Abstract
Purpose
To determine if multiparametric-MRI (mpMRI)-targeted biopsies may replace systematic biopsies to detect higher grade prostate cancer (Gleason score ≥ 7), and whether biopsy may be avoided based on mpMRI among men with Gleason 3+3 prostate cancer on active surveillance (AS).
Materials and Methods
We identified men with previously diagnosed Gleason score 3+3 prostate cancer on AS who underwent a mpMRI and a follow-up prostate biopsy. Suspicion for higher grade cancer was scored on a standardized 5-point scale. All patients received a systematic biopsy. Patients with mpMRI regions-of-interest also underwent an MRI-targeted biopsy. The detection rate of higher grade cancer was estimated for different mpMRI scores using 3 biopsy strategies: systematic, MRI-targeted, and combined.
Results
Of 206 consecutive men on AS, 135 (66%) had a mpMRI region-of-interest. Overall, higher grade cancer was detected in 72 (35%) men. Higher mpMRI score was associated with an increased probability of detecting higher grade cancer (Wilcoxon-type trend test p < 0.0001). MRI-targeted biopsy detected higher grade cancer in 23% of men. MRI-targeted biopsy alone missed higher grade cancers in 17%, 12%, and 10% of patients with mpMRI scores of 3, 4, and 5, respectively.
Conclusions
MRI-targeted biopsies increased detection of higher grade cancer for men on AS compared to systematic biopsy alone; however a clinically relevant proportion of higher grade cancer was detected only using systematic biopsy. Despite the improved detection of disease progression using MRI-targeted biopsy, systematic biopsy cannot be excluded as part of surveillance for men with low risk prostate cancer.
Keywords: Prostate cancer, active surveillance, MRI, Image-Guided Biopsy
1. Introduction
For men diagnosed with Gleason 3+3 prostate cancer, cancer-specific mortality is low, and active surveillance (AS) is widely recommended by clinical guidelines [1]. The AS strategy requires serial biopsies to detect possibly more aggressive tumors, defined by Gleason grade and tumor volume. Currently, systematic transrectal ultrasound (TRUS)-guided prostate biopsy is the standard technique, but is limited due to its tendency to misclassify cancer risk as suggested by a significant rate of upgrading (23 – 61%) after radical prostatectomy in patients meeting different published criteria for AS who undergo surgery [2,3]. In a large prospective study of men managed with AS, routine systematic biopsies during follow-up detected progression to Gleason grade ≥ 3+4 in only 9.5% after a median follow up of 6.4 years [4]. Although the baseline risk between groups was varied, the difference in rates of upgrade may suggest that some men on AS may be misclassified and harbor higher risk disease.
The role of multiparametric MRI (mpMRI) for prostate cancer detection has been evaluated in different clinical settings [5–9]. Recent studies suggest MRI-targeted biopsy may be superior than systematic biopsy for risk classification [10]. We aimed to evaluate the efficacy of prostate MRI-targeted biopsy to detect higher grade cancer (defined here as Gleason score≥3+4) and also describe the efficacy to rule out higher grade disease in patients on AS for Gleason 3+3 prostate cancer. In addition, we sought to assess whether MRI-targeted biopsies may be used instead of systematic biopsy or whether a biopsy might be avoided based on the level of suspicion of detecting cancer on imaging depicted by MRI score.
2. Methods and Materials
2.1 Patient cohort
Following institutional review board approval, we reviewed our prospective clinical database of men with low risk prostate cancer on AS. We included men with previously confirmed clinically localized Gleason grade 3+3 prostate cancer enrolled on AS, who had an mpMRI and a prostate biopsy performed between January 2014 and January 2015. All outside biopsies were re-read at our institution. Patients with a diagnosis of Gleason ≥ 3+4 prostate cancer, and those that received definitive treatment for prostate cancer were excluded. We identified 71 (34%) patients who had no Regions-of Interest (ROI) on mpMRI (mpMRI score < 3) who underwent a systematic biopsy and 135 (66%) patients with at least one ROI on mpMRI (mpMRI score of 3–5) who underwent an MRI-targeted biopsy of the ROI followed by a systematic biopsy of the remaining areas of the prostate. The median number of previous biopsies was 2. Our reporting is consistent with standards of reporting for MRI-targeted biopsy studies (START) guidelines [10].
