Abstract
Pseudoxanthoma elasticum (PXE) is a heritable disorder mainly characterized by calcified elastic fibers in cutaneous, ocular, and vascular tissues. PXE is caused by mutations in ABCC6, a gene encoding an ABC transporter predominantly expressed in liver and kidneys. The functional relationship between ABCC6 and elastic fiber calcification is unknown. We speculated that ABCC6 deficiency in PXE patients induces a persistent imbalance in circulating metabolite(s), which may impair the synthetic abilities of normal elastoblasts or specifically alter elastic fiber assembly. Therefore, we compared the deposition of elastic fiber proteins in cultures of fibroblasts derived from PXE and unaffected individuals. PXE fibroblasts cultured with normal human serum expressed and deposited increased amounts of proteins, but structurally normal elastic fibers. Interestingly, normal and PXE fibroblasts as well as normal smooth muscle cells deposited abnormal aggregates of elastic fibers when maintained in the presence of serum from PXE patients. The expression of tropoelastin and other elastic fiber-associated genes was not significantly modulated by the presence of PXE serum. These results indicated that certain metabolites present in PXE sera interfered with the normal assembly of elastic fibers in vitro and suggested that PXE is a primary metabolic disorder with secondary connective tissue manifestations.
INTRODUCTION
Pseudoxanthoma elasticum (PXE) is a complex disorder with multiorgan involvement and a progressive and uneven severity (Neldner, 1988; Chassaing et al., 2005). This disease is characterized by connective tissue alterations, including calcification of elastic fibers. PXE patients accumulate fragmented calcified elastic fibers, altered collagen fibrils, and proteoglycans in elastic tissues, resulting in lesions predominantly in the skin, retina, and arterial walls (Uitto and Shamban, 1987). Typical skin lesions appear as yellowish papules in flexural areas and are associated with loss of elasticity (Uitto and Shamban, 1987; Neldner, 1988; Uitto et al., 1998). Calcification of elastic fibers of the Bruch’s membrane of the eye results in angioid streaks that often lead to subretinal neovascularization and hemorrhages, resulting in the loss of central vision (Weenink et al., 1996). The mineralization of elastic fibers of arteries often causes cardiovascular manifestations such as premature peripheral vascular occlusive disease, intermittent claudication, and/or gastrointestinal bleeding (Mendelsohn et al., 1978; Nishida et al., 1990; Lebwohl et al., 1993). Surprisingly, it was established that the PXE phenotype derives from mutations in an ABC transporter gene called ABCC6 (Bergen et al., 2000; Le Saux et al., 2000; Ringpfeil et al., 2000; Struk et al., 2000). While mutations in similar genes such as ABCC2, -7, and -8 cause a variety of phenotypes (Riordan et al., 1989; Thomas et al., 1995; Toh et al., 1999) consistent with the known function(s) of these transporters, the functional relationship between ABCC6 and elastic fibers defects is obscure. The ABCC6 cDNA and transport activity of the encoded protein have recently been characterized (Kool et al., 1999; Ilias et al., 2002), but the true nature of the endogenous substrate(s), the overall function of ABCC6, and its influence on connective tissues are largely unknown. The elastic fiber abnormalities associated with the PXE phenotype initially suggested that PXE was a strict connective tissue disorder. In support of this original assumption, skin fibroblasts isolated from PXE patients displayed abnormal characteristics with decreased cell–cell and cell matrix adhesion properties, higher proliferation, and altered synthesis of connective tissue components, including elastin, collagen, and proteoglycans (Lebwohl et al., 1993; Baccarani-Contri et al., 1996; Quaglino et al., 2000). However, abundant ABCC6 mRNA in human and rodents is almost exclusively found in liver and kidney (Kool et al., 1999; Madon et al., 2000; Scheffer et al.,2001). Since PXE patients display seemingly normal hepatic and renal function (Uitto et al., 2001), we speculated that the deficiency of ABCC6 activity induces a persisting imbalance in certain circulating metabolite(s) that may indirectly impair the synthetic ability of matrix-producing cells or specifically alter the initial assembly of elastic fibers, thereby compromising their durability and function.
In this study, we compared the deposition of major elastic fiber components and associated gene expression in primary cultures of skin fibroblasts isolated from unaffected individuals and PXE patients maintained in the presence of fetal bovine serum (FBS), serum from normal individuals, or from PXE patients. Our results indicated that although PXE fibroblasts demonstrated elevated expression of genes encoding elastic fiber-associated proteins, these cells deposited structurally normal elastic fibers, when cultured in the presence of FBS or normal human sera. However, we found that the addition of sera from a PXE patient to the culture medium promoted the deposition of abnormal elastic fibers, not only in PXE fibroblasts but also in normal fibroblasts and normal human aortic smooth muscle cells. Our data clearly suggested that unknown circulating metabolites (or the lack thereof) modulated by ABCC6 contribute to the development of the PXE phenotype.
