Abstract
The peripheral fibers that extend from auditory neurons to hair cells are sensitive to damage, and replacement of the fibers and their afferent synapse with hair cells would be of therapeutic interest. Here, we show that RGMa, a repulsive guidance molecule previously shown to play a role in the development of the chick visual system, is expressed in the developing, newborn, and mature mouse inner ear. The effect of RGMa on synaptogenesis between afferent neurons and hair cells, from which afferent connections had been removed, was assessed. Contact of neural processes with hair cells and elaboration of postsynaptic densities at sites of the ribbon synapse were increased by treatment with a blocking antibody to RGMa, and pruning of auditory fibers to achieve the mature branching pattern of afferent neurons was accelerated. Inhibition by RGMa could thus explain why auditory neurons have a low capacity to regenerate peripheral processes: postnatal spiral ganglion neurons retain the capacity to send out processes that respond to signals for synapse formation, but expression of RGMa postnatally appears to be detrimental to regeneration of afferent hair cell innervation and antagonizes synaptogenesis. Increased synaptogenesis after inhibition of RGMa suggests that manipulation of guidance or inhibitory factors may provide a route to increase formation of new synapses at deafferented hair cells.
Keywords: RGMa, cochlear synaptogenesis, cochlear regeneration, spiral ganglion neuron
INTRODUCTION
Auditory neurons are sensitive to damage, and regeneration of the neurons and their synapse with hair cells would be an important part of a strategy for the treatment of deafness due to loss of these neurons and their synapses. Despite the greater capacity of peripheral as compared to central neurons to regenerate, the auditory nerve does not show spontaneous regenerative capacity (Starr et al., 1996; White et al., 2000; McFadden et al., 2004; Kujawa and Liberman, 2009). The loss of afferent innervation of hair cells can result from retraction of peripheral fibers after noise damage or from complete loss of neurons (White et al., 2000; Kujawa and Liberman, 2006, 2009). Methods of regenerating the neurons would therefore be important for cell replacement for the treatment of hearing loss and as a way to increase our understanding of peripheral nerve regeneration.
The spiral ganglion neurons constitute the afferent innervation of hair cells, representing the first step in the ascending pathway from the cochlea (Fuchs et al., 2003; Weisz et al., 2009). The afferent fiber thus extends from an individual synapse at the hair cell to the first central synapse in the brainstem, and the auditory pathway continues to the cortex. The hair cell synapse is glutamatergic, and the presynaptic release of vesicles occurs via a ribbon synapse found in the inner ear (Glowatzki et al., 2008). The spiral ganglion cell expresses glutamate receptors and components of postsynaptic glutamatergic specializations such as PSD95. We have recently shown that hair cell synapses can be reconstituted by neurons in culture. The neurons grew to hair cells that had been previously denervated and expressed synaptic markers at points of contact (Martinez-Monedero et al., 2006, 2008; Tong et al., 2013), although quantitative assessment of the effect of axonal growth and guidance factors on new synapses was not performed.
We hypothesized that molecules that direct axonal growth during development might be expressed postnatally and, by continuing to exert a repulsive action, could block the growth of new fibers. Thus, the neurons might be able to regenerate fibers and to form synapses but may be blocked by axonal guidance molecules. A screen for axonal guidance molecules uncovered the expression of repulsive guidance molecule a (RGMa) in the adult cochlea. We asked whether RGMa, a guidance molecule that shares no homology with other known guidance families (Monnier et al., 2002) but is recognized with high affinity by axonal guidance receptor neogenin (Matsunaga and Chedotal, 2004; Yamashita et al., 2007), had an influence on regeneration of afferent synapses in the cochlea. Blocking this inhibitory pathway increased hair cell synapses in the postnatal tissue, and the branching of the neurons reconstituted some of the patterns of the peripheral auditory neurons in the adult.
MATERIALS AND METHODS
Organ of Corti Explants
In all cultures, cells were maintained in growth medium: DMEM: F-12 (Gibco) supplemented with B27 and N2 supplements (Gibco), and 10% fetal bovine serum (Gibco). Cultures were maintained at 37°C in a humidified incubator with 5% CO2. In all experiments, the media was supplemented with 50 ng/mL NT-3 (Chemicon) and 10 ng/mL BDNF (Chemicon) after the tissue isolation and plating. The cocultures were performed by a modified version (Tong et al., 2013) of our original protocol (Martinez-Monedero et al., 2006, 2008; Flores-Otero et al., 2007), and the procedure was adapted to mouse cochlea at P3 to P5. These explant cultures provide a well-defined system in which to assess the influence of various factors on the formation of synapses. Inner and outer hair cells were carefully dissected along with their surrounding supporting cells and plated on a coated (laminin, 50 µg/mL; BD Biosciences, poly-l-ornithine, 0.01%; Sigma) cover glass in 4-well plates (Greiner) overnight in a CO2 incubator. The hair cells were removed as an intact alignment of a single row of inner hair cells and three rows of outer hair cells by cutting the auditory nerve fibers at the level of the spiral lamina. By separating these tissues, we were able to eliminate all preexisting connections. Neurons were randomly placed around the organ of Corti. For the RGMa treatment, anti-rat RGMa (10 µg/mL; Immuno-Biological Laboratories) was added to the culture medium. The RGMa antibody binds specifically to the protein (33 kDa) on Western blots (Hata et al., 2006). These cultures were used between 4 and 21 days in vitro (DIV) to allow the appropriate amount of time for neuronal processes to grow to the explants and reform connections.
