule organizing center (MTOC) to orient toward the scratch wound (Figure 5e–f, Supp. Figure VIb–c).
Figure 4. mDia knockdown inhibited SMC migration.
A) siRNA was used to knock down mDia1, mDia2, or both in primary mouse aortic SMC. Equal numbers of mouse SMCs from each knockdown group were plated onto fibronectin-coated transwell inserts and migration was stimulated for 8 hours by addition of 20 ng/ml PDGF-BB to the bottom well. Following fixation and staining with Crystal Violet, SMC migration was evaluated in three separate experiments and expressed relative to migration of control cells set to 100%. *p < 0.05. B) Confluent cultures of control (NTC) and mDia1/2 knockdown 10T1/2 cells were scratched with a P1000 pipette tip and visualized every three hours. Shown are time-points immediately (0h) and 15h after scratch wounding. C) Western blot illustrating mDia1/mDia2 knockdown in our mouse aortic SMC and 10T1/2 cell culture models 72 hr after siRNA transfection.
Figure 5. DNmDia reduced directional migration.
A) Subconfluent cultures of 10T1/2 cells were transfected with mCherry-EV (Control) or mCherry-DNmDia. Live-cell imaging was used to monitor random (unstimulated) migration of 8 cells from each group for 8h. Individual cell migration paths from a relative starting point (0,0) are shown and net distance traveled was quantified using ImageJ. B) Confluent cultures of 10T1/2 cells expressing either mCherry-EV (Control) or mCherry-DNmDia were subjected to scratch wound with a P1000 pipette tip and then placed on an inverted microscope equipped with a heated, humidified, and O2/CO2 perfused stage. Pictures taken every 5 minutes for 24 hr were assembled into movies using Quicktime. Nine cells from each group, starting at the front of the scratch wound, were tracked. The averages of their displacement towards the wound was averaged at every time point and plotted for 24 hr. Nine cells near the wound edge from each group were tracked for 24 hr. Graphs are oriented such that migration toward the scratch wound is indicated by a positive value on the x-axis. C) Average displacement of cells from “B” were plotted over time +/− SEM. Repeated measurement one-way ANOVA analysis followed by Bonferroni’s post-hoc test for individual significance demonstrated that DNmDia expression significantly inhibited migration toward the scratch wound (p <0.05). D) 10T1/2 cells expressing either mCherry or mCherry-DNmDia were subjected to scratch wound for 8 hrs, fixed, and lamellipodia orientation toward or away from the wound was scored in at least 100 cells from three separate experiments. E) 10T1/2 cells expressing either mCherry or mCherry-DNmDia were subjected to scratch wound for 8 hrs, fixed, and probed for α-Tubulin and stained with DAPI to mark MTOC orientation. At least 100 cells were counted in three separate experiments. F) Primary rat aortic SMCs expressing either mCherry or mCherry-DNmDia were subjected to scratch wound for 8 hrs, fixed, and immunostained for Golgi marker (GM)-130 and DAPI to mark orientation. At least 100 cells from three separate experiment were counted. *p <0.05.