RESULTS
PXE-derived fibroblasts produce normal elastic fibers when cultured with FBS
Immunostaining of 10-day-old cultures with antibodies specific to elastin and fibrillin 1 revealed that PXE fibroblasts maintained in the presence of 10% FBS produced an abundant network of elastic fibers (Figure 1b and d). The distribution pattern of these elastic fibers did not appreciably differ from those observed in cultures of normal cells (Figure 1a and c). The distribution patterns of immunodetectable fibronectin and chondroitin sulfate-containing moieties produced by PXE cells (Figure 1f and h) and normal fibroblasts (Figure 1e and g) were also similar. Additional immunostainings with antibodies specific to collagen type I, elastin-binding protein, biglycan, and versican did not demonstrate any significant differences in the distribution pattern of these ECM components between cultures of normal and PXE fibroblasts (data not shown).
Figure 1. Extracellular matrix components deposited by control and PXE fibroblasts.
Fibroblasts were derived from normal and PXE patients and were cultured in medium supplemented with 10% FBS. Fibroblasts were grown for 10 days and the deposited matrix network was revealed by immunofluorescence using antibodies specific to (a, b) elastin, (c, d) fibrillin-1, (e, f) fibronectin, and (g, h) chondroitin sulfate-containing glycoaminoglycan. Representative photomicrographs are presented. (b) No structural difference in the matrix network was visible between normal and PXE cells, except for the overabundance of elastin. Nuclei were counterstained with propidium iodide to reveal cell density.
Morphometric analysis (Figure 2) and statistical evaluation of immunodetected ECM components revealed that amounts of elastin, fibrillin-1, microfibril-associated glycoprotein-1 (MAGP-1), and collagen type I deposited by PXE fibroblasts cultured in the presence of FBS significantly exceeded those present in normal fibroblast cultures. Indeed, PXE cells demonstrated more elastin (+45%), fibrillin-1 (+30%), MAGP-1 (+25%), and collagen type I (+15%) than normal fibroblasts. In contrast, PXE fibroblasts seemed to produce less fibronectin (−15%) and matrix components containing chondroitin sulfate (−25%).
Figure 2. Extracellular matrix components detected by immunohistochemistry.
10-day-old cultures of normal and PXE fibroblasts supplemented with 10% FBS were used. The morphometric analysis of extracellular matrix components was performed using Image-Pro Plus software from Media Cybernetics. MAGP: microfibril-associated glycoprotein-1, CS-GAG: chondroitin sulfate-containing glycoaminoglycan. Each ECM group was analyzed from three separate cultures of three normal and three PXE fibroblast cell lines, and the area occupied by the particular immunodetectable component was quantified. The abundance of each component was then expressed as a percentage of the entire analyzed field (mean±SD (*P<0.002; **P<0.05).
To determine whether the immunodetectable elastin associated with the extracellular fibrillar network represented mature crosslinked elastin, metabolic labeling of cultures with [3H]valine followed by an assay of NaOH-insoluble elastin was used. Our results demonstrated that amounts of deposited insoluble elastin were on average increased by 41% (P<0.01) in PXE fibroblast cultures as compared to normal cells (Figure 3). These results were consistent with the immunofluorescence morphometric analysis.
Figure 3. Quantitative analysis of insoluble elastin.
The total insoluble elastin labeled with [3H]valine and produced by three normal skin fibroblasts (□) and three PXE skin fibroblasts (■) after 10 days of cultures was quantified. PXE cells deposited on average 41% more insoluble elastin than their unaffected counterparts in the presence of 10% FBS in the culture medium. Values of mean±SD from four different experiments were collected for statistical evaluation (*P<0.01).
RT-PCR experiments were performed with TaqMan probes specific to tropoelastin, fibrillin-1, MAGP-1, lysyl oxidase (LOX), LOX-like (LOXL), and collagen type I to establish whether the changes resulted directly from increased gene expression. All the tested genes were found to be significantly upregulated (P<0.01; Figure 4), except for LOXL, which presented an expression level similar to normal fibroblasts. The expression of tropoelastin showed the greatest increase (ninefold), while that of fibrillin-1, LOX, and collagen type I displayed moderate upregulation (2–3.5-fold). The level of expression clearly corroborated the increase in deposition of these proteins as detected by immunofluorescence and metabolic labeling.
Figure 4. Extracellular matrix gene expression in normal and PXE fibroblasts.
The fibroblasts were cultured in medium supplemented with 10% FBS. Total RNA was extracted after 10 days of culture and was reversed transcribed. Real-time PCRs were performed using TaqMan probes (Applied Biosystems) specific for tropoelastin (ELN), fibrillin-1 (FBN-1), microfibril-associated glycoprotein-1 (MAGP-1), lysyl oxidase (LOX), lysyl oxidase-like (LOXL), collagen type I (COL1), and the β-actin cDNAs. Units are ratio of the relative gene expression to β-actin and are shown normalized to expression in normal fibroblasts. Standard errors are indicated (n = 3, *P<0.01).
Since the age of donors from whom normal and PXE fibroblasts were derived could only be matched approximately (average 39±18 years), the level of tropoelastin gene expression and the total insoluble elastin output was verified and no significant variation was observed between our samples (Figure 3).