Spiral Ganglion Neuron Isolation
Spiral ganglion neurons for the co-culture were trypsinized and dissociated after dissection from mouse cochlea at P3 to P5 as described previously (Martinez-Monedero et al., 2006, 2008). The cells were cultured overnight in DMEM: F-12 (Gibco) supplemented with B27 and N2 supplements (Gibco) and were then collected by centrifugation. The resulting cells were triturated to a single-cell suspension and the neurons from two ears were used in a well of a 4-well plate containing two microisolated explants of the organ of Corti (separated from neurons).
Immunofluorescence
For immunofluorescence microscopy, cultures were fixed with 4% paraformaldehyde at room temperature, followed by 0.1% Triton X-100 and 15% normal goat serum at room temperature for 1 h for permeabilization and blocking. Primary antibodies—anti-CtBP2 (mouse monoclonal IgG1; BD Biosciences), anti-PSD95 (mouse monoclonal IgG2a, NeuroMab), anti-neurofilament heavy chain (chicken polyclonal; Chemicon) anti-myosin VIIa (rabbit polyclonal; Proteus), and anti-neogenin (goat polyclonal, R&D) — were applied and incubated overnight at 4°C. After rinsing three times for 10 min with 0.01 M PBS, pH 7.4, cocultures were incubated with one of the following secondary antibodies for 1 h at room temperature: Cyanine-5-conjugated goat anti-mouse IgG1 (Caltad Laboratories), biotin-conjugated goat anti-mouse IgG2a (Caltad Laboratories), Alexa 568-Streptavidin (Molecular Probes), Alexa Fluor 488 goat anti-chicken (Molecular Probes), or Alexa 350 goat anti-rabbit (Molecular Probes). Finally, after three PBS rinses, cultures were placed onto a glass microscope slide with a drop of fluorescent mounting medium (DakoCytomation), mounted with Dako fluorescent mounting medium (Dako) and viewed using a Leica confocal microscope. Images were analyzed with Metamorph software and processed with Adobe Photoshop. Labeling for CtBP2, PSD95, and neurofilament was counted in all cultures for association of the three labels, and statistical significance was determined by the Mann Whitney test for (*p < 0.05) with Sigma Plot software.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
We isolated total RNA from cerebella, spiral ganglion neurons, organs of Corti, microisolates, stria vascularis, and utricles from native tissue or after 5 days in culture (microisolates). Each experiment used TRIzol Reagent (Invitrogen). Reverse transcription (10 min at 25°C, 50 min at 42°C, and 15 min at 85°C) of the total RNA (adjusted to 1.5 µg per reaction, except for the organ of Corti, in which we used all isolated RNA; see Results) was performed in first-strand cDNA synthesis mix containing the following (after the final dilution): 50 mM Tris-HCl, 75 mM KCl, 5 mM MgCl2, 5 mM DTT adjusted to pH 8.3, 100 U of SuperScript II Reverse Transcriptase (Invitrogen), 40 U of RNaseOUT ribonuclease inhibitor, as well as 2.5 ng/µL random hexamers (Invitrogen). After the RT reaction, cDNA for RGMa was selectively amplified (in 20 µL volume in triplicate) using TaqMan Gene Expression Assays according to the Applied Biosystems protocol and subjected to quantitative PCR using ABI Prism 7000 or 7500 Sequence Detection Systems. Results were reported as fold difference relative to RGMa expression at E10 and were calculated relative to the endogenous level of 18S rRNA.
In Situ Hybridization
Mid-modiolar paraffin sections were prepared from cochleae of 4-week-old C57BL/6J. After intracardiac perfusion with 4% PFA, cochleae were perfused with Bouin’s solution (Sigma), fixed overnight, decalcified for 12 h in 0.5 M ETDA, dehydrated by alcohol for 24 h, embedded in paraffin, sectioned at 10 µm and stored at −80°C. In vitro transcription from a linearized cDNA clone of RGMa was performed to synthesize antisense/sense digoxigenin-labeled riboprobe (NC_000073.6). In situ hybridization was conducted according to a published protocol with modifications (Schaeren-Wiemers and Gerfin-Moser, 1993). Briefly, slides were baked overnight at 60°C, dewaxed in xylene, rinsed in 100% EtOH, and air-dried at room temperature. Overnight hybridization and subsequent washes were carried out at 60°C in a chamber humidified with 50% (vol/vol) formamide in 150 mM NaCl/15 mM trisodium citrate, pH 7 (1 × SSC). Hybridized probe was detected using anti-digoxygenin alkaline phosphatase conjugated antibody (1:1,000 dilution, Roche Biochemical, Indianapolis, IN) and visualized with NBT/BCIP for a blue precipitate.
RESULTS
Screen for Inhibitory Molecules
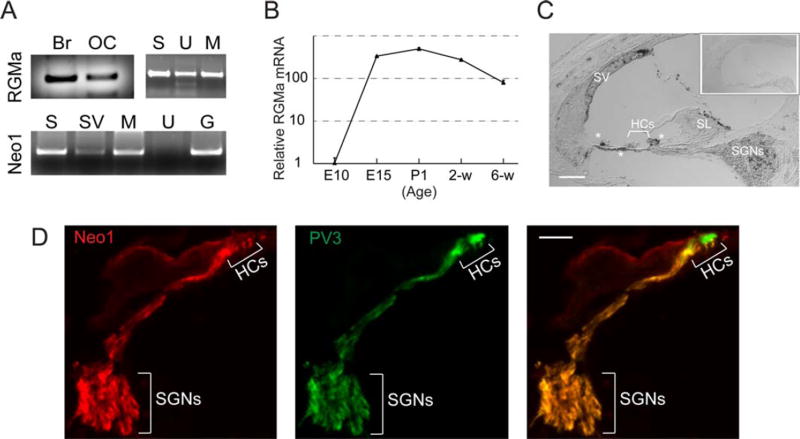
As a part of an effort to define the axonal guidance molecules that could play a role in regeneration of the synapse between afferent neurons and hair cells, we performed a screen for candidate guidance molecules expressed in both the embryonic and adult inner ear. We used both semi-quantitative and quantitative RT-PCR to assess expression. RGMa, which has not previously been examined in the auditory system, could be seen in both postnatal organ of Corti and brain. Within the inner ear it was expressed in organ of Corti and utricle as well as the spiral ganglion based on semi-quantitative RT-PCR [Fig. 1(A)]. Interestingly, the organ of Corti retained expression of RGMa when cultured for 5 days following the separation of neurons, which caused complete loss of the neural endings [Fig. 1(A), RGMa, M]. The receptor for RGMa, neogenin, was assessed and found to be expressed in neurons, and in the microisolate of organ of Corti, but not in utricle, based on RT-PCR [Fig. 1(A), Neo1]. Quantitative measurement showed that RGMa mRNA was expressed during development of the cochlea and continuously expressed after birth [Fig. 1(B)]. RGMa in postnatal organ of Corti was revealed by in situ hybridization to be expressed in the spiral ganglion, supporting cells of the sensory epithelium, cochlear mesenchymal cells associated with the basilar membrane, and the lateral wall [Fig. 1(C)]. Neogenin was localized to spiral ganglion neurons by antibody staining [Fig. 1(D)].
