Abstract
Purpose
Circulating molecules play important roles in lung cancer diagnosis. In addition, plasma lactate dehydrogenase (LDH) and creatine kinase (CK) have been shown to be closely related to tumor progression in breast cancer, prostate cancer, and colonel cancer. However, the relationships between LDH and CK levels with metastasis occurrence and the survival status of lung cancer patients remain unclear.
Experimental design
A total of 1142 lung cancer patients were enrolled in this study and were separated into negative or positive groups, according to the plasma levels of CK or LDH. Patients in both groups were assessed for clinical characteristics, metastasis occurrence, and survival status. The Cox regression model was then introduced to confirm whether CK and LDH could act as independent factors for predicting a poor prognosis.
Results
The results indicated that CK had a close relationship with bone (p < 0.05) and lymph node (p < 0.05) metastases. In addition, LDH was strongly related with bone (p < 0.05), adrenal gland (p < 0.05), and lymph node (p < 0.05) metastases. CK and LDH were also correlated with the survival status of the lung cancer patients (all p < 0.001). According to specific histological classification analysis, it was found that CK was closely related to the survival status of adenocarcinoma (ADC) and squamous cell carcinoma (SCC) patients, while LDH was only correlated with that of ADC patients. Cox regression analysis confirmed that CK and LDH could act as independent factors for predicting a poor prognosis in ADC but not SCC patients.
Conclusions
For the first time, our study confirmed the role of CK in metastasis occurrence and the survival status of lung cancer patients. In addition, it also demonstrated that CK and LDH could be used as independent factors to predict a poor prognosis in ADC patients. The identification of CK and LDH will play important roles in lung cancer diagnosis and poor outcome prediction in the future.
Introduction
Lung cancer is one of the most common cancer-related deaths worldwide, accounting for 86,380 male and 71,660 female deaths in the United States in 2015 [1]. The mortality due to lung cancer in China is 610.2 per 100,000 [2]. Moreover, the average 5-year survival rate for lung cancer is less than 15% due to late diagnosis [3]. Furthermore, the increased aging population and environmental pollution have enhanced the morbidity of lung cancer [4].
The current noninvasive diagnostic methods for lung cancer used in clinical practice include X-ray and computed tomography (CT) scans [5]. The National Lung Screening Trial (NLST), conducted in the United States, enrolled 53,454 participants. All the participants were randomly divided into a low-dose CT scan group (26,722 cases) or a chest radiography group (26,732 cases), and they all underwent annual screening. The results indicated that although the low-dose CT scan group had a 20% reduced mortality compared with the X-ray group, 96.4% of those in the low-dose CT scan group and 94.5% of those in the radiography group who had a positive diagnosis of lung cancer were found to be false positives [6]. In addition to CT and X-ray, other diagnostic methods for lung cancer are invasive, painful, and time-consuming, such as bronchoscopy and needle biopsy. Therefore, the discovery of noninvasive, nonradioactive, and rapid diagnostic methods for lung cancer is urgently needed [7].
At present, numerous tumor-related molecules, produced by cancer cells or the tumor microenvironment, have been identified in the plasma of lung cancer patients; these molecules can be used for diagnosing and monitoring disease recurrence [8]. Circulating proteins as lung cancer biomarkers, including carcinoembryonic antigen [9], Cyfra21-1 [10], CA199 [11], and CA125 [12], have been widely used in lung cancer diagnosis. However, these biomarkers have low sensitivities of 50–60% [12]. Increasing evidence has suggested that circulating lactate dehydrogenase (LDH) can predict the survival status in various cancers, including lung cancer [13], and overall survival in advanced non-small cell lung cancer (NSCLC) [14]. Creatine kinase (CK) also has been associated closely with breast cancer development and progression [15]. Moreover, inactivation of CK has been strictly correlated with oral squamous cell carcinoma (SCC) progression [16]. However, systematically analyzing the relationships between LDH and CK levels with metastasis occurrence and the survival status in lung cancer patients have not yet been reported.
In this retrospective observational study, we measured the plasma levels of CK and LDH in 1142 patients to explore whether plasma CK and LDH levels could be used as independent factors for predicting a poor prognosis in lung cancer patients.
Methods
Ethics statement
The current research protocol was approved by the Medical Ethics Committee of West China Hospital. Written consent for sampling, study, and publication was obtained from each of the included patients.
Due to the retrospective nature of the study, the informed consent of participants in these study could be waived. Parts of the survival data were obtained through phone calls, so it’s difficult to get written consent. only verbal informed consent were obtained from these subjects or their legal guardians, only for those survival data were followed up via outpatient visit, written informed consents were obtained.
Institutional review board approval for this study was also obtained from West China Hospital. All the methods used in this study were followed in accordance with the approved protocols.
Patients included in this study
This was a single-center, retrospective observational study that was conducted at the West China Hospital of Sichuan University, a tertiary academic hospital in western China. A total of 1142 lung cancer patients pathologically diagnosed in West China Hospital, from January 2008 to July 2012 with complete medical records and follow-up information, were enrolled in this study. Patients were excluded based on the exclusion criteria of without LDH and CK assays; without pathological confirmation of lung cancer; without evidence of metastasis; and lack of follow-up data.