PXE serum induces abnormal elastogenesis in cultures of normal and PXE fibroblasts
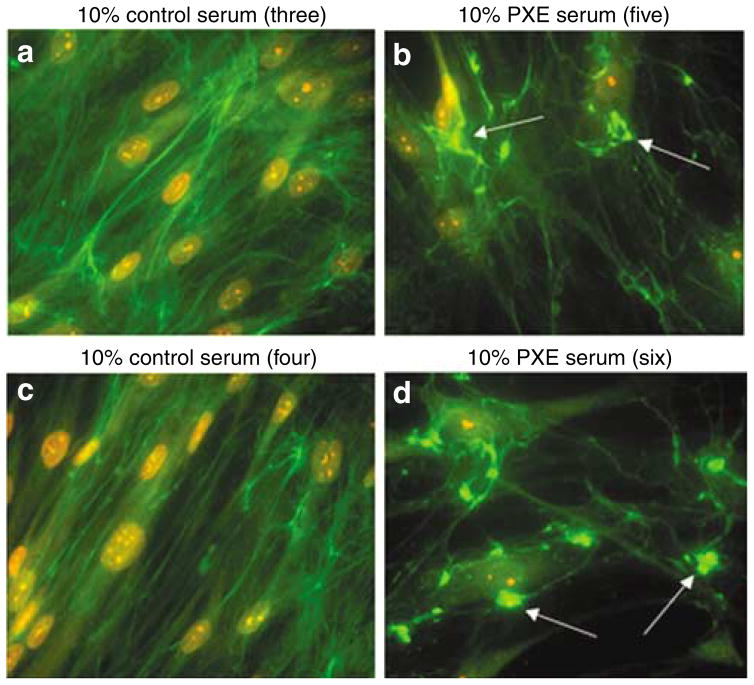
To test the hypothesis that deficiency in ABCC6 transporter activity causes an imbalance in certain circulating factors that modulate the production or durability of elastic fibers, we cultured normal and PXE fibroblasts in media supplemented with human serum derived either from unaffected individuals or from PXE patients. The expression of the relevant genes and the deposition of elastic fiber-related proteins were evaluated with the same methods as those used for cultures in the presence of FBS. Immunohistochemical assessment of 10-day-old cultures demonstrated that dermal fibroblasts derived either from normal individuals or from PXE patients deposited normal elastic fiber network when maintained in the presence of 10% normal human serum (Figure 5a and b). However, the deposition of elastic fibers by normal and PXE fibroblasts was dramatically altered when the media were supplemented with 10% serum from PXE patients (Figure 5c–h). Fibroblasts exposed to PXE serum produced fewer and thinner elastic fibers than those maintained with FBS or normal human serum. Interestingly, the reduction in quantity and thickness of normal elastic fibers in PXE serum-treated cultures seemed to be correlated, to some extent, with the appearance of amorphous elastin aggregates protruding from the fibers, as illustrated in Figure 5.
Figure 5. Immunofluorescent labeling of elastin deposited by normal and PXE fibroblasts.
The dermal fibroblasts derived from normal and PXE patients were cultured in medium supplemented with 10% serum samples from normal individuals or PXE patients. Fibroblasts were grown for 10 days and the network was revealed by immunofluorescence using an anti-elastin antibody. Representative photomicrographs of normal and PXE fibroblasts grown in normal serum are shown in panels a and b, respectively, while panels c–h represent three different PXE serum samples. Arrows point to large abnormal deposits of immunodetectable elastin. Nuclei were counterstained with propidium iodide to reveal cell density.
These alterations of elastic fibers were consistently observed in cultures treated with all PXE serum samples tested, but were not mineralized, as determined by von Kossa staining (data not shown).
Electron microscopy analysis revealed that PXE sera seemed to affect the early steps of elastic fiber formation probably by interfering with the formation of the microfibrillar scaffold. Indeed, 5-day-old cultures of normal skin fibroblasts cultured with normal human serum produced long and parallel microfibrils (Figure 6a), while fibroblasts maintained with PXE serum deposited irregular and fragmented microfibrils (Figure 6b). Electron micrographs of 10-day-old cultures maintained in the same conditions further demonstrated that cultures treated with PXE sera contained abnormally fragmented fibers in which elastin appeared to aggregate on the periphery of an irregularly assembled microfibrillar scaffold (Figure 6c and d).
Figure 6. Representative electron micrographs of cultures of skin fibroblasts maintained in 10% human sera.
Upper panel: 5-day-old cultures of normal skin fibroblasts grown with (a) normal sera and (b) from a PXE patient. While cells maintained in the presence of normal human serum produce normal microfibrills, fibroblasts treated with PXE-patient serum deposited irregular and fragmented microfibrils. Lower panel: electron micrographs showing 10-day-old cultures of normal skin fibroblasts maintained in sera from (c) unaffected individuals and (d) from a PXE subject. Cells maintained with normal serum deposit well-assembled elastic fibers, but cultures treated with PXE serum contain abnormal and fragmented fibers on an irregularly assembled microfibrillar scaffold. MF: microfibrils, EF: elastic fiber, F: fibroblasts. Image scale is indicated on panel d.
Results of metabolic labeling with [3H]valine, followed by assay of insoluble elastin, also demonstrated that cultures of PXE fibroblasts deposited more insoluble elastin than normal fibroblasts (+66%, P<0.01) when maintained in media supplemented with normal human serum. Addition of serum from PXE patients to culture media produced a similar trend (+91%, P<0.01) in both experimental groups (normal vs PXE fibroblasts); however, the stimulation of insoluble elastin deposition in the presence of PXE serum was slightly lower than that induced by normal human serum (data not shown).