Figure 1.
Expression of RGMa in inner ear. A, B: RGMa and neogenin were found in both the embryonic and newborn auditory and vestibular (utricle) organs by semiquantative (A) and quantitative (B) RT-PCR. RGMa was expressed in the cultured organ of Corti microisolate (Br: Brain; OC: organ of Corti; S: dissociated spiral ganglion; U: utricle; M: microisolated deafferented OC from P3 after 5 days; Sv: stria vascularis; G: undissociated spiral ganglion) as well as neurons. Neo-genin (Neo1) was present in the spiral ganglion neurons and the intact ganglion but not in the utricle (A). RGMa expression persisted in the organ of Corti at 6 wk based on quantitative RT-PCR reported as fold difference relative to the expression level at E10 (B). C, RGMa was localized in postnatal organ of Corti. Hybridization with an antisense probe for RGMa on a 4-week-old organ of Corti showed expression in hair cells (HCs) of the organ of Corti, the stria vascularis (SV), spiral limbus (SL), and spiral ganglion neurons (SGNs). Inset shows hybridization with a control, sense probe on a 4-week-old organ of Corti. D, Immunostaining showed neogenin1 in spiral ganglion neurons and parvalbumin 3 (PV3) in hair cells (HCs) and neurons (SGNs) (Scale bars, 20 µM). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Effect of RGMa on Growth of Fibers to the Organ of Corti
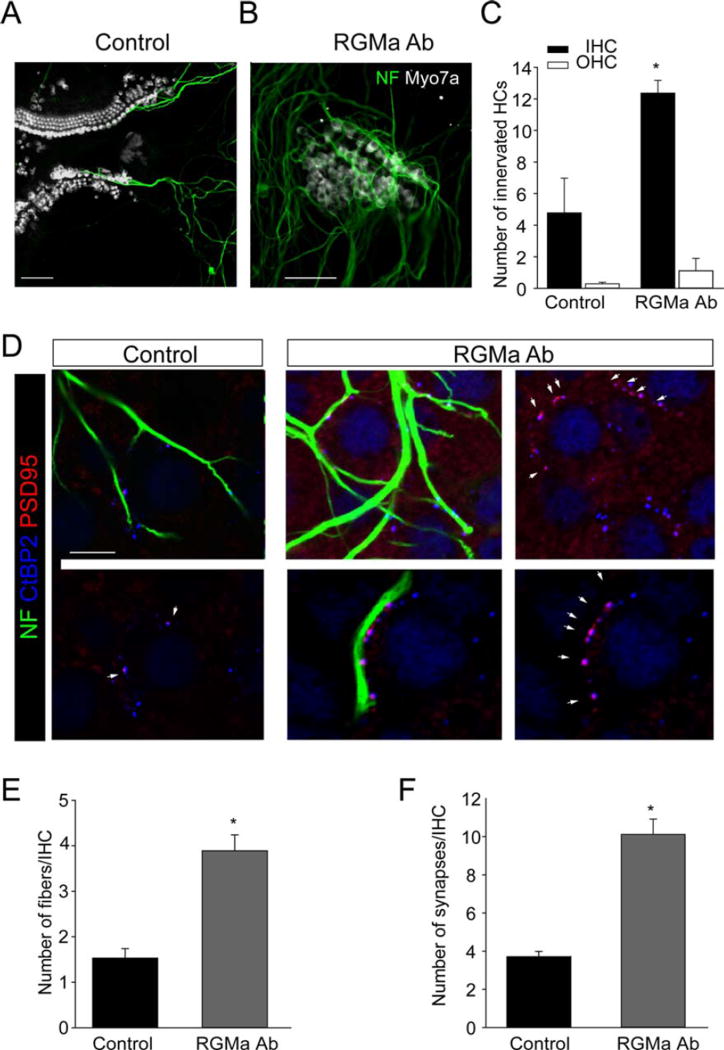
To test the effect of RGMa on the reinnervation of hair cells we used a previously described approach with a microisolate of the organ of Corti (Tong et al., 2013) as the target for new neurons and assessed the growth of fibers to the hair cells. When spiral ganglion neurons dissociated at birth were placed in the culture in close proximity to the organ of Corti, new fibers were extended and contacted cells in the organ of Corti [Fig. 2(A)]. Blocking RGMa with an antibody led to an increase in growth of fibers [Fig. 2(B)] and a significant increase in the number of hair cells contacted by fibers from the spiral ganglion neurons. Quantitative measurements revealed an increase in the number of contacts with both inner and outer hair cells in the antibody-treated as compared with control organ of Corti; the number increased from 4.77 ± 2.20 (n = 11) in the control to 12.37 ± 0.80 (n = 156) in the antibody-treated cultures for the inner hair cells [n = inner hair cells counted; three separate experiments; Fig. 2(C)].