mDia signaling maintains SMC differentiation in adults
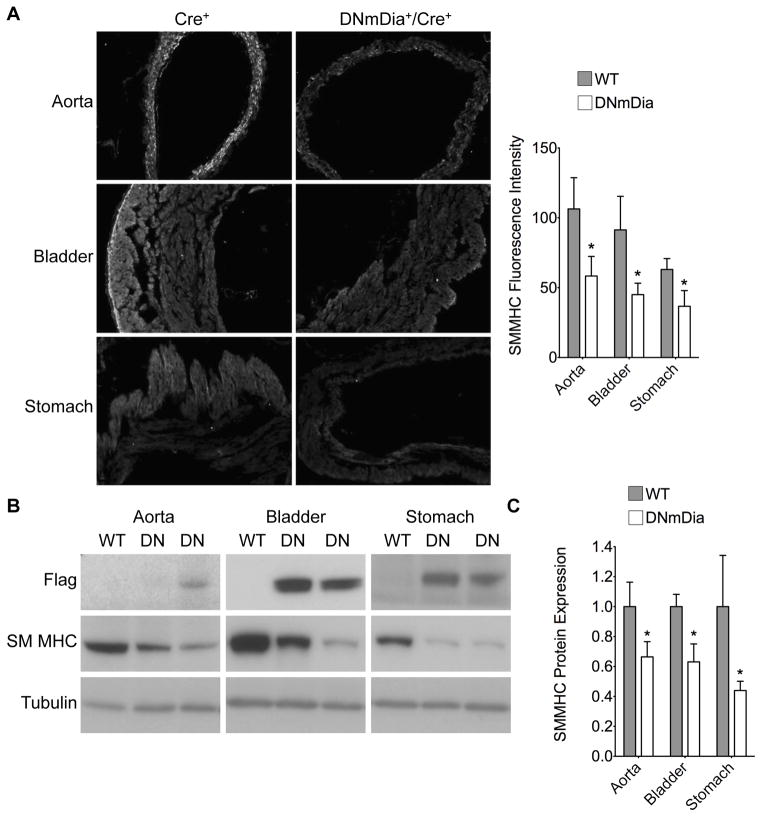
The effects of DNmDia expression in SM22-expressing cells during development were incompletely penetrant, and it is likely that stochastic differences in the timing and/or level of DNmDia expression during a critical developmental window helps explain these results. Compensatory pathways may also play a role and our demonstration that DNmDia had no effect on SMC function at baseline in adults, but delayed the re-expression of SMC differentiation marker gene expression following carotid injury supports this idea. To test this hypothesis further, we crossed DNmDia+ mice with the SMMHC-CreERT2 line that allowed us to activate DNmDia expression specifically in SMC in adult mice, effectively by-passing any compensatory pathways activated during development. As shown in Supp. Figure Ib, intraperitoneal injection of tamoxifen for 5 consecutive days activated Cre in the SMC layers of the aorta, bladder, lungs, and stomach in this model although activation of DNmDia expression was somewhat more variable (Figure 6b, Supp. Figure VII). Importantly, tamoxifen treatment of DNmDia+/SMMHC-CreERT2 mice resulted in a significant down-regulation of SM MHC gene expression in the aorta, bladder, and stomach and a significant down-regulation of all SMC differentiation marker genes in the bladder that exhibited strong and consistent DNmDia expression (Figure 6, Supp. Figure VII).
Figure 6. Induction of DNmDia in adult mice inhibited SMC differentiation marker gene expression.
Control and DNmDia+/SMMHCCre+ mice were injected with tamoxifen IP for 5 consecutive days. The indicated tissues were harvested after 3 weeks, and frozen sections were immunostained for SM MHC. A) Representative images and quantification of SM MHC staining. B) Represenative Western blots for DNmDia and SM MHC expression in aorta, bladder, and stomach from WT and DNmDia+/SMMHCCre+ mice, three weeks after tamoxifen treatment. C) SM MHC expression relative to α-tubulin was quantified using image J and then normalized to expression in WT animals set to 1. n=at least 9 animals per genotype. *p < 0.05.
DISCUSSION
To our knowledge, these results provide the first in vivo evidence that signaling through the diaphanous-related formins is critical for SMC differentiation marker gene expression and proper heart development. Our results also suggest that inhibition of mDia signaling inhibits neointima formation by attenuating directed SMC migration from the medial SMC layer of injured vessels.
Although 80% of DNmDia+/SM22Cre+ mice exhibited no detectable phenotype, a significant number died during embryonic development or perinatally. Several embryos exhibited significant hemorrhaging, suggesting that vessel integrity was affected by SMC-specific expression of DNmDia. Although the larger vessels were properly layered with SM α-actin-expressing cells, there appeared to be defects in SMC/pericyte investment of smaller vessels. Carramusa et al. demonstrated that mDia1 was required for proper E-cadherin junction formation between MCF-7 cells (37) and inhibition of EC-SMC or SMC-SMC junctions in DNmDia-expressing cells could contribute to the hemorrhage observed. This phenotype was similar to that observed in global S1P2/S1P3 receptor double knockout mice (38), which also showed a lack of SMC/pericyte investment of smaller blood vessels. Our previous studies demonstrating that S1P and S1P-dependent RhoA signaling were critical for SMC differentiation (12, 13) provide additional functional links between these two models.