Procedures
All clinical information was extracted from the medical records within 2 weeks of the date of diagnosis. The occurrence of metastasis was confirmed according to the reports of whole-body and bone CT scans and/or lymph node biopsy. The date of death was obtained during a follow-up visit or telephone inquiry. The overall survival time was calculated from the date of diagnosis to death or the last follow-up visit. Plasma concentrations of LDH and CK were measured by immunoassays in the Department of Medical Laboratory, prior to surgery or any other treatment. The cut-off values for positive LDH and CK were 215 U/L and 60 U/L, respectively.
Study design
Based on the cut-off values of LDH and CK, all enrolled lung cancer patients were divided into negative and positive groups. First, correlation analysis was performed to analyze the association between the LDH or CK level with clinical characteristics and metastatic status, respectively, in all lung cancer patients. The survival function was then analyzed to reveal the relationship between LDH or CK and survival status. Finally, the Cox regression model was used to confirm whether LDH and CK could act as independent prognostic factors for poor outcomes in lung cancer patients.
Statistical methods for data analysis
SPSS 19.0 software was used in this study. Significant differences were determined using the chi-squared test. The Kaplan—Meier test was introduced to calculate the survival conditions in the negative and positive groups. The log-rank test was used to compare the differences of survival status between the two groups. The Cox regression model for multivariate analysis was conducted to determine the association between the LDH or CK level with clinical characteristics, metastasis occurrence, and survival status; to evaluate the hazards ratio (HR) for the negative and positive groups of LDH and CK; and to identify the independent predictors of poor outcomes in lung cancer patients.
Results
CK and LDH levels were significantly correlated with metastases in lung cancer patients
A total of 1142 lung cancer patients were enrolled in this study and divided into negative and positive groups, according to the cut-off values of plasma CK and LDH. The demographic and clinical features of all enrolled patients are listed in Table 1. For the CK stratification analysis, including 678 cases in the positive group and 464 in the negative group, the results indicated that CK was closely related to the gender (p < 0.05) and tumor stage (p < 0.001). The reverse relationship was observed in bone (p < 0.05) and lymph node (p < 0.05) metastases by multiple metastasis analysis, which indicated that an increased plasma level of CK meant decreased numbers of metastasis occurrences (bone: 22.6% of CK-negative patients vs. 14.9% of CK-positive patients; lymph node: 60.6% of CK-negative patients vs. 54.3% of CK-positive patients) (Table 2).
Table 1. Demographics and clinical characteristic of enrolled lung cancer patients.
| Characteristics | No. of patients (%) Total(1142) |
|---|---|
| Gender | |
| Male Female |
790 (69.2) 352 (30.8) |
| Age | |
| <45 45–60 >60 |
97(8.5) 463 (40.5) 582 (51.0) |
| Histological classification | |
| Adenocarcinoma Squamous SCLC Others |
307 (26.9) 608 (53.2) 170 (14.9) 57 (5.0) |
| Stages | |
| I II III IV |
107 (9.4) 108 (9.5) 304 (26.6) 623 (54.5) |
| Smoke status | |
| No Yes |
492 (43.1) 650 (56.9) |
| Metastasis | |
| No Yes |
312 (27.3) 830 (72.7) |
| CK | |
| Negative Positive |
678 (59.4) 464 (40.6) |
| LDH | |
| Negative Positive |
732 (64.1) 410 (35.9) |
Abbreviations: SCLC, Small Cell Lung Cancer; CK, Creatine Kinase; LDH, lactate dehydrogenase.
Table 2. Association of clinical characteristics and metastasis after stratification analysis by plasma CK levels.
| Negative n = 678 |
Positive n = 464 |
Total n = 1142 |
p value | |
|---|---|---|---|---|
| Basic Characteristic | ||||
| Age | ||||
| <45 years 45–60 years >60 years |
62(9.1%) 274(40.4%) 342(50.5%) |
35(7.5%) 189(40.8%) 240(51.7%) |
97 463 582 |
0.629 |
| Gender | ||||
| Male Female |
443(65.3%) 235(34.7%) |
347(74.8%) 117(25.2%) |
790 352 |