Figure 2.
Inhibition of RGMa increased growth of fibers and synaptogenesis with hair cells. A, B: The growth of neurons to the deafferented organ of Corti in culture was observed for spiral ganglion neurons (A, Control), but treatment with an antibody against RGMa increased sprouting and growth of spiral ganglion neurons to hair cells after 7 days (B, RGMa Ab). Neurons were immunostained with neurofilament (NF, green) and hair cells for myosin VIIa (Myo7a, white; Scale bars, 20 µM). C: Inhibition of RGMa with an antibody increased the number of hair cells contacted by fibers from spiral ganglion neurons. D–F: Inhibition of RGMa resulted in an increase in both the number of fibers that grew to hair cells and the number of synapses quantified after immunostaining with neurofilament (NF, green), PSD95 (red), and CtBP2 (blue) in the absence (control, top, and bottom shown with and without neurofilament to reveal the synapses) or presence (RGMa Ab, images shown on the left and right with and without neurofilament to reveal the synapses) of RGMa antibody (D). CtBP2-PSD95-labeled synapses that had closely adjacent staining (indicated by the white arrowheads) were counted and were readily distinguishable from occasional background staining in the cytoplasm for PSD95 (Scale bars, 50 µM). The number of fibers (E) and synapses (F) per inner hair cell (IHC) at 7 days were increased in cultures treated with the antibody. Asterisks indicate significance (p < 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Innervation, as measured by fiber ingrowth, was dramatically increased when the antibody to RGMa was added to the cultured microisolates [Fig. 2(D,E)]. To determine whether the additional fiber ingrowth resulted in an increased rate of synapse formation in the RGMa antibody-treated cultures, we evaluated the number of synapses with hair cells by assessment of the presynaptic and postsynaptic densities. We had shown costaining of the glutamate receptor, GluR2/3, with PSD95, a component of the postsynaptic density in a previous article (Tong et al., 2013). We stained for the postsynaptic densities with a PSD95 antibody, and for the ribbon synapse with an antibody to CtBP2, which stains the ribbon protein, ribeye. Innervation of hair cells by spiral ganglion neurons resulted in the close apposition of the afferent endings (PSD95) to the hair cell ribbons (CtBP2), thus marking the synapses from both sides [Fig. 2(D)]. Some of the ribbons were clearly involved in synapses with the fibers, whereas others were unoccupied. The synapses colocalized with neurofilament antibody-positive fibers [synapses with hair cells shown in Fig. 2(D)] and were significantly increased by addition of the antibody against RGMa. The number of synapses per inner hair cell increased from 3.71 ± 0.28% (n = 38) in the control to 10.12 ± 0.80% (n = 157) in the antibody treated cultures [n = inner hair cells counted; three separate experiments; Fig. 2(F)].
Pruning of Contacts Follows the In Vivo Pattern and Is Enhanced by Inhibition of RGMa
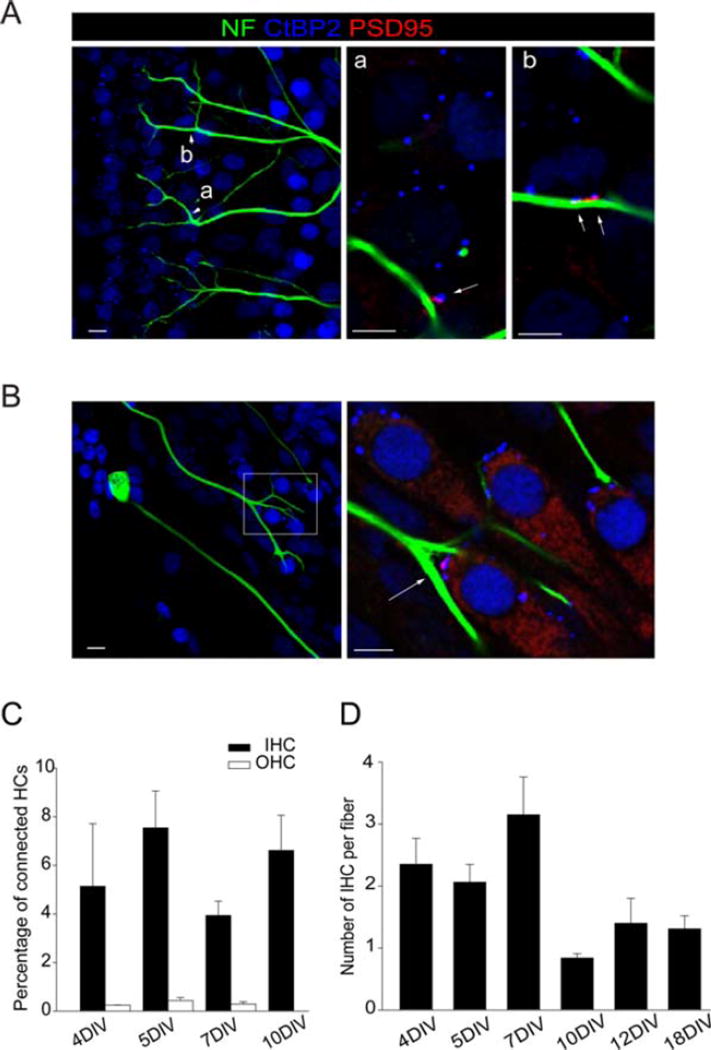
Branches of spiral ganglion neurons formed multiple synapses with inner and outer hair cells [Fig. 3(A,B)]. Approximately 4–8% of inner hair cells were innervated and did not significantly increase with time in culture (4 DIV: 5.14 ± 2.57, n = 108; 5 DIV: 7.54 ± 1.51, n = 177; 7 DIV: 3.94 ± 0.58, n = 45; 10 DIV: 6.61 ± 1.44, n = 126) (n = inner hair cells counted; four separate experiments), while very few outer hair cells were innervated even after an extended time in culture (4 DIV: 0.24 ± 0.01, n = 4; 5 DIV: 0.43 ± 0.12, n = 32; 7 DIV: 2.28 ± 0.09, n = 10) [n = outer hair cells counted: four separate experiments; Fig. 3(C)].