2.2 Imaging
Patients underwent mpMRI at least 3 months after the previous biopsy. Images were acquired under a magnetic field of 1.5-T (n = 24; 12%) with endorrectal coil, or 3-T (n = 182; 88%) without endorrectal coil. Most of the studies were performed at our institution (n = 184, 89%), outside studies were re-read at our institution. MRI systems (GE Healthcare, Wisconsin USA) and multichannel phased-array coils were used. Sequences acquired included T1-weighted images, T2-weighted images, diffusion-weighted sequences and parametric maps of apparent diffusion coefficients, and dynamic contrast-enhanced sequence. A detailed description of the planes and acquisition parameters can be found in Appendix 1. mpMRIs were evaluated as per standard clinical care by one of six members of our institution’s genitourinary (GU) radiology section, with 6 to 15 years of experience in GU radiology. mpMRI were scored on a 5-point suspicion Likert scale as previously published [11]. This scale was developed in our institution using whole-mount prostatectomy specimens as reference. It has been used in multiple studies investigating the value of prostate mpMRI [12–15], and appears to be equivalent [16–18] to the original version of the recently developed prostate imaging and reporting and data system (PI-RADS) [19], which is an expert consensus statement and still undergoing wide validation. PI-RADS is not used at our institution currently, and therefore not evaluated in this study where standard-of-care mpMRI interpretation was assessed. A shape was drawn by the radiologist on the T2-weighted images surrounding each ROI where the interpreting radiologist’s subjective degree of suspicion for the presence of cancer was at least 50% (eg, mpMRI score ≥ 3), for subsequent MRI-targeted biopsy.
2.3 Biopsy
The biopsy operator had access to the radiology report and images that were previously interpreted and annotated for targeted biopsy by the reporting radiologist. Patients with no ROI identified on mpMRI received a 14-core systematic TRUS-guided prostate biopsy consisting of samples obtained from the medial and lateral aspects of the base, middle, and apical portions of the prostate bilaterally, along with 2 samples from the transition zone. Patients with mpMRI-identified ROI received a targeted biopsy of each ROI, consisting of 2 cores obtained under visual registration (submitted separately labeled as “cognitive biopsy”) and 2 cores obtained under software registration using a computer-assisted elastic image fusion system with real-time 3D tracking technology (UroStation; Koelis, Grenoble, France) (submitted separately labeled as “fusion biopsy”), followed by a systematic biopsy using the same template as described above, but excluding areas that had been previously sampled with the MRI-targeted biopsy. Cores from each area were labeled and submitted separately. For analysis purposes, the “systematic biopsy strategy” in patients with ROI on mpMRI included the cognitive biopsy cores and the TRUS-guided systematic cores from rest of the prostate, to account for the fact that prostate areas that had been previously sampled with MRI-targeted biopsy were not re-sampled with the systematic biopsy. The “MRI-targeted biopsy strategy” included the cognitive biopsy and the fusion biopsy. The “Combined strategy” represented our surrogate gold-standard, and included the cognitive biopsy cores, the fusion biopsy cores, and the systematic cores from areas with no ROI on mpMRI. All MRI-targeted biopsies were performed by two urologists (BE and JC). All biopsy specimens were reviewed at our institution by a dedicated GU pathologist, and reports included location of the core, Gleason score, and amount of tumor in millimeters for each core.
2.4 Statistical analysis
To test the association between mpMRI score and the probability of reclassification, we used a Wilcoxon-type trend test and we displayed reclassification rates by mpMRI score. To assess the predictive value of each biopsy strategy by mpMRI score, we compared the number of patients biopsied based on MRI score, the number of higher grade cancers detected using the MRI-targeted biopsy versus the number of higher grade cancers detected using the systematic and the combined strategy and conversely, the number of higher grade cancers missed on MRI-targeted biopsy only versus the other two biopsy strategies including those who would have not been biopsied due to a low MRI score. We aimed to determine the number of higher grade cancers detected on MRI-targeted biopsy compared to the other biopsy strategies, and the number of higher grade cancers missed by the proposed MRI score thresholds of ≥3, ≥4, and 5 for biopsy. All analyses were conducted using Stata 12.0 (StataCorp, College Station, TX, USA).