Results of gene expression studies performed with RNA extracted from fibroblast cultures confirmed that PXE fibroblasts expressed significantly more tropoelastin than normal cells when maintained with normal human serum, and that the presence of PXE serum had no significant effect on mRNA synthesis. Similar results were obtained with MAGP-1 and collagen type I expression. However, fibrillin 1 and LOX showed a moderate decrease when PXE fibroblasts were exposed to PXE serum (Figure 7). Interestingly, after transient transfection with a vector carrying the ABCC6 cDNA to test whether the observed phenotype could be rescued, we found that overexpressing ABCC6 in normal and PXE fibroblasts had no influence on the level of expression of tropoelastin and the other elastic fiber-associated gene (data not shown) regardless of the type of serum used (FBS, normal or PXE). These results suggested that ABCC6 function in fibroblasts has little or no influence on elastic fiber production.
Figure 7. Extracellular matrix gene expressions in normal and PXE fibroblasts.
The fibroblasts were cultured in medium supplemented with 10% normal or PXE serum. Total RNA was extracted after 10 days of culture and was reversed transcribed. Real-time PCRs were performed using TaqMan probes (Applied Biosystems) specific for tropoelastin (ELN), fibrillin-1 (FBN-1), microfibril-associated glycoprotein-1 (MAGP-1), lysyl oxidase (LOX), lysyl oxidase-like (LOXL), collagen type I (COL1), and the β-actin cDNAs. Units are ratio of the relative gene expression to β-actin and are shown normalized to expression in normal fibroblasts. Standard errors are indicated (n = 3, **P<0.05).
PXE serum induces abnormal elastogenesis in cultures of normal smooth muscle cells
Since PXE is also characterized by calcification of elastic fibers in vascular tissues, we tested whether the presence of PXE serum would affect production of elastic fibers in cultures of smooth muscle cells (SMC) derived from normal human aorta. The results demonstrated that normal human serum stimulated the production of normal elastic fibers, whereas the presence of PXE serum grossly impaired the deposition of extracellular elastin by these normal SMC (Figure 8). The SMC cultures maintained with PXE serum showed elastin aggregates identical to those observed in cultures of dermal fibroblasts. It is noteworthy that normal SMC express higher amount of ABCC6 mRNA than skin fibroblasts (Beck et al., 2003) and yet displayed the same response to PXE serum.
Figure 8. Immunofluorescent labeling of elastin deposited by aortic smooth muscle cells.
The smooth muscle cells were derived from a normal 6-year-old individual in culture medium supplemented with 10% serum samples from normal individuals or PXE patients. The cells were grown for 5 days and the network was revealed by immunofluorescence using an anti-elastin antibody. Representative pictures of smooth muscle cells grown in normal serum are shown in panels a and c, respectively, while panels b and d represent cells cultured with PXE serum samples. Arrows point to large abnormal deposits of immunodetectable elastin. Nuclei were counterstained with propidium iodide.
DISCUSSION
ABCC6 as several other ABC transporters of the same subfamily “C” are thought to actively export metabolites from the basolateral or apical sides of polarized cells (Madon et al., 2000; Beck et al., 2003). ABCC6 is particularly abundant in renal proximal tubules and hepatocytes (Madon et al., 2000; Beck et al., 2003) and is also present in numerous epithelial cell types (Beck et al., 2005). Remarkably, nonpolarized cells such as arterial SMC, dermal fibroblasts, and other connective tissue cells that are capable of elastic fiber synthesis, present moderate or low levels of ABCC6 expression (Gorgels et al., 2005). However, the basic functions of these cells seem to be significantly affected by ABCC6 deficiency and could be responsible for the major phenotypic changes observed in PXE. However, the endogenous substrates of ABCC6 are presently not known and the pathomechanism underlying the PXE phenotype is only a matter of speculation. Indeed, it has been proposed that ABCC6 deficiency in affected skin, notably in dermal fibroblasts, could directly promote the deposition of an aberrant extracellular matrix, including abnormal elastic fibers (Beck et al., 2003; Boraldi et al., 2003). An alternate hypothesis stated that ABCC6 could instead be involved in a detoxification process in liver and/or kidney, and that in the absence of a functional transporter certain compounds accumulate or are depleted, resulting in the progressive fragmentation and systemic calcification of elastic fibers (Uitto et al., 2001). If indeed the lack of ABCC6 in kidneys or liver indirectly influences elastic fibers in the dermis, arteries, or ocular tissues, then certain metabolites must be circulating to promote the systemic alteration of extracellular matrix (Gheduzzi et al., 2003) observed in PXE patients. The experiments reported here were carried out to specifically evaluate these hypotheses.