Figure 3.
Pruning of fibers in the inner hair cell and outer hair cell areas. A, B: Hair cell synapses, stained for neurofilament (NF, green), PSD95 (red), and CtBP2 (blue). Arrows indicate triple-stained synapses (NF, PSD95 and CtBP2) in the outer (A) and inner (B) hair cell areas. Arrowheads (in A) indicate the areas (a and b) shown at higher power to the right. Box (in B) indicates area enlarged to the right. C, D: Up to 8% of the hair cells, mostly inner hair cells, were contacted after 4–10 DIV by spiral ganglion neurons (C). Quantification of synapses indicated that the number of hair cells innervated by a single neuron decreased with increasing time in culture toward a value of 1.5 (D) (Scale bars, 5 µM). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We evaluated the branching of the fibers as they formed synapses to determine how these networks matured. We traced individual fibers by confocal imaging. Branching was quantified after increasing time intervals and the number of hair cells contacted by each fiber was found to decrease toward one after 18 days [4 DIV: 2.35 ± 0.418, n = 107; 5 DIV: 2.06 ± 0.28, n = 146; 7 DIV: 3.15 ± 0.61, n 5 28; 10 DIV: 0.84 ± 0.07, n 5 188; 12 DIV: 1.4 ± 0.4, n = 11; 18 DIV: 1.31 ± 0.21, n = 17) [n = inner hair cells counted; four separate experiments; Fig. 3(D)]. These results suggested that the loss of synaptic contacts in culture might be similar to what occurs in vivo where the maturation of the initial innervation leads to a reduction in the number of synapses during development.
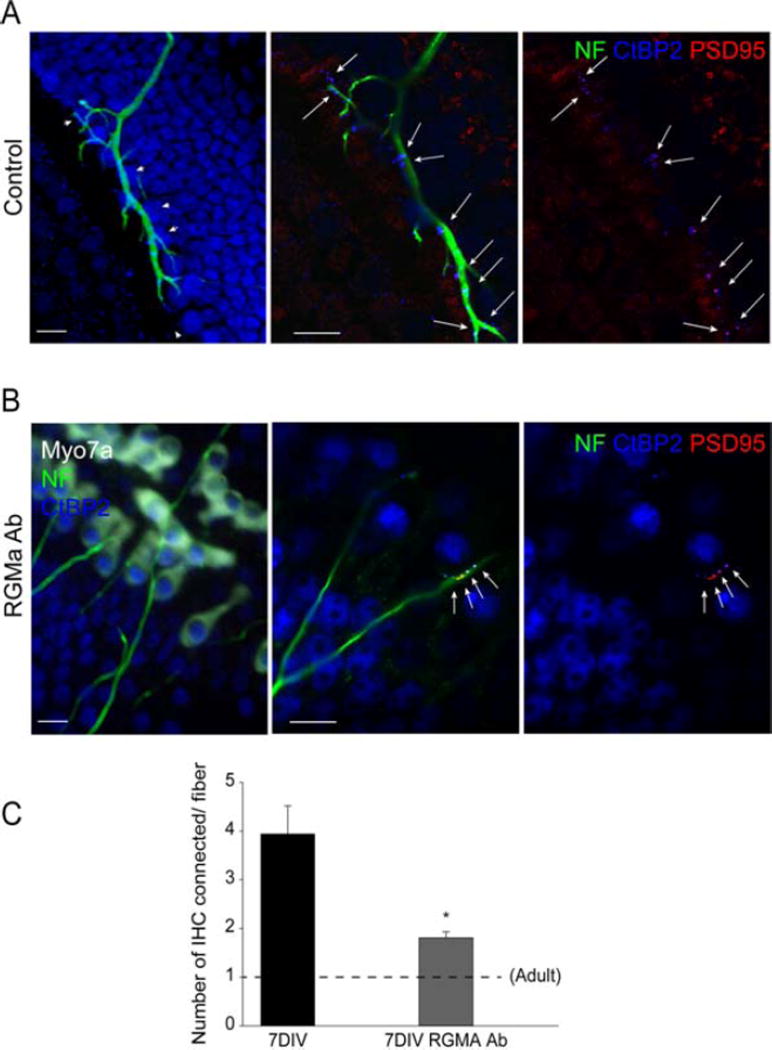
Treatment with the RGMa antibody accelerated the maturation of the branching toward the mature in vivo pattern (Fig. 4). The number of inner hair cells connected by one fiber was decreased to nearly the native adult level after 7 days in culture. The number of hair cells contacted in cultures treated with RGMa antibody decreased to 2 inner hair cells [Fig. 4(B)], from almost 4 in controls [Fig. 4(A)], as compared at the same time point [Fig. 4(C), respectively, 1.8 ± 0.12, n = 304 and 3.94 ± 0.58, n = 45; n = inner hair cells counted; three separate experiments].
Figure 4.