3. Results
Patient characteristics are displayed in Table 1. Out of the 206 patients, 135 (66%) had at least one ROI on mpMRI. In all, higher grade cancer was detected in 72 patients (35%). The rate of higher grade cancer detection on systematic biopsy was 11% for those with no ROI on mpMRI, while the rate of higher grade cancer detection on the MRI-targeted plus systematic biopsy for those with ROI on mpMRI was 47%. Among the 8 patients with no ROI on mpMRI in whom higher grade cancer was detected, seven were Gleason grade 3+4, and only one was Gleason grade 4+3 (Table 2). The probability of reclassification to Gleason grade ≥ 3+4 prostate cancer was associated with increasing level of suspicion for higher grade disease based on mpMRI score (Wilcoxon-type trend test, p < 0.0001) in Figure 1. MRI-targeted biopsy failed to detect 17% and 12% of higher grade cancers among patients with an mpMRI score of 3 and 4, respectively (Figure 2). Higher grade prostate cancer was detected in all patients with an mpMRI score of 5; however, MRI-targeted biopsy failed to detect the higher grade tumor in 1 (10%) patient (Figures 1 and 2)
Table 1.
Patient characteristics
| Characteristic | N=206 |
|---|---|
|
| |
| Age at biopsy | 63 (57, 68) |
| MRI score | |
| 1 – 2 (No lesion) | 71 (34%) |
| 3 | 18 (8.7%) |
| 4 | 107 (52%) |
| 5 | 10 (4.9%) |
| Total PSA (ng/mL) | 5.2 (3.8, 7.4) |
| Prostate volume (mL) | 40.5 (29.0, 54.0) |
| Total PSA Density (ng/mL/mL) | 0.13 (0.08, 0.19) |
| Number of positive cores on biopsy (N=150) | 2 (1, 3) |
| Length of largest positive core (mm) (N=145) | 3 (2, 5) |
Values are displayed as median (interquartile range) or frequency (percentage).
Table 2.
Number of patients with higher grade cancer detected only in systematic biopsy, and missed on MRI-targeted biopsy stratified by MRI score
| Gleason Score | MRI score
|
Total | |||
|---|---|---|---|---|---|
| 1–2 (No lesion) |
3 | 4 | 5 | ||
| 3 + 4 | 7 | 3 | 11 | 0 | 21 |
| 4 + 3 | 1 | 0 | 1 | 1 | 3 |
| 4 + 4 | 0 | 0 | 1 | 0 | 1 |
| Total | 8 | 3 | 13 | 1 | 25 |
Fig. 1.
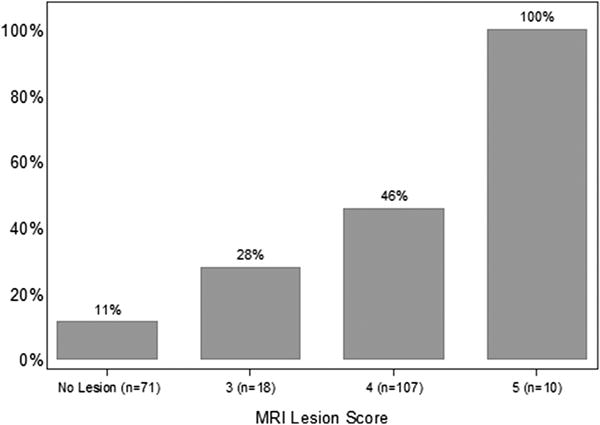
Percentage of patients who were reclassified from Gleason 3+3 to Gleason ≥ 3+4 stratified by mpMRI score. The number of patients who satisfied each mpMRI score is indicated on the x-axis.
Fig. 2.
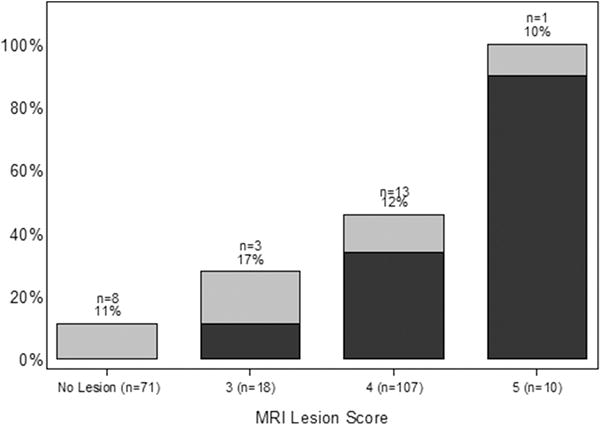
Reclassification rates from Gleason 3+3 to Gleason ≥ 3+4 stratified by mpMRI score. The black represents the higher grade cancers detected using MRI-targeted biopsy and the grey represents the higher grade cancers detected only on any biopsy. The numbers above the bars represent the number of higher grade cancers missed on MRI-targeted biopsy but were detected on systematic biopsy and the corresponding percentage.