Many deaths were attributed to heart defects that included considerable hypoplasticity. Like many SMC differentiation markers, SM22 is expressed in the myocardium during embryonic development, and our Cre-based expression model resulted in permanent and constitutive expression of DNmDia in myocardial cells potentially as early as E8.5(32). Thus, the defects in heart development observed were likely due to myocardial cell-autonomous effects of DNmDia on cell proliferation. Since the IVS forms primarily by proliferative expansion of muscular tissue of the outer curvature (39), the VSDs observed may have been due to decreased protrusion of this structure from the apical wall. However, we cannot rule out a potential effect on the endocardial cushions that contribute to the anterior portion of the IVS. A number of sarcomeric protein mutations cause cardiac septal defects (40) and it is possible that abnormal sarcomere organization due to defects in linear actin polymerization in DNmDia-expressing cells could be involved. Interestingly, neither knockdown of mDia1 and mDia2 nor expression of DNmDia had significant effects on proliferation in SMC. Gopinath et al. reported that mDia1 knockdown in c2c12 skeletal muscle cells inhibited cell cycle progression, perhaps suggesting that proliferation in striated muscles is more sensitive to mDia inhibition (41). Taken together, our results demonstrate that DRF signaling plays a functional role in normal cardiac development and blood vessel maturation. Nevertheless, additional studies using Cre driver lines that are more cardiac-specific will be needed to determine the precise role of mDia/DRF signaling on cardiomyocyte proliferation and heart development.
We did not detect differences in SMC marker gene expression in the DNmDia+/SM22Cre+ that survived to adulthood. However, we observed developmental defects in SMC investment and vessel integrity and a delay in the re-expression of SMC marker genes following carotid artery in these animals. When coupled with the decrease in SMC marker gene expression upon activation of DNmDia expression in adult SMC, these data strongly support our original hypothesis that mDia-mediated actin polymerization plays a role in the regulation of SMC phenotype in vivo. It is important to note that because DNmDia expression in our model occurs only after initial SM22 activation, we do not yet know whether mDia signaling is required for the initial differentiation of SMC or whether it is more important for SMC maturation and/or maintenance.
DNmDia+/SM22Cre+ mice exhibited reduced neointima formation following carotid artery ligation. These results are in good agreement with a study by Toure et al (42), who observed reduced neointimal formation in global mDia1 knockout mice. Since SM22α expression has been detected in some myeloid cells (43, 44) and macrophage infiltration has been shown to promote neointima formation, it is difficult to completely eliminate a role for these cells in our model. However, since the majority of neointima cells in our injury model are SMC, we believe that mDia signaling plays a SMC autonomous role in neointimal formation most likely by promoting SMC migration from the medial layer. It is well known that mDia1 and mDia2 control linear actin polymerization (36) and are required for filopodia formation and cell migration in cultured cells (45–47) and in vivo (48–50). Our data indicated that DNmDia expression had no effect on overall SMC movement, but instead inhibited directional migration. A growing body of evidence suggests that the diaphanous-related formins have important effects on microtubule organization and on cell polarity (51–57), and our demonstration that MTOC and lamellipodia positioning were altered in DNmDia expressing cells strongly supports these results. Thus, it will be critical to identify the signaling mechanisms downstream of the diaphanous-related formins that regulate cell polarity in SMCs. Interestingly, the reduction in collagen deposition in the DNmDia-expressing mice following ligation suggests that MRTF-A-mediated fibrosis may contribute to the injury response.
In summary, our data demonstrate that diaphanous-related formin-mediated actin polymerization plays a role in proper heart and vascular development, the maintenance of the differentiated SMC, and directional SMC migration. These results provide important new evidence that the Rho/actin/MRTF signaling axis plays a critical role in controlling SMC phenotype and support additional studies on this pathway during cardiovascular development and the disease progression.
Supplementary Material
SIGNIFICANCE.
We have previously shown that mDia-mediated actin polymerization regulates smooth muscle cell differentiation in vitro, but comprehensive in vivo studies of this mechanism are lacking. This study is the first to demonstrate that diaphanous-related formin-mediated actin polymerization is critical for normal heart development and for the regulation of SMC phenotype following vessel injury.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH Grants HL070953 and HL109607 (CPM), HL102446 (JMT), and T32HL069768 (LWC).