<0.05* |
| Histological classification | ||||
| Adenocarcinoma Squamous SCLC Others |
190(28.0%) 366(54.0%) 91(13.4%) 31(4.6%) |
117(25.2%) 242(54.2%) 79(17.0%) 26(5.6%) |
307 608 170 57 |
0.265 |
| Stages | ||||
| I II III IV |
50(7.4%) 54(8.0%) 173(25.5%) 401(59.1%) |
57(12.3%) 54(11.6%) 131(28.2%) 222(47.9%) |
107 108 304 623 |
<0.001** |
| Smoke status | ||||
| No Yes |
301(44.4%) 377(55.6%) |
191(41.2%) 273(58.8%) |
492 650 |
0.279 |
| Metastasis | ||||
| Brain | ||||
| No Yes |
595(87.8%) 83(12.2%) |
422(91.0%) 42(9.0%) |
1017 125 |
0.090 |
| Bone | ||||
| No Yes |
525(77.4%) 153(22.6%) |
395(85.1%) 69(14.9%) |
920 222 |
<0.05* |
| Liver | ||||
| No Yes |
613(90.4%) 65(9.6%) |
427(92.0%) 37(8.0%) |
1040 102 |
0.348 |
| Adrenal gland | ||||
| No Yes |
639(94.2%) 39(5.8%) |
442(95.3%) 22(4.7%) |
1081 61 |
0.456 |
| Lymph node | ||||
| No Yes |
267(39.4%) 411(60.6%) |
212(45.7%) 252(54.3%) |
479 663 |
<0.05* |
| Intrapulmonary | ||||
| No Yes |
594(87.6%) 84(12.4%) |
419(90.3%) 45(9.7%) |
1013 129 |
0.158 |
| Pleural | ||||
| No Yes |
579(85.4%) 99(14.6%) |
404(87.1%) 60(12.9%) |
983 159 |
0.423 |
| Mediastinal | ||||
| No Yes |
656(96.8%) 22(3.2%) |
455(98.1%) 9(1.9%) |
1111 31 |
0.183 |
*p values were calculated using the Chi-square test. (*P<0.05, **P<0.001)
LDH stratification analysis showed that an increased plasma LDH level was closely associated with gender (p < 0.05), with a decreased LDH level in female patients (33.5% of LDH-negative patients vs. 26.5% of LDH-positive patients) but an increased LDH level in male patients (66.5% of LDH-negative patients vs. 73.9% of LDH-positive patients). By histological analysis, it was shown that LDH was increased in SCLC patients (12.4% of LDH-negative patients vs. 19.2% of LDH-positive patients) but decreased in SCC patients (55.2% of LDH-negative patients vs. 49.8% of LDH-positive patients, p < 0.05). An increased plasma LDH level was also correlated to smoking history (54.6% of LDH-negative patients vs. 61% of LDH-positive patients, p < 0.05) (Table 3). According to the metastasis analysis, it was found that an enhanced LDH level was positively correlated with bone metastasis (16.5% of LDH-negative patients vs. 24.6% of LDH-positive patients, p < 0.05), adrenal gland metastasis (3.7% LDH-negative patients vs. 8.3% of LDH-positive patients, p < 0.05), lymph node metastasis (55.6% of LDH-negative patients vs. 62.4% of LDH-positive patients, p < 0.05), and mediastinal metastasis (1.9% of LDH-negative patients vs. 4.1% of LDH-positive patients, p < 0.05) (Table 3). Together, these results indicated that the plasma LDH level was closely related with the occurrence of lung cancer metastasis.
Table 3. Association of clinical characteristics and metastasis after stratification analysis by plasma LDH levels.
| Negative n = 732 |
positive n = 410 |
Total n = 1142 |
p value | |
|---|---|---|---|---|
| Basic Characteristic | ||||
| Age | ||||
| <45 years 45–60 years >60 years |
60(8.2%) 291(39.8%) 381(52.0%) |
37(9.0%) 172(42.0%) 201(49.0%) |
97 463 582 |
0.608 |
| Gender | ||||
| Male Female |
487(66.5%) 245(33.5%) |
303(73.9%) 107(26.1%) |
790 352 |
<0.05* |
| Histological classification | ||||
| Adenocarcinoma Squamous SCLC Others |
202(27.6%) 404(55.2%) 91(12.4%) 35(4.8%) |
105(25.6%) 204(49.8%) 79(19.2%) 22(5.4%) |
307 608 170 57 |
<0.05* |
| Stages | ||||
| I II III IV |
85(11.6%) 80(11.0%) 203(27.7%) 364(49.7%) |
22(5.4%) 28(6.8%) 101(24.6%) 259(63.2%) |
107 108 304 623 |
<0.001** |
| Smoke status | ||||
| No Yes |
332(45.4%) 400(54.6%) |
160(39.0%) 250(61.0%) |
492 650 |
<0.05* |
| Metastasis | ||||
| Brain | ||||
| No Yes |
662(90.4%) 70(9.6%) |
355(86.6%) 55(13.4%) |
1017 125 |
<0.05* |
| Bone | ||||
| No Yes |
611(83.5%) 121(16.5%) |
309(75.4%) 101(24.6%) |
920 222 |
<0.001** |
| Liver | ||||
| No Yes |
693(94.7%) 39(5.3%) |
347(84.6%) 63(15.4%) |
1040 102 |
<0.001** |
| Adrenal gland | ||||
| No Yes |
705(96.3%) 27(3.7%) |
376(91.7%) 34(8.3%) |
1081 61 |
<0.05* |
| Lymph node | ||||
| No Yes |
325(44.4%) 407(55.6%) |
154(37.6%) 256(62.4%) |
479 663 |
<0.05* |
| Intrapulmonary | ||||
| No Yes |
659(90.0%) 73(10.0%) |
354(86.3%) 56(13.7%) |