Inhibition of RGMa increased the rate of pruning. A, B: Spiral ganglion neurons added to the deafferented organ of Corti were cultured for 7 days followed by labeling for neurofilament (NF), PSD95, CtBP2, and myosin VIIa (Myo7a). An increased rate of loss of branches was seen in cultures treated with RGMa antibody (B), compared with control (A). C, Innervation of the sensory epithelium at 7 days nearly reached the single hair cell innervation pattern of the adult. Dashed line indicates the innervation pattern of one hair cell per spiral ganglion neuron in adult cochlea. Asterisk indicates significance (p < 0.05; Scale bars: 10 µM). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Regeneration of synaptic connections is an important goal for reversal of numerous neurodegenerative diseases. Regenerated neurons would need to connect to cochlear hair cells for functional cell replacement in the ear. In previous studies using toxins to remove the afferent neurons, we had shown that new neurons contact hair cells both in vitro and in vivo (Corrales et al., 2006; Martinez-Monedero et al., 2006, 2008; Shi et al., 2007). However, there appeared to be some block to complete synaptogenesis in the organ of Corti, and we therefore inquired what genes might prevent regeneration of peripheral auditory neurons.
Axonal guidance systems are primarily expressed in the embryo, because the initial layout of neuronal circuits during development requires directed growth of neurons in a stereotyped fashion (Tessier-Lavigne and Goodman, 1996; Mueller, 1999; Pasterkamp and Verhaagen, 2006; Yamashita et al., 2007), but the precise role of the individual molecules and how they give rise to the complex innervation patterns has not been completely established for the cochlea or for the peripheral nervous system. Many of the guidance molecules have limited expression after birth, and this corresponds with the time when afferent innervation of hair cells is completed in the first postnatal week (Huang et al., 2007). We expected that a repulsive guidance molecule that was necessary for establishing the original circuits might inhibit regeneration in the adult.
We showed that RGMa was expressed in the neonatal as well as the adult cochlea. RGMa is a glycosylphosphatidylinositol-anchored protein that binds to neogenin (Matsunaga and Chedotal, 2004; Niederkofler et al., 2004; Rajagopalan et al., 2004; Yamashita et al., 2007), but does not fall into any of the main families of axonal guidance molecules, the netrins, semaphorins, ephrins, and slits (Dickson, 2002; Moore et al., 2007). It has been demonstrated to play a role in axonal pathfinding in chick (Monnier et al., 2002), xenopus (Wilson and Key, 2006), and mouse (Brinks et al., 2004). A gradient of RGMa organizes projections of retinal neurons to the optic tectum in the chick (Matsunaga et al., 2006; Liu et al., 2009), and RGMa inhibits growth and prevents arborization of axons during the development of the laminar organization of the hippocampus (Brinks et al., 2004) and the optic tectum (Matsunaga et al., 2006), restricting branching to the correct layers.
In addition, both neonatal and adult spiral ganglion neurons expressed neogenin, the receptor for RGMa (Rajagopalan et al., 2004). Neogenin, a guidance receptor expressed in the growth cone, is a transmembrane protein with four immunoglobulin and six fibronectin III domains in its extracellular region, homologous to the guidance receptor, deleted-in-colon-cancer (DCC) (De Vries and Cooper, 2008). It binds to both RGMa and netrin-1, but binding to RGMa is higher affinity and induces growth cone collapse, whereas binding to netrin-1 attracts the growth cone. Alternative action of RGMs are mediated by BMPs (Yamashita et al., 2007), and this could be a second path for its activity in spiral ganglion neurons, but neogenin is the likely mediator of RGMa activity in the cochlea, as in guidance activity of other neurons (Matsunaga et al., 2006).
Immunostaining for RGMa revealed weak puncta in the vicinity of the basilar membrane in the cochlea. A strong signal was detected for mRNA by RT-PCR, and therefore the lack of a clear cell-associated signal for the protein could be attributable to its extracellular localization after cleavage from the cell surface, consistent with its cleavage by enzymes specific for the glycosylphosphatidylinositol linkage (Hata et al., 2006; De Vries and Cooper, 2008), as well as proteases (Tassew et al., 2012). The soluble forms of RGMa are active (Hata et al., 2006; Tassew et al., 2012). The antibody used to inhibit RGMa was directed to its C-terminal portion (Hata et al., 2006) and would have blocked both membrane-bound and soluble forms.
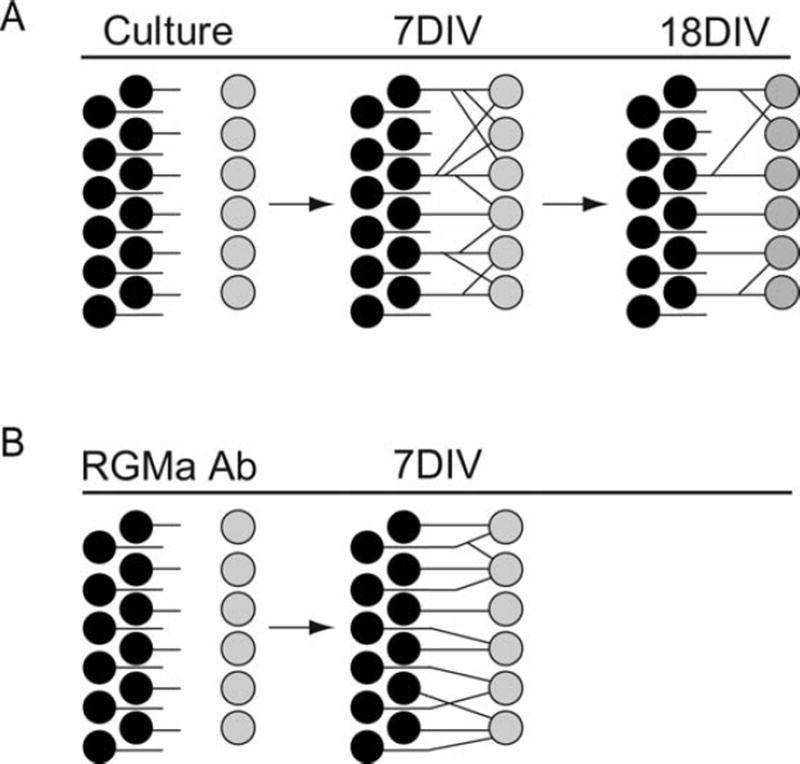
We tested whether RGMa blocked regeneration of afferent peripheral innervation of hair cells by quantitative analysis of spiral ganglion neuron fiber growth and new synapse formation. The relative increase in fibers contacting hair cells was of similar magnitude as the increase in new synapses after treatment, suggesting that new synapses were largely attributable to fiber growth. Neurons added to the cultures of the postnatal cochlea contacted hair cells, and, although synapses were formed even in the absence of manipulation [Fig. 5(A)], treatment with an antibody that blocked the repulsive activity imposed by RGMa increased spiral ganglion neuron sprouting and growth of fibers toward the organ of Corti [Fig. 5(B)]. Contacts on multiple hair cells are pruned in the developing cochlea (Echteler, 1992; Huang et al., 2007) to yield a branching pattern in which neurons contact single hair cells (Fig. 5). This pattern was established sooner after inhibition of RGMa with the antibody, and the final pattern was closer to that in the mature organ of Corti. The time course of pruning in these cultures, initiated soon after birth, was similar to the timing in vivo (Huang et al., 2007).
