Abstract
Purpose
A significant minority of colorectal cancer (CRC) patients experience clinically meaningful distress that may warrant intervention. The goal of this systematic review was to assess the impact of psychosocial interventions on quality-of-life and psychosocial outcomes for CRC patients.
Methods
A systematic search of CINAHL, MEDLINE, PsycINFO, and PsycARTICLES was undertaken to obtain relevant randomized controlled trials (RCTs) published through October 2016.
Results
Fourteen RCTs of psychosocial interventions for CRC patients were identified. Only three of these RCTs showed significant intervention effects on multiple mental health outcomes. These interventions included written and verbal emotional expression, progressive muscle relaxation training, and a self-efficacy enhancing intervention. Eight of the 14 trials, testing a range of psychoeducational and supportive care interventions, produced little to no effects on study outcomes. An evaluation of RCT quality highlighted the need for greater rigor in study methods and reporting.
Conclusion
A limited evidence base supports the efficacy of psychosocial interventions for CRC patients. Large-scale trials are needed before drawing definitive conclusions regarding intervention impact.
Keywords: Systematic review, Colorectal cancer, Interventions, Psychosocial, Psychological, Quality of life
Introduction
A significant minority of colorectal cancer (CRC) patients experience clinically meaningful anxiety or depressive symptoms or reduced mental well-being that may warrant intervention [1–4]. Worse mental health outcomes in CRC patients have been associated with younger age, lower socioeconomic status, increased perceptions of illness-related benefits, and poorer physical health outcomes (e.g., greater physical symptom distress and medical comorbidities, bowel dysfunction) [2,5–14]. Conversely, greater social support and, among Chinese CRC patients, a greater sense of personal control and collective control (i.e., control over cancer-related problems in collaboration with loved ones) have been associated with better mental health outcomes during the acute CRC survivorship period [7,10,12].
A large body of research has attempted to improve social support and coping skills among cancer patients in order to impact mental health outcomes [15]. Across meta-analyses, psychosocial interventions for cancer patients have yielded small to medium effects on distress outcomes [15–17], and studies with a distress criterion for eligibility produced larger effects [15]. However, the degree to which these studies have focused on CRC patients has not been systematically reviewed. CRC is the third most common cancer [18], and disrupted eating and bowel habits distinguish CRC from many other cancers. In qualitative research, CRC patients with altered eating and bowel habits have reported isolation from others, the loss of their professional identity, as well as the loss of privacy, dignity, and independence [19]. Furthermore, a growing body of research has documented profound changes in CRC patients’ sexual functioning, such as erectile dysfunction for men and pain during sexual intercourse for women, that negatively impact quality of life (QOL) and may result in avoidance of sexual activity [19,20]. Hoon and colleagues [21] reviewed the literature on psychosocial interventions for CRC patients and retrieved 11 studies, only four of which were randomized controlled trials (RCTs). Relevant studies may have been excluded from this review, however, as PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [22] were not followed and the search terms were limited. Additionally, various aspects of study quality were not evaluated.
The impact of lifestyle interventions on CRC patients’ QOL outcomes has also been recently reviewed [23,24]. Across five RCTs, exercise interventions were not found to affect CRC patients’ QOL or fatigue, but aerobic exercise led to improved physical fitness relative to controls [23]. Another systematic review of 12 RCTs found mixed evidence of associations between dietary changes and QOL outcomes in CRC and other cancer patients [24]. Researchers have also begun to test interventions targeting a range of health behaviors during CRC survivorship [25,26]. For example, a health coaching intervention focusing on various health behaviors (e.g., physical activity, diet, alcohol use) improved some of these behaviors (e.g., physical activity, vegetable intake) and psychosocial outcomes (e.g., posttraumatic growth, spirituality) in CRC survivors, but did not affect overall QOL relative to usual care [25,27]. Taken together, evidence for the impact of lifestyle interventions on psychosocial and QOL outcomes in CRC patients is limited, and further research is needed to link specific intervention components to these outcomes [28].
The goal of the current systematic review was to examine the effect of psychosocial interventions on QOL and psychosocial outcomes for CRC patients of all disease stages. Psychosocial interventions were defined as group and individual psychotherapy or cognitive-behavioral training that aims to modify maladaptive thoughts and behaviors. Examined psychosocial interventions also included education to reduce distress by providing information on the disease and treatment process, coping skills, or available resources. We examined RCTs with at least one psychosocial or QOL outcome. We aimed to identify psychosocial interventions with evidence of efficacy in CRC populations and to evaluate the acceptability to patients and quality of the included intervention trials. We also aimed to identify potential directions for future research and clinical practice.
Methods
Search strategy
A systematic review was performed according to the PRISMA statement [22]. Articles were identified through a search of CINAHL, MEDLINE, PsycINFO, and PsycARTICLES. We sought additional articles by hand-searching the reference lists of articles meeting the inclusion criteria. Search terms included combinations of cancer (including neoplasm and oncolog*), colorectal (including colon and rectal), and terms related to therapy (including cognitive therapy, psychotherapy, cognitive-behavio* therapy, acceptance and commitment therapy, problem-solving therapy, supportive-expressive therapy, counsel*, self-help groups, psycho-education*) or the term “intervention” and descriptive terms (including quality of life, behavio*, psycho*, distress, symptoms, and mindfulness). The date last searched was October 27, 2016.
We used several criteria to select articles for this review. Articles had to be published in peer-reviewed English-language journals and had to report outcomes of an RCT enrolling adult CRC patients or survivors of any disease stage. Studies of patients with multiple cancer types were only included if results for CRC patients could be extracted. This review focused on psychosocial interventions, including education, individual psychotherapy, cognitive-behavioral training, and group interventions. Studies focused on health behavior change (e.g., diet, exercise, smoking) and those that did not report at least one psychosocial or QOL outcome were excluded from this review.
