Abstract
Adding colloidal nanoparticles into liquid crystal media has become a promising pathway either to enhance or to introduce novel properties for improved device performance. Here we designed and synthesized new colloidal hybrid silica nanoparticles passivated with a mesogenic monolayer on the surface to facilitate their organo-solubility and compatibility in liquid crystal host. The resulting nanoparticles were identified by 1H NMR, TEM, TGA and UV-Vis techniques, and the hybrid nanoparticles were doped into a dual frequency cholesteric liquid crystal host to appraise both their compatibility with the host and the effect of the doping concentration on their electro-optical properties. Interestingly, the silica nanoparticle doped liquid crystalline nanocomposites were found to be able to dynamically self-organize into a helical configuration and exhibit multi-stability, i.e., homeotropic (transparent), focal conic (opaque) and planar states (partially transparent), depending on the frequency applied at sustained low voltage. Significantly, a higher contrast ratio between the transparent state and scattering state was accomplished in the nanoparticle-embedded liquid crystal systems.
Keywords: frequency-driven, hybrid silica nanoparticles, tunable transparency, self-organized helical superstructure, dual frequency liquid crystal
Graphical abstract
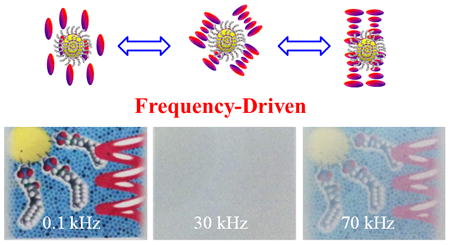
Frequency-driven self-organized helical superstructures are fabricated by incorporating novel mesogen-grafted silica nanoparticles into a dual frequency cholesteric liquid crystal host. The resultant nanocomposites were able to exhibit multiple stable states, i.e., transparent, opaque, and partially transparent, depending on the frequency applied at sustained low voltage. This work offers an impetus in developing dynamic functional soft materials potentially for diverse device applications such as smart windows.
Cholesteric liquid crystals (LCs) with a self-organized helical disposition of molecules not only exhibit fascinating scientific phenomena but also have found practical applications in devices such as temperature sensors, reflective displays, color filters, mirrorless lasers and light shutters.1 Recently, cholesteric LCs fabricated from nematic dual frequency LCs (DFLCs) with frequency dependence of the dielectric anisotropy have attracted considerable attention because both focal conic and planar configuration of such dual frequency cholesteric LCs (DFCLCs) can be switched depending on the frequency of the applied voltage, developing cholesteric reflective displays, fast switching bistable light shutters and bistable intensity modulators etc.2 On the other hand, embedded metallic, semiconducting, and carbon nanomaterials have recently been employed to modify the properties of LCs.3 It should be noted that dielectric silica (SiO2) nanoparticles are the early examples of nanoparticle dispersions into LC matrices.4 An advantage of SiO2 nanoparticles in LCs is the modulation of the refractive index of LC media, which could be exploited to develop hybrid liquid crystalline nanocomposites with improved properties.5 Though commercially available SiO2 nanoparticles were able to modify some properties of LCs, their dispersions were not stable and only very small amount of the particles can be dispersed because of their strong propensity to aggregate or poor compatibility in LC matrix.6 To circumvent this difficulty, surface functionalization of SiO2 nanoparticles has been adopted which led to some interesting results.7 Nevertheless, their functionalization with mesogenic or pro-mesogenic moieties is still a challenging task from the synthetic point of view, albeit this strategy could enable good dispersion and compatibility in LC media.8 Here we functionalized SiO2 nanoparticles with a covalently attached mesogenic monolayer through silane coupling agents and investigated their effect on the electro-optical property of a DFCLC system. The resulting hybrid nanoparticles (M-SiO2) were organo-soluble without noticeable aggregation and were able to disperse well in the DFCLC host with the concentration as high as 3.0 wt%. Interestingly, M-SiO2 nanoparticles enabled to greatly enhance the electro-optical properties compared to the pure DFCLC. The light transmittance of the M-SiO2 embedded LC nanocomposite films can be easily tuned by simply modulating the frequency of an applied constant voltage. Moreover, the M-SiO2 doped system exhibited a higher contrast ratio compared with the pure DFCLC. To the best of our knowledge, this is for the first time that the effect of mesogen-grafted SiO2 nanoparticles embedded in a dual frequency LC matrix is explored.