ABBREVIATIONS
- MRTF
myocardin-related transcription factor
- DRF
diaphanous-related formin
- SM MHC
smooth muscle myosin heavy chain
- IVS
interventricular septum
- MTOC
microtubule-organizing center
Footnotes
DISCLOSURES
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci U S A. 2005;102:8916–8921. doi: 10.1073/pnas.0503741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh J, Richardson JA, Olson EN. Requirement of myocardin-related transcription factor-B for remodeling of branchial arch arteries and smooth muscle differentiation. Proc Natl Acad Sci U S A. 2005;102:15122–15127. doi: 10.1073/pnas.0507346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Chang S, Qi X, Richardson JA, Olson EN. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol. 2006;26:5797–5808. doi: 10.1128/MCB.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Boyd K, Xu W, Ma J, Jackson CW, Fu A, Shillingford JM, Robinson GW, Hennighausen L, Hitzler JK, Ma Z, Morris SW. Acute myeloid leukemia-associated Mkl1 (mrtf-a) is a key regulator of mammary gland function. Mol Cell Biol. 2006;26:5809–5826. doi: 10.1128/MCB.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 9.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 10.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 11.Hinson JS, Medlin MD, Lockman K, Taylor JM, Mack CP. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am J Physiol Heart Circ Physiol. 2007;292:H1170–80. doi: 10.1152/ajpheart.00864.2006. [DOI] [PubMed] [Google Scholar]
- 12.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 13.Medlin MD, Staus DP, Dubash AD, Taylor JM, Mack CP. Sphingosine 1-phosphate receptor 2 signals through leukemia-associated RhoGEF (LARG), to promote smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2010;30:1779–1786. doi: 10.1161/ATVBAHA.110.209395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staus DP, Blaker AL, Taylor JM, Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol. 2007;27:478–486. doi: 10.1161/01.ATV.0000255559.77687.c1. [DOI] [PubMed] [Google Scholar]
- 15.Staus DP, Weise-Cross L, Mangum KD, Medlin MD, Mangiante L, Taylor JM, Mack CP. Nuclear RhoA signaling regulates MRTF-dependent SMC-specific transcription. Am J Physiol Heart Circ Physiol. 2014;307:H379–90. doi: 10.1152/ajpheart.01002.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 17.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeland JW, Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol Biol Cell. 2002;13:4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geneste O, Copeland JW, Treisman R. LIM kinase and diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 23.Goh WI, Ahmed S. mDia1-3 in mammalian filopodia. Commun Integr Biol. 2012;5:340–344. doi: 10.4161/cib.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, Ishizaki T, Narumiya S. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell. 2008;19:2328–2338. doi: 10.1091/mbc.E07-10-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe S, De Zan T, Ishizaki T, Yasuda S, Kamijo H, Yamada D, Aoki T, Kiyonari H, Kaneko H, Shimizu R, Yamamoto M, Goshima G, Narumiya S. Loss of a rho-regulated actin nucleator, mDia2, impairs cytokinesis during mouse fetal erythropoiesis. Cell Rep. 2013;5:926–932. doi: 10.1016/j.celrep.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Wallar BJ, Alberts AS. The formins: Active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–446. doi: 10.1016/s0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 27.Higgs HN. Formin proteins: A domain-based approach. Trends Biochem Sci. 2005;30:342–353. doi: 10.1016/j.tibs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Copeland JW, Copeland SJ, Treisman R. Homo-oligomerization is essential for F-actin assembly by the formin family FH2 domain. J Biol Chem. 2004;279:50250–50256. doi: 10.1074/jbc.M404429200. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Z, DiMichele LA, Hakim ZS, Rojas M, Mack CP, Taylor JM. Targeted focal adhesion kinase activation in cardiomyocytes protects the heart from ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2012;32:924–933. doi: 10.1161/ATVBAHA.112.245134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Z, DiMichele LA, Rojas M, Vaziri C, Mack CP, Taylor JM. Focal adhesion kinase antagonizes doxorubicin cardiotoxicity via p21(Cip1.) J Mol Cell Cardiol. 2014;67:1–11. doi: 10.1016/j.yjmcc.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French WJ, Creemers EE, Tallquist MD. Platelet-derived growth factor receptors direct vascular development independent of vascular smooth muscle cell function. Mol Cell Biol. 2008;28:5646–5657. doi: 10.1128/MCB.00441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115–2122. doi: 10.1161/ATVBAHA.108.175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MC. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4:141–147. doi: 10.4161/sgtp.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thumkeo D, Watanabe S, Narumiya S. Physiological roles of rho and rho effectors in mammals. Eur J Cell Biol. 2013;92:303–315. doi: 10.1016/j.ejcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120:3870–3882. doi: 10.1242/jcs.014365. [DOI] [PubMed] [Google Scholar]