1013 129 |
0.059 |
| Pleural | ||||
| No Yes |
639(87.3%) 93(12.7%) |
344(84.0%) 66(16.0%) |
983 159 |
0.112 |
| Mediastinal | ||||
| No Yes |
718(98.1%) 14(1.9%) |
393(95.9%) 17(4.1%) |
1111 31 |
<0.05* |
*p values were calculated using the Chi-square test. (*P<0.05, **P<0.001)
Correlation of CK or LDH with metastases of adenocarcinoma (ADC) and SCC
ADC and SCC were the two major histological classifications, accounting for 85% of the lung cancer. There were 608 ADC patients who were further separated into CK- or LDH-negative and -positive groups. CK stratification analysis, including 366 cases in the negative group and 242 cases in the positive group, showed that the plasma CK levels were increased in male patients (50.3% of CK-negative patients vs. 61.2% of CK-positive patients) and decreased in female patients (49.7% of CK-negative patients vs. 38.8% of CK-positive patients, p < 0.05) (Table 4). A decreased CK level was also closely related to increased bone metastasis (27.6% of CK-negative patients vs. 19.4% of CK-positive patients, p < 0.05). Although mediastinal metastasis analysis showed similar results as the bone metastasis analysis, the total number of cases was too small (only 15 cases) to draw a conclusion (Table 4). LDH stratification analysis showed that an increased LDH level was closely related to male patients, while a decreased LDH level was closely related to female patients, respectively. For stage analysis, it was found that the LDH level was low in stage I (12.9% of LDH-negative patients vs. 6.4% of LDH-positive patients) and stage II (10.1% of LDH-negative patients vs. 4.9% of LDH-positive patients) patients. However, the LDH level was increased dramatically in stage IV patients (57.2% of LDH-negative patients vs. 72.5% of LDH-positive patients, p < 0.05). In addition, an enhanced LDH level was associated with smoking history (37.6% of LDH-negative patients vs. 46.1% of LDH-positive patients, p < 0.05) (Table 5). For metastasis analysis, a high level of LDH was dramatically associated with an increased probability of bone (19.3% of LDH-negative patients vs. 34.3% of LDH-positive patients, p < 0.001), liver (4.8% of LDH-negative patients vs. 14.7% of LDH-positive patients, p < 0.001), and adrenal gland (2.2% of LDH-negative patients vs. 8.8% of LDH-positive patients, p < 0.001) metastases (Table 5), which clearly indicated the importance of LDH for predicting bone, liver, and adrenal gland metastases in ADC patients.
Table 4. Association of clinical characteristics and metastasis stratified by CK levels in adenocarcinoma patients.
| Negative n = 366 |
Positive n = 242 |
Total n = 608 |
p value | |
|---|---|---|---|---|
| Basic Characteristic | ||||
| Age | ||||
| <45 years 45–60 years >60 years |
43(11.7%) 139(38.0%) 184(50.3%) |
24(9.9%) 89(36.8%) 129(53.3%) |
67 228 313 |
0.684 |
| Gender | ||||
| Male Female |
184(50.3%) 182(49.7%) |
148(61.2%) 94(38.8%) |
332 276 |
<0.05* |
| Stages | ||||
| I II III IV |
26(7.1%) 27(7.4%) 66(18.0%) 247(67.5%) |
39(16.1%) 24(9.9%) 47(19.4%) 132(54.6%) |
65 51 113 379 |
<0.05* |
| Smoke status | ||||
| No Yes |
224(61.2%) 142(38.8%) |
138(57.0%) 104(43.0%) |
362 246 |
0.304 |
| Metastasis | ||||
| Brain | ||||
| No Yes |
306(83.6%) 60(16.4%) |
216(89.3%) 26(10.7%) |
294 86 |
0.050 |
| Bone | ||||
| No Yes |
265(72.4%) 101(27.6%) |
195(80.6%) 47(19.4%) |
460 148 |
<0.05* |
| Liver | ||||
| No Yes |
334(91.3%) 32(8.7%) |
225(93.0%) 17(7.0%) |
559 49 |
0.446 |
| Adrenal gland | ||||
| No Yes |
349(95.4%) 17(4.6%) |
232(95.9%) 10(4.1%) |
581 27 |
0.764 |
| Lymph node | ||||
| No Yes |
148(40.4%) 218(59.6%) |
119(49.2%) 123(50.8%) |
267 341 |
<0.05* |
| Intrapulmonary | ||||
| No Yes |
312(85.2%) 54(14.8%) |
216(89.3%) 26(10.7%) |
528 80 |
0.152 |
| Pleural | ||||
| No Yes |
294(80.3%) 72(19.7%) |
199(82.2%) 43(17.8%) |
493 115 |
0.557 |
| Mediastinal | ||||
| No Yes |
353(96.4%) 13(3.6%) |
240(99.2%) 2(0.8%) |
593 15 |
<0.05* |
*p values were calculated using the Chi-square test. (*P<0.05, **P<0.001)
Table 5. Association of clinical characteristics and metastasis stratified by LDH levels in adenocarcinoma patients.