Data extraction and analysis
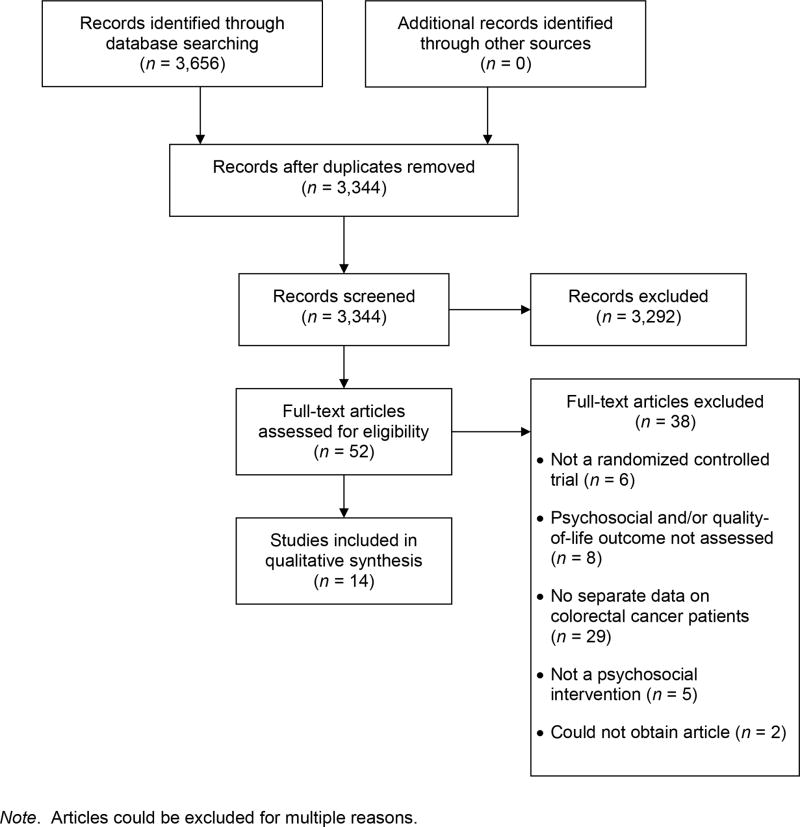
We initially excluded articles based on their titles and abstracts (Fig. 1). Then the first and second author independently reviewed potentially eligible articles, and differences were resolved through discussion. Next, the two authors independently assessed the selected studies for quality using a modified 12-item version of the PEDro scale [29] and reconciled differences in coding. Higher scores on the PEDro scale provide evidence of internal validity, generalizability, and interpretability of a trial’s results. Two items regarding the blinding of therapists and participants, respectively, were removed from the original 11-item scale ([29], http://www.pedro.org.au/english/downloads/pedro-scale/), as this blinding does not typically apply to behavioral or psychosocial intervention trials. In addition, three items were added to the original scale, as found in a prior meta-analysis of interventions for cancer patients [16]. Two of these items assessed treatment fidelity (i.e., use of manualized treatment, monitoring of treatment implementation), consistent with recommendations of the Treatment Fidelity Workgroup of the National Institutes of Health Behavior Change Consortium [30]. The third item assessed the reporting of loss to follow-up information, consistent with other reviews of interventions for cancer patients [16,31].
Fig. 1.
PRISMA flow diagram
Note. Articles could be excluded for multiple reasons.
We decided to provide a narrative of the results rather than a statistical synthesis due to the heterogeneity of outcomes. For example, outcomes included QOL, sexual functioning, unmet supportive care needs, distress, social support, and posttraumatic growth. Meta-analyses are typically not appropriate for summarizing a small number of studies with diverse outcomes that cannot be combined in a meaningful way [32]. Furthermore, the number of studies was insufficient for conducting moderation analyses based on the type of outcome. Study characteristics, findings, and methodological quality were summarized in tables.
Results
Selection of RCTs
A search of the four databases yielded 3,344 unique citations, and two authors reviewed the full text of 52 citations (see Figure 1). Fourteen unique RCTs, including a total of 2,476 participants with CRC, met the inclusion criteria and were included in this systematic review. Only 2 of the 14 RCTs had been included in the review by Hoon and colleagues [21].
Description of the RCTs
Table 1 displays demographic and cancer-related characteristics for the 14 RCTs. For the 12 trials that reported the gender of CRC participants, a minority of participants (41%) were women. In addition, across 11 studies with sufficient age data for CRC participants, the average age was 62 years. In the eight trials that reported ethnicity, participants were primarily European, Chinese, or Caucasian American.
Table 1.
Characteristics of included studies
| Study citation | Number of participants | Female gender (%) | Predominant ethnicity (%) | Mean age | Cancer site and stage | Mean time since diagnosis | Point in treatment at baseline | QOL eligibility criterion |
|---|---|---|---|---|---|---|---|---|
| Beaver et al., 2012 [35] | N = 50 | 42% | Not reported | 73.0 | 58% colon and 42% rectum; stage not reported, but 100% had no evidence of recurrent disease | 21 months | Post-treatment | None |
| Carmack et al., 2011 [33] | N = 40 | 63% | 63% Caucasian | 56.2 | Colon and rectum; 22% stage I, 18% stage II, 60% stage III | 45 months | Post-treatment | T score ≥ 63 on BSI Global Severity Index or at least 2 of 9 symptom dimensions |
| Cheung et al., 2003 [43] | N = 59 | 32% | 100% Chinese | 58.2 | Colon and rectum; stage not reported | Not reported | Post-stoma surgery | None |
| Edgar et al., 2001 [36] | N = 225 (BC, n = 146; colon cancer, n = 79) | 82% (BC and colon cancer combined) | Not reported | 56.5 (BC and colon cancer combined) | 65% breast and 35% colon; stage not reported | Not reported, but 100% diagnosed within prior 4 months at time of enrollment | Mixed | None |
| Haase et al., 2005 [44] | N = 60 | 38% | Not reported | 65.0 | 25% colon and 75% rectum; 8% stage 0, 35% stage I, 27% stage II, 30% stage III | Not reported | Pre-surgery | None |
| Harrison et al., 2011 [37] | N = 74 | 39% | 69% Australian | 64.6 | 58% colon, 34% rectum, and 8% rectosigmoid; ACPDS: 27% stage A, 27% stage B, 31% stage C, 7% stage D, and 8% noncancerous (identified after surgery and randomization) | Not reported | Pre-surgery | None |
| Hendren et al., 2012 [38] | N = 319 (BC, n = 270; CRC, n = 49) | 92% (BC and CRC combined) | 68% Caucasian (BC and CRC combined) | 57.0 (BC and CRC combined) | 85% breast, 15% colon/rectum; stages reported for BC and CRC combined: 8% stage 0, 32% stage I, 35% stage II, 21% stage III, 4% stage IV | Not reported | Mixed | None |
| Lee et al., 2010 [45] | N = 166 | 34% | 100% Chinese | 59.7 | Colon and rectum; 59% late stage | 24 months | Mixed | None |
| O’Connor et al., 2014 [39] | N = 76 | 36% | Not reported | 73.7% aged 59 and older | Rectum; Duke’s stage: 5% stage A, 55% stage B, 34% stage C, 1% stage D, and 4% premalignant | Not reported | Pre-surgery | None |
| Reese et al., 2014 [34] | N = 18 | 33% | 89% Caucasian | 52.6 | 44% colon and 56% rectum; 17% stage I or IIA, 28% stage IIIB or IIIC, and 56% stage IV | 23 months | Post-treatment | Either noticed changes in sex life or “not at all” to “somewhat” satisfied with sex life |
| Ross et al., 2005 [40] | N = 249 | 51% | 100% Danish | 68.5a | Colon and rectum; Duke’s stage: 12% stage A, 39% stage B, 30% stage C, 16% distant metastases, 0.4% carcinoid tumor, and 2% unknown | Not reported | Post-surgery | None |
| White et al., 2012 [41] | N = 648 | 38% | Not reported | 64.6 | Colon and rectum; 19% stage I, 46% stage II, and 29% stage IIIA/IIIB (some missing data) | 3 months | Mixed | None |
| Young et al., 2013 [42] | N = 756 | 44% | Not reported | 67.8 | 67% colon and 33% rectum; ACPDS: 26% stage A, 32% stage B, 29% stage C, 10% stage D, 2% noncancerous (identified after randomization), and .8% missing | Not reported | Post-surgery | None |
| Zhang et al., 2014 [46] | N = 152 | 36% | 100% Chinese | 53.3 | 49% colon and 51% rectum; 39% stage II and 61% stage III | Not reported, but 100% had received a CRC diagnosis within the past 6 months | Scheduled to receive adjuvant chemotherapy after surgery | None |
Note. ACPS, Australian clinicopathological stage; BC, breast cancer; BSI, Brief Symptom Inventory; CRC, colorectal cancer; QOL, quality of life.