In this study, we investigated the effects of serum samples derived from PXE subjects and from unaffected individuals on the deposition of extracellular matrix components in cultures of normal and PXE-derived dermal fibroblasts. All tested fibroblasts were derived from lesional skin biopsies of different PXE patients and from equivalent skin areas of unaffected donors. Results of immunohistochemistry, metabolic labeling, and gene expression studies showed that PXE fibroblasts cultured in medium supplemented with FBS produced an abundant extracellular matrix characterized by the presence of a structurally normal network of elastic fibers (Figures 1–3). The increased deposition of major elastic fiber components as well as other ECM molecules by PXE fibroblasts was associated with an overall increase in ECM gene expression, with the notable exception of LOXL (Figure 4). The high level of expression of these genes in vitro suggested a permanent phenotypic shift of PXE fibroblasts that appeared independent of the environment of the cells. Interestingly, the LOXL gene that encodes an enzyme essential for elastic fiber homeostasis (Maki et al., 2002; Hornstra et al., 2003; Liu et al., 2004) was not elevated in PXE fibroblasts. This would suggest that these cells produced an under-crosslinked elastin, and thus less durable fibers that could possibly be responsible for the abnormal PXE phenotype, as suggested previously (Baccarani-Contri et al.,1996; Bacchelli et al., 1999; Quaglino et al., 2000). However, our experiments showed that PXE fibroblast produced fully polymerized elastin. This fact would suggest that, despite their apparent abnormal in vitro phenotype, PXE fibroblasts might only play a limited role towards the elastic fiber defects observed in PXE patients. In addition, the presence of PXE serum equally resulted in the abnormal production of elastic fibers in cultures of normal and PXE fibroblasts and normal SMC. Thus, it appears that the impaired elastogenesis occurring in these conditions is independent of the overproduction of elastic fiber components observed in PXE fibroblasts and the expression of ABCC6, as shown by the rescue experiments.
The current understanding of elastic fiber formation in the pericellular space indicates that the secreted tropoelastin molecules must be assembled on a preformed microfibrillar scaffold in order to be crosslinked into insoluble and resilient elastin by LOX (Bedell-Hogan et al., 1993). The major component of this scaffold is fibrillin-1, which assembles into head-to-tail periodically beaded structures (Kielty et al., 2002; Rock et al., 2004). MAGP-1 is thought to bind the beaded regions of the fibrillin-containing microfibrils and subsequently promote the accretion of tropoelastin (Clarke and Weiss 2004). The microfibrilar scaffold thus critically influences tropoelastin deposition by providing the foundation and organization of mature elastic fibers (Sherratt et al., 2003). One can then reasonably assume that any defect in the microfibrils assembly is very likely to alter the normal formation of elastic fibers. Indeed, mutations in the fibrillin 1 gene lead to Marfan syndrome (Dietz and Pyeritz, 1995).
Consistent with this assumption, our results indicated that the abnormal elastic fibers we observed were unlikely to result from the insufficient supply of one or more of the elastic fiber components or crosslinking activity. More specifically, the ultrastructural examination of the abnormal elastic fibers suggested that a defective microfibrilar scaffold (Figure 6b) was responsible for the observed alterations. Indeed, one can speculate that the secreted tropoelastin molecules would be unable to assemble properly along the disorganized microfibrils and would be forced to aggregate into the amorphous structures we observed. It is interesting to note that the deposited elastin was crosslinked as efficiently as in controls, indicating that the secreted tropoelastin molecules assumed a structure compatible with the progressive crosslinking activity of LOX (Bellingham et al., 2001) even in the absence of orderly assembly on the microfibrils. We propose that such aggregates containing crosslinked but improperly anchored elastin are reminiscent of the structurally invalid elastic fibers observed in PXE patients, even though these structures were not calcified in the experimental conditions we used. The lack of mineralization was not unexpected, since the cell culture media were not favorable to mineralization (Sugitani et al., 2003) and also because samples of serum from PXE patients contained levels of calcium and phosphate within the normal range. Moreover, it is known that mineralization of elastic fibers does not occur on early stages of their assembly (Davis 1993) and that, in PXE patients as well as in mouse models of PXE, the mineralization of elastic fibers occurs progressively in the second decade of their life (Neldner 1988) or at about 3–5 weeks of age (Gorgels et al., 2005; Klement et al., 2005).
The overall clinical manifestations of the PXE phenotype suggest that the lack of ABCC6 function relates to deterioration of elastic fibers. Although the in vitro conditions of our experiments do not reflect in vivo conditions, results presented in this report showed unambiguously that metabolites in the serum of PXE patients interfered with the initial assembly of elastic fibers produced by normal human skin fibroblasts and normal aortic SMC. It is the first indication that the pathology of PXE derives from the defective modulation of circulating factors by ABCC6 transport activity probably in kidney and/or liver.
We hope that this report will initiate a series of comprehensive analytical studies that may eventually identify these serum factors and precisely determine the level of their action during the complicated process of elastic fiber formation. We also anticipate that the identification of factors interfering with elastic fiber assembly would lead to the development of therapeutic measures alleviating the symptoms of this still puzzling disease.
MATERIALS AND METHODS
Fibroblasts, smooth muscle cells, and serum samples
Primary fibroblasts were derived from biopsies of affected skin from three PXE patients and equivalent biopsies from three unaffected individuals (Table 1). These skin samples were obtained with the patients’ consent and were generous gifts of Drs F.M. Pope, Z. Urban, and from PXE International, Inc. All donors were females, except one male PXE patient. The dates of birth of five patients and unaffected subjects were known, whereas the ages of two subjects could not be determined. The smooth muscle cells were derived from a single 6-year-old female without evidence of pathology. The serum samples were collected with informed consents of the donors and were prepared by centrifugation from coagulated blood collected in tubes with no additives. The serum samples were subsequently frozen and stored at −20°C. For the experiments, both individual and pooled serum samples were used in separate cultures. The institutional review boards of the participating Universities or institutions approved the collection and use of these samples and experiments adhered to the Declaration of Helsinki Principles. The ABCC6 genotype of fibroblasts and serum donors was determined according to published criteria and methods (Le Saux et al., 2001; Chassaing et al., 2004).