| Negative N = 404 |
Positive N = 204 |
Total N = 408 |
p value | |
|---|---|---|---|---|
| Basic Characteristic | ||||
| Age | ||||
| <45 years 45–60 years >60 years |
41(10.1%) 146(36.1%) 217(53.8%) |
26(12.7%) 82(40.2%) 96(47.1%) |
67 228 313 |
0.274 |
| Gender | ||||
| Male Female |
205(50.7%) 199(49.3%) |
127(62.3%) 77(37.7%) |
332 276 |
<0.05* |
| Stages | ||||
| I II III IV |
52(12.9%) 41(10.1%) 80(19.8%) 231(57.2%) |
13(6.4%) 10(4.9%) 33(16.2%) 148(72.5%) |
65 51 113 379 |
<0.05* |
| Smoke status | ||||
| No Yes |
252(62.4%) 152(37.6%) |
110(53.9%) 94(46.1%) |
362 246 |
<0.05* |
| Metastasis | ||||
| Brain | ||||
| No Yes |
353(87.4%) 51(12.6%) |
169(82.8%) 35(17.2%) |
522 86 |
0.130 |
| Bone | ||||
| No Yes |
326(80.7%) 78(19.3%) |
134(65.7%) 70(34.3%) |
460 148 |
<0.001** |
| Liver | ||||
| No Yes |
385(95.3%) 19(4.7%) |
174(85.3%) 30(14.7%) |
559 49 |
<0.001** |
| Adrenal gland | ||||
| No Yes |
395(97.8%) 9(2.2%) |
186(91.2%) 18(8.8%) |
581 27 |
<0.001** |
| Lymph node | ||||
| No Yes |
184(45.5%) 220(54.5%) |
83(40.7%) 121(59.3%) |
267 341 |
0.254 |
| Intrapulmonary | ||||
| No Yes |
356(88.1%) 48(11.9%) |
172(84.3%) 32(15.7%) |
528 80 |
0.190 |
| Pleural | ||||
| No Yes |
336(83.2%) 68(16.8%) |
157(77.0%) 47(23.0%) |
493 115 |
0.065 |
| Mediastinal | ||||
| No Yes |
397(98.3%) 7(1.7%) |
196(96.1%) 8(3.9%) |
593 15 |
0.100 |
*p values were calculated using the Chi-square test. (*P<0.05, **P<0.001)
A total of 307 patients were diagnosed as having SCC in this study. The results indicated that an increased plasma CK level was closely related to a decreased occurrence of bone metastasis (16.8% of CK-negative patients vs. 6.8% of CK-positive patients), while other metastases, including brain, liver, lymph node, et al., as well as clinical characteristics, such as age, gender, stage, and smoking history, showed no relationship with the CK level in SCC patients (S1 Table). It was also found that the LDH level was not related to clinical characteristics or metastasis occurrence in SCC patients (S2 Table).
Both CK and LDH levels were related to survival in all lung cancer, ADC, and SCC patients
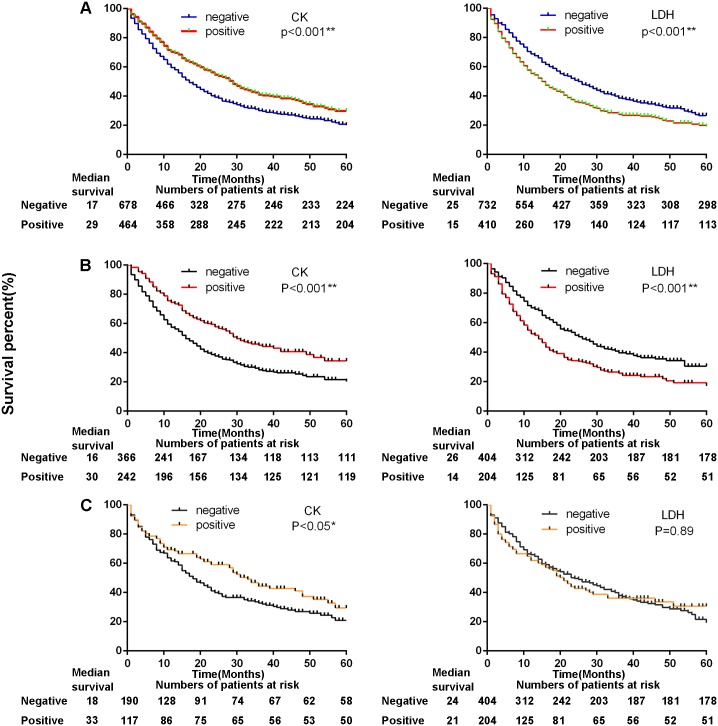
For all lung cancer patients (follow-up time range 1–60 months), the results indicated that a decreased plasma CK level was closely related to a poor survival status (p < 0.001) (Fig 1A). In addition, the patients with an increased LDH level showed poor outcomes (p < 0.001) (Fig 1A). It was also found that a decreased plasma CK level (p < 0.001) and an increased LDH level (p < 0.001) were strongly associated with a poor survival status in ADC patients (Fig 1B). However, the results confirmed that only the CK level was associated with the survival status in SCC patients (p < 0.05) (Fig 1C).
Fig 1. The survival status of non-small cell lung cancer patients correlated with CK and LDH.
A, All lung cancer patients. Median observation period: CK (Negative 17 months;Positive 29 months); LDH (Negative 25 month;Positive 15 months). B, ADC patients. Median observation period: CK (Negative 16 months;Positive 30 months); LDH (Negative 26 month;Positive 14 months). C, SCC patients. Median observation period: CK (Negative 18 months;Positive 33 months); LDH (Negative 24 month;Positive 21 months). CK, creatine kinase; LDH, lactate dehydrogenase; ADC, adenocarcinoma; SCC, squamous cell carcinoma.