Value represents weighted average of median ages.
Regarding medical characteristics, 12 of the 14 trials enrolled both colon and rectal cancer patients. Only 2 of the 12 trials that reported disease stage enrolled primarily late stage or stage IV participants. In addition, only five trials reported the average time since diagnosis, which ranged from 3 months to 45 months. The point in treatment at baseline also varied widely across studies, ranging from pre-surgery to post-treatment.
Only two trials had a QOL criterion for eligibility [33,34]. One trial enrolled patient who showed significant distress (i.e., met a clinical cutoff for distress on the Brief Symptom Inventory’s Global Severity Index or 2 of 9 primary symptom dimensions) [33]. The other trial enrolled patients who endorsed change in their sex life since cancer or its treatment or lower levels of sexual satisfaction [34].
Table 2 shows intervention characteristics, control or comparison groups, and outcome measures. Eight trials evaluated educational or supportive care interventions [35–42]. Other tested interventions included relaxation (e.g., progressive muscle relaxation [PMR]; n = 2) [43,44], written and verbal emotional expression (n = 1) [33], an Eastern Body-Mind-Spirit intervention (n = 1) [45], intimacy enhancement (n = 1) [34], and a self-efficacy enhancing intervention (n = 1) [46]. The majority of studies (10/14) used an individual delivery approach, and the number of sessions ranged from 1 to 12, with the exception that three studies did not have a standard number of sessions. Five studies had in-person sessions, five had telephone sessions, and four involved a combination of in-person and telephone sessions. Interventions were delivered by nurses, physicians, mental health professionals, and trained volunteers. Most studies (10/14) compared the intervention to standard care, and only one study included a comparison arm that controlled for time and attention given to participants. Studies most often employed validated questionnaires of QOL or distress as primary outcomes.
Table 2.
Intervention description and results of included studies
| Study citation | Intervention type | Intervention delivery | Intervention length | Control or comparison group | Outcome measures | Results |
|---|---|---|---|---|---|---|
| Beaver et al., 2012 [35] | Psychoeducation, social support | CRC nurse practitioner via telephone | Number of contacts and timeframe varied based on patient needs; the average patient was enrolled for 12 months | Hospital follow-up conducted by surgeons, registrars, junior doctors, or CRC nurse practitioners | STAI and GHQ-12; administered at baseline and 1 follow-up that varied based on patient needs | No significant between-group differences at follow-up. |
| Carmack et al., 2011 [33] | Combined written and verbal expression; 2 mailings of educational materials related to support needs of cancer patients | Master’s level interventionists via group format (average of 8 participants per group) | 12, 60-minute sessions delivered over 4 months (9 weekly sessions, 2 bi-monthly sessions, 1 final session at 4 months) | Standard care and 2 mailings of educational materials related to support needs of cancer patients | BSI, CES-D, and EORTC-QLQ; administered at baseline, 2 months (mid-intervention), and 4 months (post-intervention) | Compared to control arm, intervention arm reported reduced global symptom distress and depression at 2 months; however, differences in QOL were non-significant at 2 months. Intervention arm also reported reduced global symptom distress and depression as well as improved emotional functioning at 4 months. |
| Cheung et al., 2003 [43] | Progressive muscle relaxation (PMR) training | Therapist delivered the intervention at the patient’s bedside in 1 briefing and 1 training session; a therapist or research nurse called patients every 2 weeks to monitor PMR practice | 2 training sessions with 20-minutes of PMR delivered during hospitalization; bi-weekly phone follow-ups conducted over remaining 10 weeks | Standard care | C-STAI, C-QOL-Colostomy, and WHOQOL-BREF-HK; administered at 1, 5, and 10 weeks post-surgery | Compared to control arm, intervention arm reported decreased anxiety and improved domains of QOL, including psychological health, at 5 and 10 weeks. No between-group differences in CRC-specific QOL reported at 5 weeks; however, intervention group reported improved CRC-specific QOL at 10 weeks. |
| Edgar et al., 2001 [36] | Psychoeducation and coping skills training | Two nurses, a social worker, and a psychologist delivered 3 of the intervention arms in person: 1) individual sessions, 2) group sessions, and 3) peer support groups. | 5, 90-minute sessions over 6 months | Two comparison groups: 1) peer support group and 2) no treatment | POMS and FACT-C; administered at baseline and every 4 months over a 12-month period (4 time points overall) | No significant between-group differences for any of the outcomes in CRC patients. |
| Haase et al., 2005 [44] | Guided imagery and PMR | Patients were given either a 12-minute guided imagery tape or a 12-minute PMR tape; there was no additional contact with study team members. | Patients instructed to listen to the tape at least 3 times per day starting 2 days before surgery and continuing through 7 days post-surgery | Standard care | Analgesic consumption, pulmonary function, postoperative ileus, VAS-Pain, and VAS-fatigue; administered daily for 7 days post-surgery | No significant between-group differences for any of the outcomes. |
| Harrison et al., 2011 [37] | Supportive care including unmet needs assessment, education, and emotional support | CRC nurse-delivered via telephone | 5 phone calls over 6 months post-surgery (days 3 and 10, and 1, 3, and 6 months) | Standard care | SCNS-SF, Ca-SUN, FACT-C, and health service utilization; administered at baseline and 1, 3, and 6 months | No significant between-group differences in supportive care needs, CRC-specific QOL, or health service utilization at any time point; however, the authors argued that between-group differences were clinically relevant. |
| Hendren et al., 2012 [38] | Patient navigation | Trained, non-medical study personnel via multiple in-person and telephone encounters | Number of contacts varied based on patient needs | Standard care | FACT-C; administered at baseline and 3, 6, 9, and 12 months | No significant between-group differences across time points. |
| Lee et al., 2010 [45] | Eastern Body-Mind-Spirit psychosocial intervention and health education print materials on CRC and its treatment | Trained facilitators via in-person groups (10 to 12 patients per group) | 5 weekly 180-minute sessions | Control group (content not specified) and health education print materials | C-CECS, C-MMAC, C-PTGI, and C-SF-36; administered at baseline, immediately post-intervention, and 4, 8, and 12 months post-intervention | Compared to control group, intervention group reported improved post-traumatic growth and attitudes toward cancer immediately post-intervention; however, there were no between-group differences on QOL or emotional control immediately post-intervention. There were no significant between-group differences on any outcomes at the other time points. |
| O’Connor et al., 2014 [39] | Tailored information packets on various aspects of rectal cancer and its treatment | Stoma care nurse specialists guided the patients through the packets in-person and conducted assessments either in-person or via telephone | 1 in-person meeting with nurse | Standard care | HADS, RNLI, and PSCaTE; administered at baseline, post-intervention (after surgery before hospital discharge), and 6-months after discharge | Intervention group reported lower anxiety at the last follow-up; however, there were no between-group differences on depression or readjustment at either time point. |