Restricting the number of biopsies performed to those with ROI on mpMRI would reduce the number of biopsies performed, but would lead to an increased number of missed higher grade cancers (Table 3). For example, if we used an mpMRI score ≥ 3 as a threshold to trigger prostate biopsy, then the number of biopsies would be reduced by 35%, the number of patients that would fail to be reclassified with higher grade cancer would be 39 per 1000 patients and the number reclassified would be 311 per 1000 patients. Furthermore, relying on an MRI-targeted biopsy alone would increase the number of higher grade cancers that would have failed to be detected. For example, if a clinician were to use only the MRI-targeted biopsy strategy (fusion and cognitive targeting of ROI) in patients with ROI on mpMRI, 11% of those not biopsied (because of no ROI on mpMRI) would have higher grade cancer and 13% of patients with ROI on mpMRI would have higher grade cancer in areas not targeted by mpMRI; this strategy would fail to detect higher grade cancer in 12% of all patients.
Table 3.
Using different mpMRI score thresholds as the indication for biopsy, the number of patients biopsied higher grade cancers (Gleason score ≥ 7) detected and missed by biopsy strategy per 1000 men are shown. The higher grade cancers missed using the combined biopsy strategy (MRI-targeted + systematic) are those missed due to mpMRI score threshold. The higher grade cancers missed using MRI-targeted biopsy technique include those missed due to mpMRI score threshold and those missed in regions of the prostate not targeted. Higher grade cancers missed on systematic biopsy include those missed due to low mpMRI score and those that were detected only in the cores obtained only using MRI-targeted biopsy.
| MRI Score Threshold | Biopsy Type | Number Biopsied | Biopsies Avoided | Number Reclassified | Number Missed |
|---|---|---|---|---|---|
| All patients | MRI Target + Systematic | 1000 (100%) | 0 (0%) | 350 (35%) | 0 (0%) |
| MRI Score ≥ 3 | MRI Target + Systematic MRI Target Systematic |
655 (66%) | 345 (35%) | 311 (31%) 228 (23%) 277 (28%) |
39 (4%) 121 (12%) 73 (7%) |
| MRI Score ≥ 4 | MRI Target + Systematic MRI Target Systematic |
568 (57%) | 432 (43%) | 286 (29%) 218 (22%) 257 (26%) |
63 (6%) 131 (13%) 92 (9%) |
| MRI Score = 5 | MRI Target + Systematic MRI Target Systematic |
49 (5%) | 951 (95%) | 49 (5%) 44 (4%) 39 (4%) |
301 (30%) 306 (31%) 311 (31%) |
Due to rounding, the number of cancers caught and missed do not always sum up to the number detected based on any mpMRI score. MRI target = 2 cores obtained under visual registration + 2 cores obtained under software registration.
4. Discussion
In a contemporary cohort of men with Gleason 3+3 prostate cancer on AS who underwent mpMRI with systematic biopsy plus MRI-targeted biopsy in those with ROI on MRI, the probability of detecting higher grade cancer was associated with the level of suspicion determined by mpMRI score. Among men with no ROI on mpMRI who were reclassified based on Gleason grade, almost all were discovered to harbor Gleason grade 3+4 prostate cancer. Only one of these patients was discovered to harbor a Gleason 4+3 cancer on systematic biopsy. In patients with ROI on mpMRI, combining MRI-targeted and systematic biopsy techniques resulted in the highest detection rate for higher grade cancer. However, in this group of patients with ROI on mpMRI, we discovered that 10% to 17% had higher grade cancer in areas without ROI on mpMRI. In these men, the MRI-targeted biopsy technique failed to detect the higher grade cancer. These findings can be attributed to tumor characteristics such as low tumor volume; patient characteristics resulting in artifacts that reduce image quality, such as increased body mass index; issues related to mpMRI interpretation; or technical issues related to inadequate fusion of the ultrasound and mpMRI images. Above all, these results support the current clinical approach of combining MRI-targeted biopsy and systematic biopsy techniques. If the decision to perform prostate biopsy was determined by mpMRI score, then raising the threshold to an mpMRI score 4 or 5 would result in an unacceptably high number of men with undiagosed higher grade cancer.