- 38.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 39.Schleich JM, Abdulla T, Summers R, Houyel L. An overview of cardiac morphogenesis. Arch Cardiovasc Dis. 2013;106:612–623. doi: 10.1016/j.acvd.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 40.McNally E, Dellefave L. Sarcomere mutations in cardiogenesis and ventricular noncompaction. Trends Cardiovasc Med. 2009;19:17–21. doi: 10.1016/j.tcm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Gopinath SD, Narumiya S, Dhawan J. The RhoA effector mDiaphanous regulates MyoD expression and cell cycle progression via SRF-dependent and SRF-independent pathways. J Cell Sci. 2007;120:3086–3098. doi: 10.1242/jcs.006619. [DOI] [PubMed] [Google Scholar]
- 42.Toure F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279–1293. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Z, Li C, Frieler RA, Gerasimova AS, Lee SJ, Wu J, Wang MM, Lumeng CN, Brosius FC, 3rd, Duan SZ, Mortensen RM. Smooth muscle protein 22 alpha-cre is expressed in myeloid cells in mice. Biochem Biophys Res Commun. 2012;422:639–642. doi: 10.1016/j.bbrc.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sui Y, Park SH, Xu J, Monette S, Helsley RN, Han SS, Zhou C. IKKbeta links vascular inflammation to obesity and atherosclerosis. J Exp Med. 2014;211:869–886. doi: 10.1084/jem.20131281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellegrin S, Mellor H. The rho family GTPase rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goh WI, Lim KB, Sudhaharan T, Sem KP, Bu W, Chou AM, Ahmed S. mDia1 and WAVE2 proteins interact directly with IRSp53 in filopodia and are involved in filopodium formation. J Biol Chem. 2012;287:4702–4714. doi: 10.1074/jbc.M111.305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282:25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 49.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanizaki H, Egawa G, Inaba K, Honda T, Nakajima S, Moniaga CS, Otsuka A, Ishizaki T, Tomura M, Watanabe T, Miyachi Y, Narumiya S, Okada T, Kabashima K. Rho-mDia1 pathway is required for adhesion, migration, and T-cell stimulation in dendritic cells. Blood. 2010;116:5875–5884. doi: 10.1182/blood-2010-01-264150. [DOI] [PubMed] [Google Scholar]
- 51.Ishizaki T, Morishima Y, Okamoto M, Furuyashiki T, Kato T, Narumiya S. Coordination of microtubules and the actin cytoskeleton by the rho effector mDia1. Nat Cell Biol. 2001;3:8–14. doi: 10.1038/35050598. [DOI] [PubMed] [Google Scholar]
- 52.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 53.Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, Monypenny J, Ishizaki T, Bito H, Nozaki K, Hashimoto N, Matsuda M, Narumiya S. The rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing apc and c-src. Mol Cell Biol. 2006;26:6844–6858. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goulimari P, Knieling H, Engel U, Grosse R. LARG and mDia1 link Galpha12/13 to cell polarity and microtubule dynamics. Mol Biol Cell. 2008;19:30–40. doi: 10.1091/mbc.E06-11-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong B, Zhang SS, Gao W, Su H, Chen J, Jin F, Bhargava A, Chen X, Jorgensen L, Alberts AS, Zhang J, Siminovitch KA. Mammalian diaphanous-related formin 1 regulates GSK3beta-dependent microtubule dynamics required for T cell migratory polarization. PLoS One. 2013;8:e80500. doi: 10.1371/journal.pone.0080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daou P, Hasan S, Breitsprecher D, Baudelet E, Camoin L, Audebert S, Goode BL, Badache A. Essential and nonredundant roles for diaphanous formins in cortical microtubule capture and directed cell migration. Mol Biol Cell. 2014;25:658–668. doi: 10.1091/mbc.E13-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan J, Lordier L, Meyran D, Rameau P, Lecluse Y, Kitchen-Goosen S, Badirou I, Mokrani H, Narumiya S, Alberts AS, Vainchenker W, Chang Y. The formin DIAPH1 (mDia1) regulates megakaryocyte proplatelet formation by remodeling the actin and microtubule cytoskeletons. Blood. 2014 doi: 10.1182/blood-2013-12-544924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.