| Reese et al., 2014 [34] | Intimacy enhancement | Therapist delivered via telephone to patient-partner dyads | 4 weekly 50-minute sessions | Wait-list control | ISS, DSCS, MSIS, FSFI, IIEF, SFQ-Medical Impact subscale, and self-efficacy for communicating about sex, dealing with sexual difficulties, and enjoying intimacy; administered at baseline and post-intervention | Compared to control group, patients in the intervention group reported better female and male sexual function, self-efficacy for enjoying intimacy, and less medical impact on sexual function; however, no between-group differences in sexual distress or intimacy and negative effects on sexual communication and two self-efficacy items. Compared to control group, partners in the intervention group reported improvement in all outcomes. |
| Ross et al., 2005 [40] | Home visits providing emotional and informational support and encouraging patients to use social network | A nurse or medical doctor visited the patient in his or her home | 10, 60-minute home visits over first 2 years of discharge (5 visits in first 2–3 months, and then visits at 4, 7, 11, 16, and 24 months) | Standard care | HADS and EORTC-QLQ; administered at 3, 6, 12, and 24 months after hospital discharge | No significant between-group differences on any outcomes across all time points, with the exception of less fatigue for the intervention group compared to controls at 3-month follow-up. |
| White et al., 2012 [41] | Supportive care | Trained volunteers via telephone | The number of contacts varied based on patient needs. | Standard care | SCNS, HADS, CRC symptom checklist, use of health services, and MOS-SS; administered at baseline and 3, 6, and 9 months | No significant between-group differences on any primary outcomes across all time points, except for a greater reduction in the prevalence of elevated anxiety in the intervention group compared to controls. |
| Young et al., 2013 [42] | Supportive care including unmet needs assessment, education, and emotional support | Nurses via telephone | 5 phone calls over 6 months post-surgery (days 3 and 10, and 1, 3, and 6 months) | Standard care | SCNS-SF34, FACT-C, DT, and health service utilization; administered at baseline and 1, 3, and 6 months post-discharge | No significant between-group differences on any of the outcomes across all time points. |
| Zhang et al., 2014 [46] | Self-efficacy enhancing intervention | Oncology nurses delivered the intervention in-person and via telephone | 1, 60-minute in-person psychoeducation session; 4 monthly 20–40 minute telephone sessions; a handbook with self-efficacy improvement strategies; a 30-minute relaxation audio recording; all delivered over 6 months | Standard care | SICPA, C-MDASI, C-HADS, and C-FACT-G; administered at baseline and 3 and 6 months post-intervention | Compared to control group, intervention group reported improved self-efficacy as well as decreased symptom severity and interference, anxiety, and depression at both follow-ups. However, no between-group differences in QOL. |
Note. BSI, Brief Symptom Inventory; Ca-SUN, Cancer Survivors’ Unmet Needs Measure; C-CECS, Chinese version of the Courtauld Emotional Control Scale; CES-D, Center for Epidemiologic Studies Depression Scale; C-FACT-C, Chinese version of the Functional Assessment of Cancer Therapy-Colorectal; C-HADS, Chinese version of the Hospital Anxiety and Depression Scale; C-MDASI, Chinese version of the M.D. Anderson Symptom Inventory; C-MMAC, Chinese version of the Mini-Mental Adjustment to Cancer Scale; C-PTGI, Chinese version of the Post-Traumatic Growth Inventory; C-QOL-Colostomy, Chinese version of the Quality of Life index for Colostomy Scale; CRC, colorectal cancer; C-SF-36, Chinese version of the Short Form-36 Health Survey; C-STAI, Chinese version of the State-Trait Anxiety Inventory; DSCS, Dyadic Sexual Communication Scale; DT, Distress Thermometer; EORTC-QLQ, European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire; FSFI, Female Sexual Function Index; FACT-C, Functional Assessment of Cancer Therapy-Colorectal; GHQ-12, General Health Questionnaire-Short Version; HADS, Hospital Anxiety and Depression Scale; ISS, Index of Sexual Satisfaction; IIEF, International Index of Erectile Functioning; MOS-SS, Medical Outcome Study-Social Support scale; MSIS, Miller Social Intimacy Scale; POMS, Profile of Mood States; PSCaTE, Patient Satisfaction with Cancer Treatment Education; QOL, quality of life; RNLI, Reintegration to Normal Living Index; SCNS-SF, Supportive Care Needs Survey Short Form; SFQ, Sexual Function Questionnaire; SICPA, Stanford Inventory of Cancer Patient Adjustment; STAI, State-Trait Anxiety Inventory; VAS, Visual Analog Scale; WHOQOL-BREF-HK, Hong Kong Chinese version of the World Health Organization Quality of Life Measure-Abbreviated Version.
PEDro criteria [29] were used to evaluate the quality of each of the 14 studies. Table 3 shows the coding of the criteria, including the specification of eligibility criteria, quality of randomization procedures, blinding of assessors to treatment information, adequacy of follow-up, data analysis and reporting, and treatment fidelity monitoring. Trials met between 7 and 12 of the 12 quality criteria. Two trials did not meet the allocation concealment criterion [34,44]. Three trials did not have comparable groups at baseline regarding prognostic indicators, such as cancer stage [35,36,39]. Nine trials did not report blinding assessors to treatment information [34,35,37–39,41–43,45]. Four studies did not have measures of key outcomes on more than 85% of participants [34–36,45]. Two trials did not report having a treatment manual [40,45], and eight trials did not report monitoring treatment implementation [34,37–40,43–45].
Table 3.
Quality of included studies
| Study Citation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physiotherapy Evidence Database (PEDro) criterion | Beaver et al. (2012) | Carmack et al. (2011) | Cheung et al. (2003) | Edgar et al. (2001) | Haase et al. (2005) | Harrison et al. (2011) | Hendren et al. (2012) | Lee et al. (2010) | O’Connor et al. (2014) | Reese et al. (2014) | Ross et al. (2005) | White et al. (2012) | Young et al. (2013) | Zhang et al. (2014) |
| Eligibility criteria were specified | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Subjects were randomly assigned to treatment groups | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Allocation was concealed | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| The groups were similar at baseline regarding most important prognostic indicators | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| All assessors who measured at least one key outcome were blinded to treatment information | No | Yes | No | Yes | Yes | No | No | No | No | No | Yes | No | No | Yes |
| Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to treatment groups | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| All participants for whom outcome measures were available received the treatment or control intervention as allocated or, when this was not done, data for at least one key outcome was analyzed by “intention to treat” (including imputation) | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The results of between-group statistical comparisons were reported for at least one key outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The study provided both point measures and measures of variability for at least one key outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| The study had an adequate treatment fidelity protocol, including manualized treatment | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| The study had an adequate treatment fidelity protocol, including monitoring of treatment implementation | Yes | Yes | No | Yes | No | No | No | No | No | No | No | Yes | Yes | Yes |
| Loss to follow-up information was provided | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes |
| Total number of criteria met | 9 | 12 | 9 | 10 | 10 | 10 | 8 | 7 | 9 | 8 | 8 | 11 | 11 | 12 |
Note. “Yes” indicates that the criterion was evidenced in the article. “No” indicates that the criterion is not evidenced or could not be determined in the article.