In line with our results, Hu et al [20] reported that in men who met the Epstein criteria for insignificant cancer, MRI-targeted biopsy resulted in frequent detection of tumors exceeding the Epstein criteria (36%), and upgrading to Gleason score ≥ 7 prostate cancer (23%). In addition, all men with a ROI on mpMRI labeled as mpMRI score 5 were reclassified. In a study evaluating the rate of reclassification based on mpMRI score for men who underwent systematic biopsy only, Vargas et al [21] reported an association between mpMRI score and reclassification to Gleason score ≥ 7 prostate cancer. However, it is unclear in prior studies if the ROI detected on mpMRI was the index tumor due to a lack of targeted-biopsy techniques. The use of mpMRI as a substitute for biopsy is appealing as in this population the number of previous biopsies is a known risk factor for infectious complications [22]. Siddiqui et al [10] reported that using a nomogram based on mpMRI data will decrease the number of confirmatory biopsies in patients on AS by 68%. In our study, if only men with ROI on mpMRI (score 3–5) were biopsied using the combined strategy, the number of biopsies would decrease by a 34%, but higher grade cancers would fail to be detected in 4% of patients. In a study with fewer men evaluating the role of mpMRI for men on AS, Da Rosa el al [23] reported that MRI-targeted biopsy could detect higher grade cancer using fewer cores compared to systematic biopsy. In addition, the authors noted that no men were reclassified with higher grade prostate cancer if no ROI were seen on mpMRI. In contrast, we report higher grade cancer discovered on systematic biopsy in 11% (95% CI: 5–21%) of men with no ROI detected on mpMRI. A difference in our study was that more cores were obtained during systematic biopsy directed anteriorly in the transition zone. Our findings support the role of mpMRI in AS to assess risk and increase detection of higher grade prostate cancer by helping guide biopsies (Fig. 3). However, we caution againt using mpMRI alone to risk stratify because we identified a small but clinically significant number of Gleason ≥7 cancer in patients with no ROI identified on mpMRI, futhermore, we caution against using only MRI-targeted biopsy becase we detected a small but clinically significant number of Gleason≥7 cancer on systematic biopsy in areas with no ROI on mpMRI.
Fig.3.
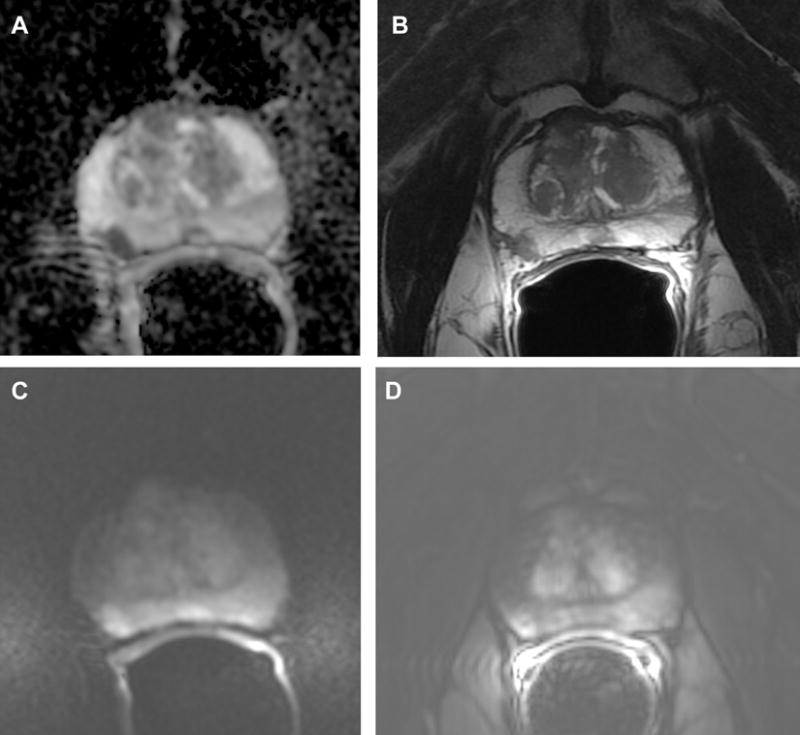
Prostate mpMRI images from a patient with MRI score 4, and biopsy confirmed Gleason 4+4 3a. Apparent-diffusion coefficient (ADC); 3b. Axial T2-weighted image; 3c. Diffusion weighted image; 3d. Dynamic contrast-enhanced image
Some limitations of our study should be noted. Physicians performing the biopsies reviewed mpMRI findings prior to performing systematic biopsies (Fig. 4). Among patients with ROI on mpMRI, the systematic biopsy was influenced by searching for hypoechoic areas in these suspicious regions of the gland, and the “systematic biopsy strategy” included the cognitive cores from the ROI plus systematic cores from non-suspicious areas. Therefore, the systematic biopsy results may overestimate the diagnostic accuracy systematic biopsy in a setting without access to mpMRI data. Had we compared targeted versus systematic biopsy without including the cognitive cores in the systematic strategy, our results would have been biased in favor of targeted biopsy. We did not have whole-mount prostatectomy specimens to determine the positive and negative predictive value of each biopsy technique. Finally, while Gleason score is widely accepted as a marker of aggressiveness, our definition of higher grade cancer (Gleason score ≥ 7) represents a heterogenous cohort and the clinical outcomes of not detecting these tumors is still unclear.