Figure 5.
Patterns of hair cell innervation mature with time in culture and were accelerated by blocking RGMa. Hair cells were initially innervated with multiple branches and refinement of the branching was observed at times up to 18 DIV in the organ of Corti cultures (A). Treatment with an antibody to RGMa increased the number of synapses and enhanced the rate of reduction in branches resulting in a pattern more like that of in vivo neurons (B).
A large body of literature has attempted to find the cause of inadequate neuronal regeneration after neuronal damage. Little regeneration occurs in the central nervous system, but some replacement can be achieved by transplantation (Brustle and McKay, 1996; Lie et al., 2004; Okano et al., 2007). Peripheral nervous system axons have a capacity to regenerate (Sanes and Lichtman, 1999; O’Brien et al., 2009), although the environment in the adult tissue may be less favorable than in the embryo (Harel and Stritt-matter, 2006; O’Brien et al., 2009; Sanes and Yamagata, 2009; Zou et al., 2009). Therapeutic approaches to overcome inhibitory molecules have been suggested: motor neurons show increased ability to grow to their targets when chondroitin sulfate is removed (Curinga et al., 2007) or antibodies to Nogo or other inhibitors of regrowth are used (Fischer et al., 2004; Zhang et al., 2008; Cao et al., 2010). The limited ability of neurons to regrow to form synapses in the adult has been partially overcome by treatments that increase axon sprouting or expression of synaptic molecules (Fischer et al., 2004; Harel and Strittmatter, 2006; Paradis et al., 2007; Cao et al., 2010).
There have been few studies of the importance of axonal guidance molecules in the adult (Harel and Strittmatter, 2006; Pasterkamp and Verhaagen, 2006). A role for inhibitory guidance molecules has been suggested in spinal cord regeneration (Hata et al., 2006; Kaneko et al., 2006; Pasterkamp and Verhaagen, 2006) and in regeneration of sensory neurons (O’Brien et al., 2009). Guidance molecules in the adult could act, as in the embryo, to aid neurons to their synaptic sites, but may also prevent growth of axons to a site that was innervated in the embryo. Upregulation of inhibitory guidance molecules after injury could be detrimental for axonal regeneration. RGMa was found to play a negative role in regeneration of neurons in a rat model of spinal cord injury: blocking of RGMa led to increased neuronal growth and partial recovery of function (Hata et al., 2006). RGMa antibody treatment overcame the inhibition of synaptogenesis of cortico-spinal neurons (Kyoto et al., 2007) and neurite branching (Yoshida et al., 2008) similar to what we demonstrate here.
New approaches to the regeneration of auditory neurons would be attractive because of the poor recovery from loss of hair cell innervation. Regrowth of auditory neurons after loss due to noise or toxin damage has never been clearly demonstrated (Starr et al., 2004; Kujawa and Liberman, 2009). Although there is a possibility of some regeneration of afferent synapses immediately postinjury (Lerner-Natoli et al., 1997), the retraction of peripheral fibers due to noise damage results in hearing loss, and a lack of fiber growth or synaptogenesis prevents spontaneous recovery (Kujawa and Liberman, 2006, 2009).
The limited regeneration capacity of many neurons is thought to result from the loss of inherent capacity for neurite outgrowth by neurons, combined with the loss of molecules for axonal guidance in the adult (Harel and Strittmatter, 2006; Pasterkamp and Verhaagen, 2006; Loers and Schachner, 2007; Zou et al., 2009) and the overexpression of inhibitory molecules at the sites of damage to the mature nervous system (Fischer et al., 2004; Loers and Schachner, 2007; Zhang et al., 2008; Cao et al., 2010). Postnatal expression of RGMa and neogenin was demonstrated here, and postnatal expression of neogenin has been demonstrated in the neurogenic regions of the brain (De Vries and Cooper, 2008). The data here indicate that the lack of regeneration of synapses between afferent neurons and hair cells is caused in part by guidance molecules in the adult cochlea.
Acknowledgments
Contract grant sponsor: National Institute on Deafness and other Communicative Disorders (NIDCD); contract grant numbers: RO1 DC007174 and P30 DC005209.
The authors thank the Capita Foundation and Robert Boucai for support, and Dr. Shyan-Yuan Kao for technical help with in situ hybridization.