Synthesis of results
Results of the 14 RCTs appear in Table 2. Six of the trials produced null effects of the intervention across all outcome variables [35–38,42,44]. These trials tested diverse interventions, including psychoeducation, supportive care, patient navigation, and training in relaxation and other coping skills. Two trials, one testing supportive home visits by medical professionals and the other testing telephone-delivered support from volunteers, produced effects on only one study outcome [40,41].
Three trials showed an intervention effect on multiple mental health outcomes [33,43,46]. The examined interventions included a group-based written and verbal emotional expression intervention [33], individual PMR training [43], and a self-efficacy enhancing intervention for individuals [46]. Two of these intervention trials met all of the quality criteria (12/12) [33,46], with the other trial meeting 9 of the criteria [43].
The remaining three trials showed improvement in certain outcomes related to mental health and QOL [34,39,45]. Specifically, a group-based Eastern Body-Mind-Spirit intervention led to higher levels of posttraumatic growth and more positive attitudes towards cancer, but did not affect other QOL outcomes [45]. Nurse-administered information packets on rectal cancer and its treatment, with one exception, did not affect mental health outcomes [39]. Finally, a feasibility study of intimacy enhancement for patient-partner dyads impacted certain sexual outcomes (e.g., male and female sexual function) and not others (e.g., sexual distress) [34].
Table 4 shows feasibility and acceptability outcomes, including accrual and retention rates, intervention adherence, and participant satisfaction. Accrual rates ranged from less than 10% using a passive recruitment approach to 100% of eligible patients. Retention rates were generally high, with 10 studies having measures of key outcomes from more than 85% of participants, as noted previously. Intervention adherence was variable across studies, but was generally high for phone-based interventions. Acceptability of the interventions was assessed with measures of patient satisfaction in six of the trials [33–35,39,42,44]. Overall, high levels of satisfaction with the interventions were reported, including educational and supportive care interventions [35,39,42], written and verbal expression [33], guided imagery and PMR [44], and intimacy enhancement for patient-partner dyads [34].
Table 4.
Feasibility and acceptability outcomes of included studies
| Study citation | Accrual rate | Retention rate | Intervention adherencea | Participant satisfaction |
|---|---|---|---|---|
| Beaver et al., 2012 [35] | 72% (65/90) of eligible patients consented. | Across the intervention and hospital follow-up groups, 77% completed a follow-up that varied based on patient needs. | 31/32 (97%) received telephone-based psychoeducation/social support; 31/33 (94%) received hospital follow-up. | Both groups reported high levels of satisfaction with information and services. |
| Carmack et al., 2011 [33] | MD Anderson Cancer Center: 38% (105/274) of potentially eligible patients consented to screening; Kelsey-Seybold Clinic: 10% (38/375) returned consent forms and 47% (15/32) of potentially eligible patients consented to screening. Of the 63 eligible patients across sites, 40 completed the baseline assessment. | Across the intervention and control groups, 88% completed follow-up at 2 months (mid-intervention), and 98% completed follow-up at 4 months (post-intervention). | For written/verbal expression group intervention, mean attendance was 7.12/12 sessions. | On average, participants in the intervention group reported that they would definitely recommend the program to other patients. |
| Cheung et al., 2003 [43] | All eligible patients (n=63) consented. | Across the intervention and control groups, 94% provided complete data at 1, 5, and 10 weeks post-surgery. | On average, PMR was practiced 1.67 times per week across the 10-week period. | None reported. |
| Edgar et al., 2001 [36] | 33% (225/667) of eligible patients consented. | Across the four groups, 84% were retained over a 12-month time period (4 time points). | For individual psychoeducation/coping skills training, mean attendance was 4/6 sessions. For group psychoeducation/coping skills training or peer support group, mean attendance was 2.5/6 sessions. | None reported. |
| Haase et al., 2005 [44] | 80% (74/93) of eligible patients were randomized. | Across the three groups, 81% were retained during daily assessments for 7 days post-surgery. | 20/22 (91%) received guided imagery intervention; 22/29 (76%) received PMR intervention. | Over 90% of patients in the intervention conditions reported that they would recommend the intervention to other patients. |
| Harrison et al., 2011 [37] | 86% (75/87) of eligible patients consented. | Across the intervention and control groups, 80% completed an unmet needs measure and 77% completed a quality of life measure at 6 months. | Receipt of the telephone-based supportive care intervention ranged from 92% to 95% through the 3-month point and was 84% at 6 months. | None reported. |
| Hendren et al., 2012 [38] | 49% (324/661) of eligible patients who could be reached via phone consented. 319/324 were cancer patients. | Across the intervention and control groups, 94% completed the 3-month follow-up; completion rates were not reported for 6, 9, and 12-month follow-ups. | Not reported. | None reported. |
| Lee et al., 2010 [45] | Not reported. | Across the intervention and control groups, 70% completed all 5 assessments over a 12-month period. | Not reported. | None reported. |
| O’Connor et al., 2014 [39] | 98% (85/87) of eligible patients were randomized. | Across the intervention and control groups, 89% completed the post-intervention follow-up and 86% completed the 6-month follow-up. | 43/47 (91%) received the tailored information packets on rectal cancer and its treatment. | Compared to control group, intervention group was more satisfied with information at both follow-ups. |
| Reese et al., 2014 [34] | 29% (23/79) of eligible couples were randomized. | Across the intervention and control groups, 78% of couples completed the post-intervention assessment. | 10/13 couples (77%) received a telephone-delivered intimacy enhancement intervention. | Overall, participants found the program to be helpful in improving intimacy and relevant. |
| Ross et al., 2005 [40] | 64% (265/413) of contacted patients were randomized and 6% (16/265) were found to be ineligible after randomization. | Across the intervention and control groups, 84% to 88% of patients who were alive completed follow-ups at each of 4 time points up to 24 months post-hospital discharge. | 77/125 (62%) received all 10 supportive care home visits, 34 (27%) had 6–9 home visits, 12 (10%) had 1–5 home visits, and 2 (2%) had no home visits. | None reported. |
| White et al., 2012 [41] | 79% (717/905) of potentially eligible patients agreeing to researcher contact consented. | Across the intervention and control groups, follow-up participation rates at 3, 6, and 9 months were 93%, 87%, and 82%, respectively. | 95% (290/306) completed at least one supportive care phone call. Number of calls varied based on patient needs. | None reported. |
| Young et al., 2013 [42] | Not reported. | Across the intervention and control groups, follow-up participation rates at 1, 3, and 6 months were 92%, 89%, and 87%, respectively. | Completion of supportive care phone calls at each of 5 time points over 6 months post-surgery ranged from 84% to 93%. | Most intervention participants reported being highly satisfied with the assistance received from the research nurse. |
| Zhang et al., 2014 [46] | 87% (152/174) of eligible patients consented. | Across the intervention and control groups, 89% completed the 3-month follow-up and 80% completed the 6-month follow-up. | Not reported. | None reported. |
A broad definition of intervention adherence was used, including frequency of skills practice, session attendance, and intervention receipt.