Table 1.
Fibroblast cells and serum samples
| Cells | Diagnosis | Mutations | Age (years) |
|---|---|---|---|
| Fibroblast P24 (F) | Normal | wt/wt | 54 |
| Fibroblast 9063 (F) | Normal | wt/wt | 25 |
| Fibroblasts 9007 (F) | Normal | wt/wt | ? |
| Aortic SMC (F) | Normal | wt/wt | 6 |
| Fibroblast P411 (F) | PXE | IVS8+2delTG/del23-29 | 44 |
| Fibroblast HGS (F) | PXE | ?/? | 21 |
| Fibroblast U21 (M) | PXE | R1141X/R1141X | ? |
| Serum samples | |||
| Control #1 (M) | Normal | wt/wt | 45 |
| Control #2 (M) | Normal | wt/wt | 34 |
| Control #3 (pool)1 | Normal | wt/wt | Av. 68 |
| Control #4 (pool)1 | Normal | wt/wt | Av. 18 |
| PXE 1 (F) | PXE | R391G/del23-29 | 50 |
| PXE 2 (F) | PXE | IVS21+1 G>T/4104delC | 51 |
| PXE 3 (F) | PXE | IVS13-29 T>A/R1141X | 41 |
| PXE 4 (M) | PXE | ?/? | 52 |
| PXE 5 (F) | PXE | ?/? | 69 |
| PXE 6 (M) | PXE | Y768X/Y768X | 18 |
Control #3: pool of 10 samples, age >65, control #4: pool of 15 samples age <30. Av.: average, SMC: smooth muscle cell.
Cell cultures
The fibroblasts derived from the skin biopsies were routinely passaged by trypsinization. All experiments were performed with skin fibroblasts at passages 4–6. Cells were maintained in DMEM containing 1% antibiotics/antimycotics and supplemented with either 10% FBS from GIBCO Life Technologies (Burlington, ON) or 10% heat-inactivated serum from unaffected subjects or PXE patients. Transient transfections were performed using the GeneJammer transfection reagent (Stratagene, La Jolla, CA) as described by the manufacturer. Briefly, cells were plated in 24-well plates at 2×105 cells per well and incubated at 37°C until 80% confluence. Cells were rinsed once with serum-free medium without antibacterial agents before transfection. Cells were then transfected with 1 μg of pCDNA3.1 vector carrying the ABCC6 cDNA. After 3 hours, the transfection mixture was removed and replaced with complete growth medium. After 72 hours, cells were harvested for RNA extraction.
Immunostaining
PXE and normal human fibroblasts were plated in six-well plates (6 cm2/well) at an initial level of 100,000 cells/dish and subsequently maintained in medium supplemented with appropriate serum for 10-days. Each well contained a 4 cm2 glass coverslip that was subsequently used for immunostainings. Media were changed every 72 hours (at days −4 and −7). After 10 days of culture, confluent cells were fixed in cold 100% methanol at −20°C for 30 minutes and blocked with 1% normal goat serum for 1 hour at room temperature. Cultures used for immunohistochemical assessment of microfibrillar components (MAGP-1) were fixed in 0.5% paraformaldehyde for 15 minutes, treated with phosphate-buffered saline containing 50 mM of dithiothreitol for an additional 10 minutes, and alkylated with 100 mM iodoacetamide for 15 minutes. Finally, these cultures were blocked in phosphate-buffered saline containing 0.1 M ammonium chloride. The cells were then incubated for 1 hour with different antibodies: 20 μg/ml of specific polyclonal antibodies recognizing tropoelastin (Elastin product, Owensville, MI), fibrillin-1 (Biomeda Corp., Foster City, CA), MAGP-1 (Elastin product, Owensville, MI), collagen type I (generous gift of Dr Larry W. Fischer from The National Institute of Health, Bethesda, MD), and elastin-binding protein (Hinek et al., 1993), as well as with 10 μg/ml of monoclonal antibodies to fibronectin (Chemicon, Temecula, CA), chondroitin sulfate (Sigma, St Louis, MO), and LOX (gift from Dr Csiszar to AH). All cultures were then incubated for an additional hour with the appropriate fluorescein-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Sigma, St Louis, MO). Nuclei were counterstained with propidium iodide. For negative controls the same procedure was followed, but the primary antibody was omitted. All cultures were then mounted in elvanol, and examined with a Nikon Eclipse E1000 microscope. The images were obtained with a cooled CCD camera (QImaging, Retiga EX). The morphometric analysis of extracellular matrix components was then performed using Image-Pro Plus software from Media Cybernetics (Silver Springs, MD) as described previously (Hinek et al., 2000).