Multivariate Cox regression analysis was conducted to identify poor prognostic factors
The multivariate Cox regression model was introduced to identify whether CK or LDH could be used as independent factors for predicting a poor prognosis in lung cancer patients. Our results found that the HR increased to 1.438 in patients aged older than 65 years old (95% CI: 1.091–1.895, p < 0.05) and to 1.195 in patients with a smoking history (95% CI: 1.028–1.389, p < 0.05) for all lung cancer patients. An advanced stage (III, HR: 2.171 and IV, HR: 2.842) and the occurrence of metastasis (HR: 1.326) also could serve as independent factors for predicting poor outcomes. For the two factors of CK and LDH used in this study, the HR decreased to 0.748 in the CK-positive group (95% CI: 0.643–0.871, p < 0.05), while it increased to 1.282 in the LDH-positive group (95% CI: 1.103–1.488, p < 0.05) (Table 6).
Table 6. Multivariate analysis for all lung cancer patients.
| Multivariate HR (95% CI) | p value | |
|---|---|---|
| Age | ||
| <45 45–65 >65 |
[1][Reference] 0.913(0.689–1.210) 1.438(1.091–1.895) |
0.527 <0.05* |
| Smokes | ||
| No Yes |
[1][Reference] 1.195(1.028–1.389) |
<0.05* |
| Stages | ||
| I II III IV |
[1][Reference] 1.377 (0.886–2.142) 2.171(1.481–3.182) 2.842 (1.956–4.130) |
0.155 <0.001** <0.001** |
| Metastasis | ||
| No Yes |
[1][Reference] 1.326(1.094–1.607) |
|
| CK | ||
| Negative Positive |
[1][Reference] 0.748(0.643–0.871) |
<0.001** |
| LDH | ||
| Negative Positive |
[1][Reference] 1.282(1.103–1.488) |
<0.05* |
*p values were calculated using the Cox-proportional hazard model. (*P<0.05, **P<0.001)
Specific histological classification analysis was performed. The results were similar for ADC patients compared with all lung cancer patients. Moreover, an age ≥ 65 years old, a smoking history (HR: 1.228, 95%CI: 1.001–1.506), stage (II, HR: 2.149; III, HR: 4.267; IV, HR: 5.363), and metastasis (HR: 1.248, 95% CI: 0.941–1.656) could be used to predict a poor outcome in ADC patients. It was found that the HR decreased to 0.657 in the CK-positive group (95% CI: 0.529–0.815), while the HR increased to 1.442 in the LDH-positive group for the ADC patients (95% CI: 1.168–1.778) (Table 7). However, the results in this study revealed that CK and LDH could not be used as independent factors to predict a poor prognosis in SCC patients (Table 8). Therefore, our data only confirmed that CK and LDH were important for predicting poor outcomes in ADC patients.
Table 7. Multivariate analysis for ADC patients.
| Multivariate HR (95% CI) | p value | |
|---|---|---|
| Age | ||
| <45 45–65 >65 |
[1][Reference] 0.938(0.664–1.326) 1.373(0.983–1.919) |
0.719 0.063 |
| Smokes | ||
| No Yes |
[1][Reference] 1.228(1.001–1.506) |
0.049 |
| Stages | ||
| I II III IV |
[1][Reference] 2.149 (1.019–4.530) 4.267(2.219–8.202) 5.363 (2.845–10.110) |
<0.05* <0.001** <0.001** |
| Metastasis | ||
| No Yes |
[1][Reference] 1.248(0.941–1.656) |
0.125 |
| CK | ||
| Negative Positive |
[1][Reference] 0.657(0.529–0.815) |
<0.001** |
| LDH | ||
| Negative Positive |
[1][Reference] 1.442(1.168–1.778) |
<0.05* |
*p values were calculated using the Cox-proportional hazard model. (*P<0.05, **P<0.001)
Table 8. Multivariate analysis for SCC patients.
| Multivariate HR (95% CI) | p value | |
|---|---|---|
| Age | ||
| <45 45–65 >65 |
[1][Reference] 1.824(0.654–5.089) 3.832(1.387–10.586) |
0.251 0.010 |
| Smokes | ||
| No Yes |
[1][Reference] 0.819(0.573–1.172) |
0.276 |
| Stages | ||
| I II III IV |
[1][Reference] 0.857(0.442–1.660) 1.075(0.602–1.920) 1.106 (0.612–1.996) |
0.647 0.808 0.739 |
| Metastasis | ||
| No Yes |
[1][Reference] 1.765(1.241–2.512) |
<0.05* |
| CK | ||
| Negative Positive |
[1][Reference] 0.742(0.547–1.005) |
0.054 |
| LDH | ||
| Negative Positive |
[1][Reference] 0.933(0.690–1.262) |
0.652 |
*p values were calculated using the Cox-proportional hazard model. (*P<0.05, **P<0.001)
Discussion
The present study had a large sample size to assess the association of LDH or CK levels with lung cancer prognosis. In this study, we found that CK and LDH were independently and closely related to metastasis occurrence and the survival status in lung cancer patients. In detail, CK was strongly associated with bone metastasis (p < 0.05) and lymph node (p < 0.05) metastasis, while LDH correlated with bone metastasis (p < 0.05), adrenal gland metastasis (p < 0.05), lymph node metastasis (p < 0.05), and mediastinal metastasis (p < 0.05). The lung cancer patients were then stratified by cancer type, ADC or SCC, according to the histological classification. It was found that CK was related to bone metastasis (p < 0.05) and mediastinal metastasis (p < 0.05) and that LDH was associated closely with bone metastasis (p < 0.001), liver metastasis (p < 0.001), and adrenal gland metastasis (p < 0.001) for ADC patients; however, only CK showed a relationship with bone metastasis (p < 0.05), while LDH did not show a relationship with metastasis or clinical characteristics for SCC patients. The Kaplan—Meier survival curves revealed that CK and LDH also showed a close relationship with the survival status for ADC patients but not for SCC patients.