Discussion
This systematic review yielded only 14 RCTs of psychosocial interventions for CRC patients, despite the high prevalence of this cancer type [18]. This review identified 12 more studies than a prior review of psychosocial interventions for CRC patients which included non-RCT designs [21]. Of the 14 RCTs in this review, only three showed significant effects of the intervention on multiple mental health outcomes. These interventions included written and verbal emotional expression [33], PMR training [43], and a self-efficacy enhancing intervention [46]. Three additional intervention trials showed an impact on outcomes related to mental health and QOL, including studies testing an Eastern Body-Mind-Spirit intervention [45], nurse-administered information packets on rectal cancer and its treatment [39], and an intimacy enhancement intervention for patient-partner dyads [34]. The remaining eight trials, examining a wide range of interventions (e.g., psychoeducation, supportive care, coping skills training), produced little to no effects on study outcomes. Taken together, there is limited empirical support for psychosocial interventions for CRC patients, and further work is needed to address the unique QOL concerns of this population, such as embarrassing side effects of treatment and sexual dysfunction.
The literature on lifestyle interventions for CRC patients also has found limited evidence of effects on psychosocial and QOL outcomes [23,24]. Methodological issues, such as biased sampling, attrition, and contamination across study conditions, may have contributed to null findings. Testing the separate and combined impact of psychosocial and lifestyle interventions on QOL outcomes in larger, methodologically rigorous trials would advance the science of supportive care interventions for this population. Greater attention to patients’ perceptions of intervention acceptability is needed, as lifestyle intervention trials often have low uptake [47].
Only six trials in the current review assessed patient satisfaction with the intervention and, in all cases, patients generally expressed a high degree of satisfaction, regardless of the evidence for intervention efficacy. Social desirability and other biases may contribute to high satisfaction ratings. Alternatively, CRC patients may have experienced benefits from the intervention not captured by current assessments, such as increased social support and coping tools. The generally high retention rates across studies are consistent with this explanation.
The current results should be interpreted in light of a number of methodological limitations. In particular, some studies did not report monitoring treatment implementation or blinding assessors to treatment condition, thus increasing the risk of detection bias. Other PEDro criteria [29] (e.g., allocation concealment, having comparable groups at baseline regarding prognostic indicators) also were not met in multiple trials. Thus, quality indicators were quite variable across studies and highlight the need for greater rigor in reporting and methodology. Additionally, some studies had low accrual rates and small sample sizes, which limited statistical power for detecting effects.
Other directions for future research warrant consideration. First, inclusion of attention control groups would allow for analysis of intervention effects above and beyond the provision of standard support. Second, testing interventions delivered via the Internet and other technology platforms may help expand their reach to patients with physical impairments and those in rural areas. Third, intervention approaches with evidence of efficacy in other populations with chronic physical illness, such as third wave cognitive behavioral therapies [48,49], may be tailored to CRC patients and tested in RCTs. Third wave cognitive behavioral therapies emphasize mindfulness, acceptance, cognitive flexibility, and patient values and include interventions such as Acceptance and Commitment Therapy, Meta-Cognitive Therapy, and mindfulness-based therapies [49]. Additionally, a focus on understanding intervention mechanisms or factors underlying their efficacy would result in more efficacious interventions [50]. These mechanisms could include changes in self-efficacy or confidence in using coping skills targeted by the intervention, acceptance of unwanted thoughts and feelings, or enhanced social support as well as physiological mechanisms (e.g., decreased arousal to negative thoughts and feelings about cancer).
As psychosocial interventions for CRC patients continue to be tested across cultures, consistent reporting of ethnicity will enable cross-cultural comparisons. In addition, greater inclusion of ethnic minorities will allow for the examination of culturally tailored interventions as well as the degree to which interventions are effective across ethnocultural groups.
In addition to increasing ethnic diversity, enrolling samples with clinically meaningful levels of distress will ensure that findings generalize to those with the greatest need for support services. Only two studies in this review had a QOL criterion for study entry, which parallels the broader literature with cancer patients. Indeed, one meta-analysis found that only 10% of psychosocial intervention studies with cancer patients restricted eligibility to those with some degree of distress [15].
Based on results of the current review and the broader literature on common problems in CRC patients [3], future intervention research should address the following outcome domains. First, novel interventions to reduce stigma and self-blame in CRC patients are needed, given their associations with depressive symptoms [51]. CRC may be stigmatizing for a number of reasons. For example, incontinence and other defecation-related problems may contribute to disturbance in body image, social isolation, and QOL impairment [52]. Furthermore, difficulty adjusting to changes in roles (e.g., loss of employment) and sexual dysfunction may increase perceptions of stigma [20]. Preliminary evidence suggests that stigma may be targeted via an acceptance-based cognitive behavioral approach in lung cancer [53]; such interventions may be modified to address the challenges of CRC patients and tested in RCTs. Second, interventions to address the unique sexual concerns of CRC patients and their partners warrant further development and evaluation, given the prevalent and persistent sexual side effects of treatment [20,54] and limited sexual health intervention research with cancer populations [55]. Finally, the broader cancer literature suggests that supported self-management of various treatment side effects may promote patients’ active engagement in their care and mental and physical QOL [56]. Preventing negative QOL outcomes through the early provision of coping tools may be a promising direction for future research.
Regarding practice implications, results point to a limited evidence base for psychosocial interventions with respect to improving psychosocial and QOL outcomes in CRC patients; thus, few clinical recommendations can be made at this time. Caution should be used when applying findings from the broader literature on psycho-oncologic interventions to CRC patients, as breast cancer patients and women are over-represented [15]. In addition, CRC patients have unique psychosocial needs (e.g., isolation, embarrassment) related to altered eating and bowel habits and sexual dysfunction that warrant clinical attention. Tailoring support services to address the mental health and QOL concerns of CRC patients is an important goal for clinical care and future research endeavors.
Acknowledgments
Catherine Mosher’s work was supported by the National Cancer Institute [Grant numbers: K07CA168883 and K05CA175048]. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
For this type of study formal consent is not required.