RNA expression studies
Fibroblasts were cultured for 10 days in the same conditions as those used for immunostaining, except for transient transfection assays. RNA was extracted using TRI reagent. Reverse transcription of total RNA (1 μg) from fibroblasts was performed using the Superscript first-strand synthesis kit (Invitrogen, Carlsbad, CA) and random hexamer primers. The generated cDNA was subsequently used as template for real-time PCR amplification with a DNA Engine Opticon 2 (MJ Research/Bio-Rad, Waltham, MA). TaqMan probes specific for beta-actin, tropoelastin, fibrillin-1, MAGP-1, LOX, and LOXL and collagen type I were purchased from Applied Biosystems (Foster City, CA). The PCR cycling conditions started with two incubation steps at 50°C for 2 minutes and 95°C for 2 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minute with plate read.
Insoluble elastin assay
In each experiment, fibroblasts from unaffected individuals and PXE patients were densely plated in 60-mm culture dishes (100,000 cells/dish) to quickly reach confluence. After 7 days in culture, an aliquot of 20 μCi of [3H]valine (Amersham Canada Ltd, Oakville, ON) was added to fresh media. Cultures were then incubated for the next 3 days. Quadruplicate cultures, 10-day-old, belonging to each experimental group were terminated and insoluble elastin was assessed as described previously (Hinek and Wilson, 2000). After the removal of the media, cell layers containing insoluble elastin deposited in ECM were washed in 0.1 M acetic acid, then scraped in 0.1 N NaOH, sedimented by centrifugation, and boiled in 0.5 ml of 0.1 N NaOH for 45 minutes to dissolve all matrix components except elastin. The resulting pellets containing the insoluble elastin were then dissolved by boiling in 200 μl of 5.7 N HCl for 1 hour, and the aliquots were mixed with scintillation fluid and counted. Aliquots taken from each culture were also used for DNA determination using the DNeasy Tissue System from Qiagen (Valencia, CA). Final results reflecting amounts of metabolically labeled insoluble elastin were expressed as CPM/μg DNA.
Electron microscopy
Cultures of human fibroblasts, 5- and 10-day-old, maintained with serum from normal individuals and from PXE patients were prepared for electron microscopic examination. The cells were fixed in the presence of 2% glutaraldehyde dissolved in 0.1 M cacodylate buffer containing 0.25% tannic acid and post-fixed with 1% osmium tetroxide in the same buffer. The samples were then dehydrated in ethanol and embedded in Spurr low-viscosity resin, which assures high contrast of elastin even when thin sections are stained with uranyl acetate and lead citrate (Hinek et al., 1976).
Acknowledgments
We are very grateful to all the patients and their families whose cooperation made this study possible. This work was supported by the National Institutes of Health grant RR16453 and the McKee funds of the Hawaii Community Foundation (20041635) to O. Le Saux and by the Canadian Institute of Health Research (grant PG 13920), the Stroke Foundation of Ontario (grant NA 4381), and a Career Investigator Award (CI 4198) to A. Hinek.
Abbreviations
- FBS
fetal bovine serum
- LOX
lysyl oxidase
- LOXL
lysyl oxidase-like
- MAGP-1
microfibril-associated glycoprotein-1
- PXE
Pseudoxanthoma elasticum
- SMC
smooth muscle cell
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Baccarani-Contri M, Boraldi F, Taparelli F, De Paepe A, Ronchetti IP. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am J Pathol. 1996;148:569–77. [PMC free article] [PubMed] [Google Scholar]
- Bacchelli B, Quaglino D, Gheduzzi D, Taparelli F, Boraldi F, Trolli B, et al. Identification of heterozygote carriers in families with a recessive form of pseudoxanthoma elasticum (PXE) Mod Pathol. 1999;12:1112–23. [PubMed] [Google Scholar]
- Beck K, Hayashi K, Dang K, Hayashi M, Boyd CD. Analysis of ABCC6 (MRP6) in normal human tissues. Histochem Cell Biol. 2005;123:517–28. doi: 10.1007/s00418-004-0744-3. [DOI] [PubMed] [Google Scholar]
- Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem. 2003;51:887–902. doi: 10.1177/002215540305100704. [DOI] [PubMed] [Google Scholar]
- Bedell-Hogan D, Trackman P, Abrams W, Rosenbloom J, Kagan H. Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase. J Biol Chem. 1993;268:10345–50. [PubMed] [Google Scholar]
- Bellingham CM, Woodhouse KA, Robson P, Rothstein SJ, Keeley FW. Self aggregation characteristics of recombinantly expressed human elastin polypeptides. Biochem Biophys Acta. 2001;1550:6–19. doi: 10.1016/s0167-4838(01)00262-x. [DOI] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–31. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- Boraldi F, Quaglino D, Croce MA, Garcia Fernandez MI, Tiozzo R, Gheduzzi D, et al. Multidrug resistance protein-6 (MRP6) in human dermal fibroblasts. Comparison between cells from normal subjects and from Pseudoxanthoma elasticum patients. Matrix Biol. 2003;22:491–500. doi: 10.1016/j.matbio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881–92. doi: 10.1136/jmg.2004.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing N, Martin L, Mazereeuw J, Barrie L, Nizard S, Bonafe JL, et al. Novel ABCC6 mutations in pseudoxanthoma elasticum. J Investig Dermatol. 2004;122:608–13. doi: 10.1111/j.0022-202X.2004.22312.x. [DOI] [PubMed] [Google Scholar]