Cox multivariate regression analysis indicated that CK and LDH could act as independent factors for predicting a poor prognosis in ADC patients. The HR decreased to 0.749 in the CK-positive group, while it increased to 1.281 in the LDH-positive group. According to specific subtype analysis, CK showed a low HR of 0.657 and LDH showed an HR of 1.385 in the corresponding positive groups for ADC patients. However, neither CK nor LDH were independent factors for a poor prognosis in SCC patients. Together, these results indicated the importance of CK and LDH in the diagnosis and prediction of a poor prognosis for ADC patients. In our study, CK and LDH were considered as auxiliary diagnostic factors. We believed that the diagnostic value of CK, LDH cannot be compared with the traditional biomarker CEA, Cyfra21-1, CYF, etc. So we just analyzed the value of CK and LDH in the diagnosis and prognosis of lung cancer.
LDH is an important enzyme that catalyzes the hydrogen transfer reaction between lactic acid and pyruvic acid [17], and it is widely distributed in various tissues of the body. The circulating level of LDH is elevated in malignant tumors [18]. The hypoxic microenvironment of a tumor can increase the conversion of pyruvic acid to lactic acid; therefore, unregulated LDH levels ensure proficient anaerobic glycolytic metabolism in the tumor environment, leading to reduced oxygen dependence [19]. An enhanced serum LDH level has been reported as a poor prognostic factor in patients with various malignant tumors, including breast cancer, T-cell lymphoma [20], pancreatic carcinoma [21], and renal cell carcinoma [22]. The LDH levels were significantly higher in metastatic stage IV lung cancer patients who were enrolled in a study of 329 lung cancer patients [23]. In addition, by analyzing 309 advanced-stage NSCLC patients treated with erlotinib, it was found that the change in LDH level during the first month was a surrogate marker for the efficacy of erlotinib in advanced NSCLC [24].
CK is an enzyme that is expressed by various cells and tissues and plays an important role in energy transduction in tissues, particularly in cardiac and skeletal muscle [25]. CK is one of the most important biomarkers that may reflect the status of innate immunity [26]. The study results indicated that the plasma CK levels were dramatically lower compared with positive group. The reason of plasma CK level decreased in lung cancer patients may be due to the CK played an important role in the growth of tumor cell and we hypothesized that growth tumor cells recruited circulating CK and finally resulted in the decline level of CK in circulation. Moreover, by analysis of 823 breast cancer patients, it was found that the serum CK levels were dramatically lower, compared with healthy controls, and were negatively correlated with the stage and tumor size of breast cancer [15].
Previously reported studies on LDH enrolled a relatively small patient sample size, comprising approximately 500 cases or fewer, and included no specific histological classification analysis such as ADC and SCC. To date, the correlation between CK and lung cancer has not been reported. In this retrospective analysis, 1142 patients were enrolled to analyze the correlation between the CK or LDH level with clinical characteristics, metastasis occurrence, as well as survival status. For the first time, our study analyzed the association between CK and lung cancer and confirmed the close relationship between CK and bone metastasis; systematically analyzed the role of LDH in metastasis occurrence and a poor outcome for lung cancer patients, particularly ADC and SCC patients; found that CK could act as an independent factor for predicting a poor prognosis in lung cancer patients, especially ADC patients; and confirmed that LDH is only closely related to a poor prognosis for ADC, not SCC.
The limitation of this study was that this was a one-center and retrospective study, and no consistent data were available to monitor the changes of CK and LDH levels with tumor progression. Furthermore, all analyses were conducted before surgery or other therapy; therefore, the relationship between the CK or LDH level and the therapeutic effects could not be evaluated.