References
- 1.Dunn J, Ng SK, Holland J, Aitken J, Youl P, Baade PD, Chambers SK. Trajectories of psychological distress after colorectal cancer. Psychooncology. 2013;22:1759–1765. doi: 10.1002/pon.3210. [DOI] [PubMed] [Google Scholar]
- 2.Hou WK, Law CC, Yin J, Fu YT. Resource loss, resource gain, and psychological resilience and dysfunction following cancer diagnosis: a growth mixture modeling approach. Health Psychol. 2010;29:484–495. doi: 10.1037/a0020809. [DOI] [PubMed] [Google Scholar]
- 3.Mosher CE, Winger JG, Given BA, Helft PR, O’Neil BH. Mental health outcomes during colorectal cancer survivorship: a review of the literature. Psychooncology. 2016;25:1261–1270. doi: 10.1002/pon.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers SK, Meng X, Youl P, Aitken J, Dunn J, Baade P. A five-year prospective study of quality of life after colorectal cancer. Qual Life Res. 2012;21:1551–1564. doi: 10.1007/s11136-011-0067-5. [DOI] [PubMed] [Google Scholar]
- 5.Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol. 2004;22:4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Hou WK, Law CC, Fu YT. Does change in positive affect mediate and/or moderate the impact of symptom distress on psychological adjustment after cancer diagnosis? A prospective analysis. Psychol Health. 2010;25:417–431. doi: 10.1080/08870440802559375. [DOI] [PubMed] [Google Scholar]
- 7.Hou WK, Wan JH. Perceived control mediates the prospective impact of relationship quality in the year after colorectal cancer diagnosis. Ann Behav Med. 2012;43:129–138. doi: 10.1007/s12160-011-9303-z. [DOI] [PubMed] [Google Scholar]
- 8.Simon AE, Thompson MR, Flashman K, Wardle J. Disease stage and psychosocial outcomes in colorectal cancer. Colorectal Dis. 2009;11:19–25. doi: 10.1111/j.1463-1318.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Gagen J, Cress RD, Drake CM, Romano PS, Yost KJ, Ayanian JZ. Quality-of-life and surgical treatments for rectal cancer–a longitudinal analysis using the California Cancer Registry. Psychooncology. 2010;19:870–878. doi: 10.1002/pon.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steginga SK, Lynch BM, Hawkes A, Dunn J, Aitken J. Antecedents of domain-specific quality of life after colorectal cancer. Psychooncology. 2009;18:216–220. doi: 10.1002/pon.1388. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Sharp DM, Walker LG, Monson JR. Predictors of early postoperative quality of life after elective resection for colorectal cancer. Ann Surg Oncol. 2007;14:3435–3442. doi: 10.1245/s10434-007-9554-x. [DOI] [PubMed] [Google Scholar]
- 12.Lynch BM, Steginga SK, Hawkes AL, Pakenham KI, Dunn J. Describing and predicting psychological distress after colorectal cancer. Cancer. 2008;112:1363–1370. doi: 10.1002/cncr.23300. [DOI] [PubMed] [Google Scholar]
- 13.Emmertsen KJ, Laurberg S. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013;100:1377–1387. doi: 10.1002/bjs.9223. [DOI] [PubMed] [Google Scholar]
- 14.Occhipinti S, Chambers SK, Lepore S, Aitken J, Dunn J. A longitudinal study of post-traumatic growth and psychological distress in colorectal cancer survivors. PLoS ONE. 2015;10:e0139119. doi: 10.1371/journal.pone.0139119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta-analysis. J Clin Oncol. 2013;31:782–793. doi: 10.1200/JCO.2011.40.8922. [DOI] [PubMed] [Google Scholar]
- 16.Hart SL, Hoyt MA, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, Steel JL, Cuijpers P, Mohr DC, Berendsen M, Spring B, Stanton AL. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst. 2012;104:990–1004. doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider S, Moyer A, Knapp-Oliver S, Sohl S, Cannella D, Targhetta V. Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis. J Behav Med. 2010;33:1–14. doi: 10.1007/s10865-009-9227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Cancer Society. Cancer facts and figures 2017. American Cancer Society; Atlanta, GA: 2017. [Google Scholar]
- 19.Rozmovits L, Ziebland S. Expressions of loss of adulthood in the narratives of people with colorectal cancer. Qual Health Res. 2004;14:187–203. doi: 10.1177/1049732303260874. [DOI] [PubMed] [Google Scholar]
- 20.Traa MJ, De Vries J, Roukema JA, Den Oudsten BL. Sexual (dys)function and the quality of sexual life in patients with colorectal cancer: a systematic review. Ann Oncol. 2011;23:19–27. doi: 10.1093/annonc/mdr133. [DOI] [PubMed] [Google Scholar]
- 21.Hoon LS, Chi Sally CW, Hong-Gu H. Effect of psychosocial interventions on outcomes of patients with colorectal cancer: a review of the literature. Eur J Oncol Nurs. 2013;17:883–891. doi: 10.1016/j.ejon.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Cramer H, Lauche R, Klose P, Dobos G, Langhorst J. A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care (Engl) 2014;23:3–14. doi: 10.1111/ecc.12093. [DOI] [PubMed] [Google Scholar]
- 24.Kassianos AP, Raats MM, Gage H, Peacock M. Quality of life and dietary changes among cancer patients: a systematic review. Qual Life Res. 2015;24:705–719. doi: 10.1007/s11136-014-0802-9. [DOI] [PubMed] [Google Scholar]
- 25.Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, Aitken JF, Meng X, Courneya KS. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol. 2013;31:2313–2321. doi: 10.1200/JCO.2012.45.5873. [DOI] [PubMed] [Google Scholar]
- 26.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, Cohen HJ. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkes AL, Pakenham KI, Chambers SK, Patrao TA, Courneya KS. Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: a randomized controlled trial. Ann Behav Med. 2014;48:359–370. doi: 10.1007/s12160-014-9610-2. [DOI] [PubMed] [Google Scholar]
- 28.Winger JG, Mosher CE, Rand KL, Morey MC, Snyder DC, Demark-Wahnefried W. Diet and exercise intervention adherence and health-related outcomes among older long-term breast, prostate, and colorectal cancer survivors. Ann Behav Med. 2014;48:235–245. doi: 10.1007/s12160-014-9598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centre for Evidence-Based Physiotherapy. PEDro Scale. Centre for Evidence-Based Physiotherapy. 2009 http://www.pedro.org.au/. Accessed November 2 2016.