Fig. 4.
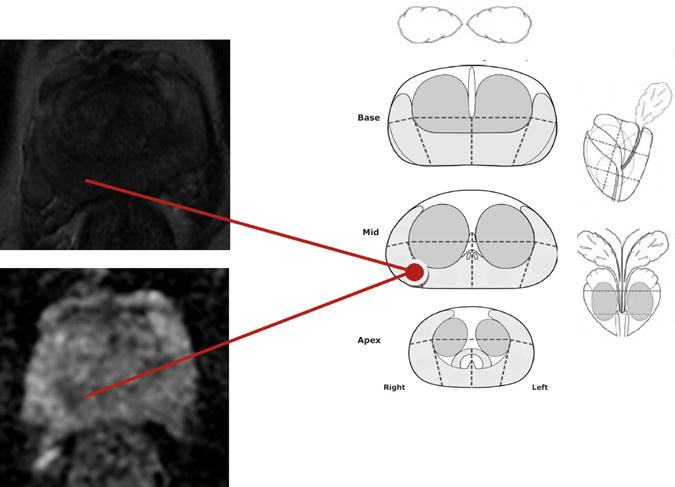
Diagram completed prior to cognitive biopsy. This patient had an MRI score of 4 and the MRI-targeted biopsy confirmed Gleason 4+4
Our study is strengthened by the inclusion of a wide population of AS patients beyond the traditional Epstein criteria, and hybrid validation (cognitive+fusion). This may explain the high overall reclassification rate compared to previous studies.
5. Conclusions
Among men with low risk prostate cancer on AS, a higher mpMRI score is associated with a higher probability of reclassification to Gleason ≥ 3+4 disease. The combination of MRI-targeted biopsy and systematic biopsy yields the highest number of higher grade cancers detected. However, across mpMRI scores, a clinically relevant proportion of Gleason ≥ 3+4 tumors are detected only using systematic biopsy. Until the technology of MRI-targeted biopsy can be improved, systematic biopsy is recommended in all patients regardless of mpMRI findings, in addition to targeted biopsy in patients with ROI on mpMRI to identify higher Gleason-grade prostate cancer in men on AS.
Acknowledgments
Funding: This study was supported the Sidney Kimmel Center for Prostate and Urologic Cancers, the NIH/NCI Cancer Center Support Grant P30 CA008748, and by David H. Koch through the Prostate Cancer Foundation.
List of Abbreviations
- AS
Active surveillance
- GU
Genitourinary
- mpMRI
multiparametric magnetic resonance imaging
- ROI
Regions-of-Interest
- TRUS
Transrectal Ultrasound
Appendix 1. mpMRI: planes acquired and acquisition parameters
The following sequences were acquired: transverse T1-weighted images; transverse, coronal, and sagittal T2-weighted images; and transverse diffusion-weighted sequences and parametric maps of apparent diffusion coefficients. Most patients (88%) also had a dynamic contrast-enhanced, three-dimensional, T1-weighted, spoiled gradient-echo sequence after intravenous injection of 0.1 mmol of gadopentetate dimeglumine (Magnevist™, Berlex Laboratories, Montville, New Jersey, USA) per kilogram of body weight. Acquisition parameters (range) for T1-weighted images were repetition time (TR) (400–1583.34 msec), echo time (TE) (6.016–15.756 msec), slice thickness (3–7 mm), interslice gap (0 – 2 mm), and field of view (FOV) (256×256 – 512×512 mm); for T2-weighted images were TR (2820–8410 msec), TE (88–125.4 msec), slice thickness (3–4 mm), interslice gap (0 mm) and FOV (256×256 – 512×512 mm); and for diffusion-weighted imagery were TR (3500–8200 msec), TE (60.5–101.8 msec), slice thickness (3–4 mm), interslice gap (0 mm), and FOV (256×256 – 512×512); b values used were 0 and 1000 sec/mm2. Acquisition parameters were similar between in-house and outside studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Attestation: All authors attest to approving the final manuscript. BE, MA, DDS, and PR have reviewed the original study data and data analysis and attest to the integrity of the original data and the analysis reported in this manuscript. BE and MA are the archival authors who are responsible for maintaining the study records.