References
- Brinks H, Conrad S, Vogt J, Oldekamp J, Sierra A, Deitinghoff L, Bechmann I, et al. The repulsive guidance molecule RGMa is involved in the formation of afferent connections in the dentate gyrus. J Neurosci. 2004;24:3862–3869. doi: 10.1523/JNEUROSCI.5296-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, McKay RD. Neuronal progenitors as tools for cell replacement in the nervous system. Curr Opin Neurobiol. 1996;6:688–695. doi: 10.1016/s0959-4388(96)80104-8. [DOI] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Receptors for myelin inhibitors: Structures and therapeutic opportunities. Mol Cell Neurosci. 2010;43:1–14. doi: 10.1016/j.mcn.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of corti. J Neurobiol. 2006;66:1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curinga GM, Snow DM, Mashburn C, Kohler K, Thobaben R, Caggiano AO, Smith GM. Mammalian-produced chondroitinase AC mitigates axon inhibition by chondroitin sulfate proteoglycans. J Neurochem. 2007;102:275–288. doi: 10.1111/j.1471-4159.2007.04530.x. [DOI] [PubMed] [Google Scholar]
- De Vries M, Cooper HM. Emerging roles for neogenin and its ligands in CNS development. J Neurochem. 2008;106:1483–1492. doi: 10.1111/j.1471-4159.2008.05485.x. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci U S A. 1992;89:6324–6327. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–14034. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol. 2003;13:452–458. doi: 10.1016/s0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Grant L, Fuchs P. Hair cell afferent synapses. Curr Opin Neurobiol. 2008;18:389–395. doi: 10.1016/j.conb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller BK, Yamashita T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: Evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after temporary noiseinduced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoto A, Hata K, Yamashita T. Synapse formation of the cortico-spinal axons is enhanced by RGMa inhibition after spinal cord injury. Brain Res. 2007;1186:74–86. doi: 10.1016/j.brainres.2007.10.038. [DOI] [PubMed] [Google Scholar]
- Lerner-Natoli M, Ladrech S, Renard N, Puel JL, Eybalin M, Pujol R. Protein kinase C may be involved in synaptic repair of auditory neuron dendrites after AMPA injury in the cochlea. Brain Res. 1997;749:109–119. doi: 10.1016/s0006-8993(96)01306-6. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Liu X, Hashimoto M, Horii H, Yamaguchi A, Naito K, Yamashita T. Repulsive guidance molecule b inhibits neurite growth and is increased after spinal cord injury. Biochem Biophys Res Commun. 2009;382:795–800. doi: 10.1016/j.bbrc.2009.03.115. [DOI] [PubMed] [Google Scholar]
- Loers G, Schachner M. Recognition molecules and neural repair. J Neurochem. 2007;101:865–882. doi: 10.1111/j.1471-4159.2006.04409.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Corrales CE, Cuajungco MP, Heller S, Edge AS. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66:319–331. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008;68:669–684. doi: 10.1002/dneu.20616. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Chedotal A. Repulsive guidance molecule/neogenin: a novel ligand-receptor system playing multiple roles in neural development. Dev Growth Differ. 2004;46:481–486. doi: 10.1111/j.1440-169x.2004.00768.x. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Nakamura H, Chedotal A. Repulsive guidance molecule plays multiple roles in neuronal differentiation and axon guidance. J Neurosci. 2006;26:6082–6088. doi: 10.1523/JNEUROSCI.4556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, Mann M, Flad M, et al. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–395. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- Mueller BK. Growth cone guidance: First steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci. 2004;24:808–818. doi: 10.1523/JNEUROSCI.4610-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien GS, Martin SM, Sollner C, Wright GJ, Becker CG, Portera-Cailliau C, Sagasti A. Developmentally regulated impediments to skin reinnervation by injured peripheral sensory axon terminals. Curr Biol. 2009;19:2086–2090. doi: 10.1016/j.cub.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361:1499–1511. doi: 10.1098/rstb.2006.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller BK, et al. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: In situ hybridization using digoxigenin-labelled cRNA probes. Histo-chemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Shi F, Corrales CE, Liberman MC, Edge AS. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur J Neurosci. 2007;26:3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- Starr A, Isaacson B, Michalewski HJ, Zeng FG, Kong YY, Beale P, Paulson GW, et al. A dominantly inherited progressive deafness affecting distal auditory nerve and hair cells. J Assoc Res Otolaryngol. 2004;5:411–426. doi: 10.1007/s10162-004-5014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Tassew NG, Charish J, Seidah NG, Monnier PP. SKI-1 and Furin generate multiple RGMa fragments that regulate axonal growth. Dev Cell. 2012;22:391–402. doi: 10.1016/j.devcel.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tong M, Brugeaud A, Edge AS. Regenerated synapses between postnatal hair cells and auditory neurons. J Assoc Res Otolaryngol. 2013;14:321–329. doi: 10.1007/s10162-013-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141:12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Wilson NH, Key B. Neogenin interacts with RGMa and netrin-1 to guide axons within the embryonic vertebrate forebrain. Dev Biol. 2006;296:485–498. doi: 10.1016/j.ydbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Mueller BK, Hata K. Neogenin and repulsive guidance molecule signaling in the central nervous system. Curr Opin Neurobiol. 2007;17:29–34. doi: 10.1016/j.conb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Yoshida J, Kubo T, Yamashita T. Inhibition of branching and spine maturation by repulsive guidance molecule in cultured cortical neurons. Biochem Biophys Res Commun. 2008;372:725–729. doi: 10.1016/j.bbrc.2008.05.124. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang Q, Zhang JH, Qin X. NgR acts as an inhibitor to axonal regeneration in adults. Front Biosci. 2008;13:2030–2040. doi: 10.2741/2821. [DOI] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]