- 30.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 31.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 32.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons, Ltd; Chichester, UK: 2009. When does it make sense to perform a meta-analysis? pp. 357–364. [Google Scholar]
- 33.Carmack CL, Basen-Engquist K, Yuan Y, Greisinger A, Rodriguez-Bigas M, Wolff RA, Barker T, Baum G, Pennebaker JW. Feasibility of an expressive-disclosure group intervention for post-treatment colorectal cancer patients: results of the Healthy Expressions study. Cancer. 2011;117:4993–5002. doi: 10.1002/cncr.26110. [DOI] [PubMed] [Google Scholar]
- 34.Reese JB, Porter LS, Regan KR, Keefe FJ, Azad NS, Diaz LA, Herman JM, Haythornthwaite JA. A randomized pilot trial of a telephone-based couples intervention for physical intimacy and sexual concerns in colorectal cancer. Psycho-Oncology. 2014;23:1005–1013. doi: 10.1002/pon.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaver K, Campbell M, Williamson S, Procter D, Sheridan J, Heath J, Susnerwala S. An exploratory randomized controlled trial comparing telephone and hospital follow-up after treatment for colorectal cancer. Colorectal Dis. 2012;14:1201–1209. doi: 10.1111/j.1463-1318.2012.02936.x. [DOI] [PubMed] [Google Scholar]
- 36.Edgar L, Rosberger Z, Collet JP. Lessons learned: Outcomes and methodology of a coping skills intervention trial comparing individual and group formats for patients with cancer. Int J Psychiatry Med. 2001;31:289–304. doi: 10.2190/U0P3-5VPV-YXKF-GRG1. [DOI] [PubMed] [Google Scholar]
- 37.Harrison JD, Young JM, Solomon MJ, Butow PN, Secomb R, Masya L. Randomized pilot evaluation of the supportive care intervention “CONNECT” for people following surgery for colorectal cancer. Dis Colon Rectum. 2011;54:622–631. doi: 10.1007/DCR.0b013e31820bc152. [DOI] [PubMed] [Google Scholar]
- 38.Hendren S, Griggs JJ, Epstein R, Humiston S, Jean-Pierre P, Winters P, Sanders M, Loader S, Fiscella K. Randomized controlled trial of patient navigation for newly diagnosed cancer patients: effects on quality of life. Cancer Epidemiol Biomarkers Prev. 2012;21:1682–1690. doi: 10.1158/1055-9965.EPI-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connor G, Coates V, O’Neill S. Randomised controlled trial of a tailored information pack for patients undergoing surgery and treatment for rectal cancer. Eur J Oncol Nurs. 2014;18:183–191. doi: 10.1016/j.ejon.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Ross L, Thomsen BL, Karlsen RV, Boesen EH, Johansen C. A randomized psychosocial intervention study on the effect of home visits on the well-being of Danish colorectal cancer patients–the INCA Project. Psychooncology. 2005;14:949–961. doi: 10.1002/pon.899. [DOI] [PubMed] [Google Scholar]
- 41.White VM, Macvean ML, Grogan S, D’Este C, Akkerman D, Ieropoli S, Hill DJ, Sanson-Fisher R. Can a tailored telephone intervention delivered by volunteers reduce the supportive care needs, anxiety and depression of people with colorectal cancer? A randomised controlled trial. Psychooncology. 2012;21:1053–1062. doi: 10.1002/pon.2019. [DOI] [PubMed] [Google Scholar]
- 42.Young JM, Butow PN, Walsh J, Durcinoska I, Dobbins TA, Rodwell L, Harrison JD, White K, Gilmore A, Hodge B, Hicks H, Smith S, O’Connor G, Byrne CM, Meagher AP, Jancewicz S, Sutherland A, Ctercteko G, Pathma-Nathan N, Curtin A, Townend D, Abraham NS, Longfield G, Rangiah D, Young CJ, Eyers A, Lee P, Fisher D, Solomon MJ. Multicenter randomized trial of centralized nurse-led telephone-based care coordination to improve outcomes after surgical resection for colorectal cancer: the CONNECT intervention. J Clin Oncol. 2013;31:3585–3591. doi: 10.1200/JCO.2012.48.1036. [DOI] [PubMed] [Google Scholar]
- 43.Cheung YL, Molassiotis A, Chang AM. The effect of progressive muscle relaxation training on anxiety and quality of life after stoma surgery in colorectal cancer patients. Psychooncology. 2003;12:254–266. doi: 10.1002/pon.638. [DOI] [PubMed] [Google Scholar]
- 44.Haase O, Schwenk W, Hermann C, Muller JM. Guided imagery and relaxation in conventional colorectal resections: a randomized, controlled, partially blinded trial. Dis Colon Rectum. 2005;48:1955–1963. doi: 10.1007/s10350-005-0114-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee AM, Ho JW, Chan CL. Efficacy of psychosocial intervention in improving quality of life and psychological well-being of Chinese patients with colorectal cancer: a randomised controlled trial. Hong Kong Med J. 2010;16:20–24. [PubMed] [Google Scholar]
- 46.Zhang M, Chan SW, You L, Wen Y, Peng L, Liu W, Zheng M. The effectiveness of a self-efficacy-enhancing intervention for Chinese patients with colorectal cancer: a randomized controlled trial with 6-month follow up. Int J Nurs Stud. 2014;51:1083–1092. doi: 10.1016/j.ijnurstu.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Adams RN, Mosher CE, Blair CK, Snyder DC, Sloane R, Demark-Wahnefried W. Cancer survivors’ uptake and adherence in diet and exercise intervention trials: an integrative data analysis. Cancer. 2015;121:77–83. doi: 10.1002/cncr.28978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hann KEJ, McCracken LM. A systematic review of randomized controlled trials of Acceptance and Commitment Therapy for adults with chronic pain: Outcome domains, design quality, and efficacy. J Contextual Behav Sci. 2014;3:217–227. [Google Scholar]
- 49.Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware, and active: Contextual approaches as an emerging trend in the behavioral and cognitive therapies. Annu Rev Clin Psychol. 2011;7:141–168. doi: 10.1146/annurev-clinpsy-032210-104449. [DOI] [PubMed] [Google Scholar]
- 50.Stanton AL, Luecken LJ, MacKinnon DP, Thompson EH. Mechanisms in psychosocial interventions for adults living with cancer: Opportunity for integration of theory, research, and practice. J Consult Clin Psychol. 2013;81:318–335. doi: 10.1037/a0028833. [DOI] [PubMed] [Google Scholar]
- 51.Phelan SM, Griffin JM, Jackson GL, Zafar SY, Hellerstedt W, Stahre M, Nelson D, Zullig LL, Burgess DJ, van Ryn M. Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psychooncology. 2013;22:65–73. doi: 10.1002/pon.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grumann MM, Noack EM, Hoffmann IA, Schlag PM. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149–156. doi: 10.1097/00000658-200102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chambers SK, Baade P, Youl P, Aitken J, Occhipinti S, Vinod S, Valery PC, Garvey G, Fong KM, Ball D, Zorbas H, Dunn J, O’Connell DL. Psychological distress and quality of life in lung cancer: the role of health-related stigma, illness appraisals and social constraints. Psychooncology. 2015;24:1569–1577. doi: 10.1002/pon.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Earle CC, Chretien Y, Morris C, Ayanian JZ, Keating NL, Polgreen LA, Wallace R, Ganz PA, Weeks JC. Employment among survivors of lung cancer and colorectal cancer. J Clin Oncol. 2010;28:1700–1705. doi: 10.1200/JCO.2009.24.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brotto LA, Yule M, Breckon E. Psychological interventions for the sexual sequelae of cancer: A review of the literature. J Cancer Surviv. 2010;4:346–360. doi: 10.1007/s11764-010-0132-z. [DOI] [PubMed] [Google Scholar]
- 56.McCorkle R, Ercolano E, Lazenby M, Schulman-Green D, Schilling LS, Lorig K, Wagner EH. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin. 2011;61:50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]