IRB: The data used in this study were reviewed by the IRB and granted a Waiver of Authorization determined to be exempt from human subject research consent requirement.
References
- 1.Mohler J, Armstrong AJ, Bahnson RR, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:1081–7. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 2.Conti SL, Dall’era M, Fradet V, Cowan JE, Simko J, Carroll PR. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–33. doi: 10.1016/j.juro.2008.11.107. [DOI] [PubMed] [Google Scholar]
- 3.Lee MC, Dong F, Stephenson AJ, Jones JS, Magi-Galluzzi C, Klein EA. The Epstein criteria predict for organ-confined but not insignificant disease and a high likelihood of cure at radical prostatectomy. Eur Urol. 2010;58:90–5. doi: 10.1016/j.eururo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 5.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33:17.e1–7. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Abd-Alazeez M, Ahmed HU, Arya M, et al. Can multiparametric magnetic resonance imaging predict upgrading of transrectal ultrasound biopsy results at more definitive histology? Urol Oncol. 2014;32:741–7. doi: 10.1016/j.urolonc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Bonekamp D, Bonekamp S, Mullins JK, Epstein JI, Carter HB, Macura KJ. Multiparametric magnetic resonance imaging characterization of prostate lesions in the active surveillance population: incremental value of magnetic resonance imaging for prediction of disease reclassification. J Comput Assist Tomogr. 2013;37:948–56. doi: 10.1097/RCT.0b013e31829ae20a. [DOI] [PubMed] [Google Scholar]
- 9.Puech P, Rouvière O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy–prospective multicenter study. Radiology. 2013;268:461–9. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544–52. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–84. doi: 10.1148/radiol.11102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wibmer A, Vargas HA, Sosa R, Zheng J, Moskowitz C, Hricak H. Value of a standardized lexicon for reporting levels of diagnostic certainty in prostate MRI. AJR Am J Roentgenol. 2014;203:W651–7. doi: 10.2214/AJR.14.12654. [DOI] [PubMed] [Google Scholar]
- 14.Vargas HA, Akin O, Shukla-Dave A, et al. Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology. 2012;265:478–87. doi: 10.1148/radiol.12120041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazaheri Y, Shukla-Dave A, Hricak H, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging–correlation with pathologic findings. Radiology. 2008;246:480–8. doi: 10.1148/radiol.2462070368. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology. 2013 Nov;269(2):482–92. doi: 10.1148/radiol.13122233. [DOI] [PubMed] [Google Scholar]
- 17.Vaché T, Bratan F, Mège-Lechevallier F, Roche S, Rabilloud M, Rouvière O. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology. 2014;272:446–55. doi: 10.1148/radiol.14131584. [DOI] [PubMed] [Google Scholar]
- 18.Rosenkrantz AB, Lim RP, Haghighi M, Somberg MB, Babb JS, Taneja SS. Comparison of interreader reproducibility of the prostate imaging reporting and data system and likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol. 2013;201:W612–8. doi: 10.2214/AJR.12.10173. [DOI] [PubMed] [Google Scholar]
- 19.PI-RADS: prostate imaging and reporting and data system, version 2. American College of Radiologists Web site; 2015. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf. Accessed August 18, 2015. [Google Scholar]
- 20.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192:385–90. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188:1732–8. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehdaie B, Vertosick E, Spaliviero M, et al. The impact of repeat biopsies on infectious complications in men with prostate cancer on active surveillance. J Urol. 2014;191:660–4. doi: 10.1016/j.juro.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 23.Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging. 2014;41:220–5. doi: 10.1002/jmri.24710. [DOI] [PubMed] [Google Scholar]