In conclusion, Our results indicated that CK decreased while LDH increased in early stage of lung cancer, which suggested the diagnostic role of CK and LDH in lung cancer. At present, none of the biomarkers could be used alone for molecular diagnosis of tumors. Combination of multiple factors such as CEA, NSE, Cyfra21-1, as well as CK and LDH, together with imaging results, could provide more objective and accurate diagnosis. Plasma CK and LDH levels were identified as independent poor prognostic factors for ADC patients. The confirmation of these factors will help physicians to perform individualized treatment and interpret the contribution of CK and LDH to survival differences in clinical practice. Our results also suggest that further prospective studies should be performed with a large population size.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank department of medical record for providing clinical information of patients.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65: 5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Chen WC, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66: 115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Eng J Med. 2006; 355: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 4.Sun N, Chen ZL, Tan FW, Zhang BH, Yao R, Zhou CC et al. Isocitrate dehydrogenase 1 is a novel plasma biomarker for the diagnosis of non-small cell lung cancer. Clin. Can Res. 2013; 19:5136–5145. [DOI] [PubMed] [Google Scholar]
- 5.Alberle DR, Adams AM, Berg CD, Black WC, Clapp JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365: 395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulshine JL, D’Amico TA. Issues with implementing a high-quality lung cancer screening program. CA Cancer J Clin. 2014; 64: 351–363. [DOI] [PubMed] [Google Scholar]
- 7.Boiselle PM. Computed tomography screening for lung cancer.JAMA.2013; 309: 1163–1170. doi: 10.1001/jama.2012.216988 [DOI] [PubMed] [Google Scholar]
- 8.Vansteenkiste J, Dooms C, Mascaux C, Nackaerts K. Screening and early detection of lung cancer. Ann Oncol. 2012; (Suppl 10): x320–x327. [DOI] [PubMed] [Google Scholar]
- 9.Sakao Y, Tomimitus S, Takeda Y, Natsuaki M, Itoh T. Carcinoembryonicantigen as a predictive factor for postoperative tumor relapse in early-stage adenocarcinoma. Eur J Car-Tho Surg. 2004; 25: 520–522. [DOI] [PubMed] [Google Scholar]
- 10.Alatas F, Alatas O, Metintas M, Colak O, Harmanci E, Demir S. Diagnostic vaue of CEA, CA153, CA199, Cyfra21-1, NSE and TSA assay in pleural effusion. Lung Cancer.2001. 31: 9–16. [DOI] [PubMed] [Google Scholar]
- 11.Molina R, Auge JM, Escudero JM, Marrades R, Viñolas N, Carcereny E et al. CA125, CA199, CA153 and TAG-72.3 as tumor markers in patients with lung cancer: comparison with Cyfra21-1, CEA, SCC and NSE. Tum Biol. 2008; 29: 371. [DOI] [PubMed] [Google Scholar]
- 12.Salgia R, Harpole D, Herndon JA, Pisick E, Elias A, Skarin AT. Role of serum tumor markers CA125 and CEA in non-small cell lung cancer. Anticancer Res. 2001; 21: 1241–1246. [PubMed] [Google Scholar]
- 13.Danner BC, Didilis VN, Wiemeyer S, Stojanovic T, Kitz J, Emmert A et al. Long-term survival is linked to serum LDH and partly to tumor LDH-5 in NSCLC. Anticancer Res. 2010; 30: 1347–1351. [PubMed] [Google Scholar]
- 14.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC et al. Lactate dehydrogenase-5 (LDH-5) over expression in non-small-cell lung cancer tissues is linked to tumor hypoxia, angiogenic factor production and poor prognosis. Br. J Cancer. 2003; 89: 877–885. doi: 10.1038/sj.bjc.6601205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan H, Xia K, Zhou WB, Xue JQ, Liang XQ, Cheng L et al. Low serum creatine kinase levels in breast cancer patients: a case-control study. PlosOne. 2013; 8: e62112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onda T, Uzawa K, Endo Y, Bukawa H, Yokoe H, Shibahara T et al. Ubiquitous mitochondrial creatine kinase downregulated in oral squamous cell carcinoma. Br. J Cancer.2006; 94: 698–709. doi: 10.1038/sj.bjc.6602986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11: 325–337. doi: 10.1038/nrc3038 [DOI] [PubMed] [Google Scholar]
- 18.Somaiah N, Simor GR. Molecular targeted agents and biologic therapies for non-small cell lung cancer. J Thorac. Oncol. 2010; 5: S434–454. doi: 10.1097/01.JTO.0000391362.10517.1f [DOI] [PubMed] [Google Scholar]
- 19.Philip B, Ito K, Moreno-S’anchez R, Ralph SJ. HIF expression and the role of hypoxic microenvironments within primary tumors as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013; 34: 1699–1707. doi: 10.1093/carcin/bgt209 [DOI] [PubMed] [Google Scholar]
- 20.Yi JH, Kim JH, Baek KK, Lim T, Lee DJ, Ahn YC et al. Elevated LDH and paranasal sinus involvement are risk factors for central nervous system involvement in patients with peripheral T-cell lymphoma. Ann. Oncol.2011; 22: 1636–1643. doi: 10.1093/annonc/mdq645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E et al. Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am. J Clin. Oncol. 2001;24: 547–550, [DOI] [PubMed] [Google Scholar]
- 22.Girgis H, Masui O, White NM, Scorilas A, Rotondo F, Seivwright A et al. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol. Cancer. 2014; 13: 101 doi: 10.1186/1476-4598-13-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DS, Park KR, Kim SJ, Chung MJ, Lee YH, Chang JH et al. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: predictive value of metastases and relation to survival outcomes. Tumor Biol. 2016; 37: 619–625. [DOI] [PubMed] [Google Scholar]
- 24.Fiala O, Pesek M, Finek J, Topolcan O, Racek J, Svaton M et al. Change in serum lactate dehydrogenase is associated with outcome of patients with advanced-stage NSCLC treated with Erlotinib. Anticancer Res.2016; 36: 2459–2466. [PubMed] [Google Scholar]
- 25.Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol. Cell Biochem. 1994; 133–134: 193–220. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Li H, Wang X, Gao X, Liu X. Regulation of T cell development and activation by creatine kinase B. PLoS One. 2009; 4: e5000, doi: 10.1371/journal.pone.0005000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.