- Clarke AW, Weiss AS. Microfibril-associated glycoprotein-1 binding to tropoelastin: multiple binding sites and the role of divalent cations. Eur J Biochem. 2004;271:3085–90. doi: 10.1111/j.1432-1033.2004.04246.x. [DOI] [PubMed] [Google Scholar]
- Davis EC. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry. 1993;100:17–26. doi: 10.1007/BF00268874. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet. 1995;4:1799–809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- Gheduzzi D, Sammarco R, Quaglino D, Bercovitch L, Terry S, Taylor W, et al. Extracutaneous ultrastructural alterations in pseudoxanthoma elasticum. Ultrastruct Pathol. 2003;27:375–84. [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–73. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Investig. 1993;91:1198–205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Thyberg J, Friberg U. Heterotopic transplantation of isolated aortic cells, an electron microscopical study. Cell Tissue Res. 1976;172:59–79. doi: 10.1007/BF00226049. [DOI] [PubMed] [Google Scholar]
- Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000;156:925–38. doi: 10.1016/S0002-9440(10)64961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–93. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–7. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–28. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59:175–82. [PubMed] [Google Scholar]
- Le Saux O, Beck K, Sachsinger C, Silvestri C, Treiber C, Goring HH, et al. A spectrum of abcc6 mutations is responsible for pseudoxanthoma elasticum. Am J Hum Genet. 2001;69:749–64. doi: 10.1086/323704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25:223–7. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Schwartz E, Lemlich G, Lovelace O, Shaikh-Bahai F, Fleischmajer R. Abnormalities of connective tissue components in lesional and non-lesional tissue of patients with pseudoxanthoma elasticum. Arch Dermatol Res. 1993;285:121–6. doi: 10.1007/BF01112912. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Madon J, Hagenbuch B, Landmann L, Meier PJ, Stieger B. Transport function and hepatocellular localization of mrp6 in rat liver. Mol Pharmacol. 2000;57:634–41. doi: 10.1124/mol.57.3.634. [DOI] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Mendelsohn G, Bulkley BH, Hutchins GM. Cardiovascular manifestations of Pseudoxanthoma elasticum. Arch Pathol Lab Med. 1978;102:298–302. [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Int J Dermatol. 1988;27:98–100. doi: 10.1111/j.1365-4362.1988.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Nishida H, Endo M, Koyanagi H, Ichihara T, Takao A, Maruyama M. Coronary artery bypass in a 15-year-old girl with pseudoxanthoma elasticum. Ann Thorac Surg. 1990;49:483–5. doi: 10.1016/0003-4975(90)90265-8. [DOI] [PubMed] [Google Scholar]
- Quaglino D, Boraldi F, Barbieri D, Croce A, Tiozzo R, Pasquali Ronchetti I. Abnormal phenotype of in vitro dermal fibroblasts from patients with Pseudoxanthoma elasticum (PXE) Biochim Biophys Acta. 2000;1501:51–62. doi: 10.1016/s0925-4439(00)00007-7. [DOI] [PubMed] [Google Scholar]
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA. 2000;97:6001–6. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Marson A, et al. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. J Biol Chem. 2004;279:23748–58. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Pijnenborg ACLM, Schuurman E, Hu XF, Wijnholds J, Bergen AAB. Third FEBS Advanced lecture Course “ATP-Binding Cassette (ABC) Proteins: From Genetic Disease to Multidrug Resistance”. 2001. Mar, MRP6 (ABCC6) is localized at the basolateral membranes of hepatocytes. [Google Scholar]
- Sherratt MJ, Baldock C, Haston JL, et al. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J Mol Biol. 2003;332:183–93. doi: 10.1016/s0022-2836(03)00829-5. [DOI] [PubMed] [Google Scholar]
- Struk B, Cai L, Zach S, Ji W, Chung J, Lumsden A, et al. Mutations of the gene encoding the transmembrane transporter protein ABC-C6 cause pseudoxanthoma elasticum. J Mol Med. 2000;78:282–6. doi: 10.1007/s001090000114. [DOI] [PubMed] [Google Scholar]
- Sugitani H, Wachi H, Murata H, Sato F, Mecham RP, Seyama Y. Characterization of an in vitro model of calcification in retinal pigmented epithelial cells. J Atheroscler Thromb. 2003;10:48–56. doi: 10.5551/jat.10.48. [DOI] [PubMed] [Google Scholar]
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–9. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- Toh S, Wada M, Uchiumi T, Inokuchi A, Makino Y, Horie Y, et al. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin–Johnson syndrome. Am J Hum Genet. 1999;64:739–46. doi: 10.1086/302292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Boyd CD, Lebwohl MG, Moshell AN, Rosenbloom J, Terry S. International centennial meeting on pseudoxanthoma elasticum: progress in PXE research. J Investig Dermatol. 1998;110:840–2. doi: 10.1046/j.1523-1747.1998.00188.x. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment–genome interface? Mol Med Today. 2001;7:13–7. doi: 10.1016/s1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]
- Uitto J, Shamban A. Heritable skin diseases with molecular defects in collagen or elastin. Dermatol Clin. 1987;5:63–84. [PubMed] [Google Scholar]
- Weenink AC, Dijkman G, de Meijer PH. Pseudoxanthoma elasticum and its complications: two case reports. Neth J Med. 1996;49:24–9. doi: 10.1016/0300-2977(95)00079-8. [DOI] [PubMed] [Google Scholar]