The synthesis of M-SiO2 nanoparticles is straightforward in a facile manner as depicted in Scheme 1. The commercially available SiO2-OH nanoparticles were reacted with (3-amino propyl)-triethoxy silane (APTES) in ethanol to afford the intermediate SiO2-NH2 nanoparticles followed by coupling with mesogenic 4-octyl-4-biphenylcarboxylic acid through the amide bond linkage. The resulting nanoparticles M-SiO2 were characterized by proton nuclear magnetic resonance (1H NMR), thermogravimetric analysis (TGA), transmission electron microscopy (TEM), energy dispersive X-ray spectrum (EDX) and UV-Vis analysis. The M-SiO2 nanoparticles were able to well disperse in organic solvents such as THF and CHCl3 even after being stored for several weeks at room temperature. 1H NMR spectrum of M-SiO2 nanoparticles displayed the presence of aromatic signals at lower fields, with a chemical shift around 7.5 ppm associated with the protons of the aromatic groups of the covalently attached mesogen (Figure S1), whereas these signals were not present in the spectrum of SiO2-NH2.9 From the TGA analysis (Figure S2), the SiO2-OH nanoparticles had a total loss of 1.78 wt%, which is associated to the presence of silanol groups on their surface. The TGA curve of the SiO2-NH2 presents one decomposition step at ∼410 °C related to the chemical attachment of APTES molecules onto the surface. The total weight loss was 6.65 wt%, indicating that ∼4.8 wt% of APTES was attached to the SiO2 nanoparticle surface. In contrast, the TGA plot of the M-SiO2 nanoparticles displays a two-step degradation curve, indicating progressive decomposition of surface groups, one due to the mesogens at ∼220 °C and the other due to the APTES groups at ∼410 °C; and the total loss was 9.74%.
Scheme 1.
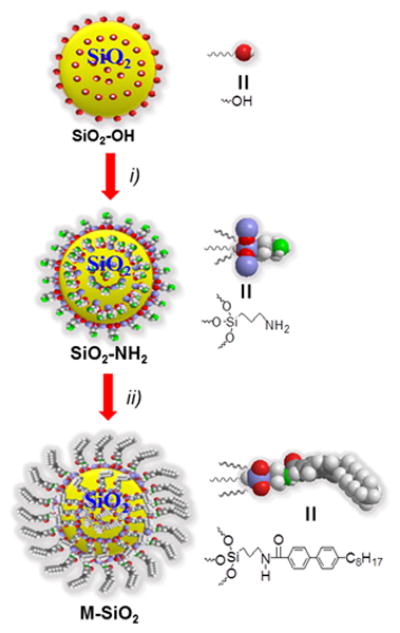
Synthesis of mesogen-grafted silica nanoparticles (M-SiO2). i) (3-amino propyl)-triethoxy silane, ethanol, and ii) 4-octyl-4-biphenylcarboxylic acid, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride, THF.
The M-SiO2 nanoparticles exhibited fluorescence at certain UV range, which could be used as a simple technique to monitor the presence of the SiO2 nanoparticles in solution, as demonstrated in Figure 1a. The SiO2-OH nanoparticles were water soluble whereas the M-SiO2 nanoparticles were soluble in organic solvents after surface modification. UV-induced fluorescence clearly demonstrates good dispersion of M-SiO2 nanoparticles in the organic solvent. Transmission electron microscope (TEM) images (Figure 1b) also showed apparent well-dispersion of M-SiO2 nanoparticles, demonstrating no noticeable aggregation in solution compared with the precursor SiO2-OH and SiO2-NH2 nanoparticles. The serious overlap of SiO2-OH nanoparticles was observed in the TEM grid due to the strong hydrogen bonding of the silanol groups. In contrast, when the mesogen was linked, no nanoparticle overlap was observed in the TEM image, which indicates the improvement of nanoparticle dispersion. The size of the M-SiO2 nanoparticles was 26.97±2.92 nm, calculated by TEM considering 500 nanoparticles. The size distribution is showed in Figure S4 (top). Additionally, the presence of the nitrogen, oxygen and silicon in the functionalized nanoparticles was observed from energy dispersive X-ray (EDX) spectrum (see the supporting information Figure S4 (bottom)).
Figure 1.
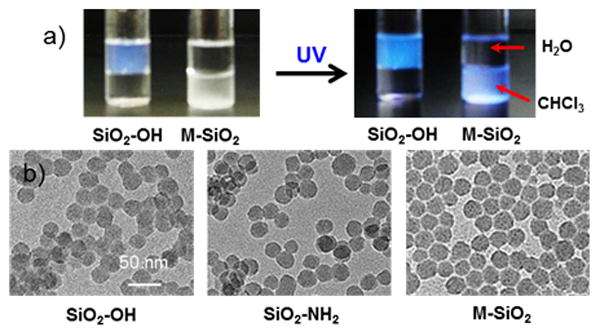
(a) Photographs of SiO2 nanoparticle solutions before (SiO2-OH) and after ligand exchange (M-SiO2) in a water-chloroform mixture with and without 365 nm UV irradiation, and (b) TEM images of SiO2-OH, SiO2-NH2 and M-SiO2 nanoparticles.
To explore the effect of M-SiO2 nanoparticles on the properties of DFCLC host, 0.5, 1.0, 1.5, 2.0 and 3.0 wt% M-SiO2 nanoparticles were respectively doped into the DFCLC with a pitch length of around 1.6 μm. The DFCLC used here is comprised commercially available chiral dopant S811 (6 wt%) and 94 wt% nematic DFLC (DP002-016, TN-I = 104 °C, Δn = 0.268). The M-SiO2 doped DFCLC nanocomposites were capillary-filled into the LC cells assembled from untreated glass substrates with 15 μm cell gap. Their liquid crystalline behaviors at room temperature were observed under a polarized optical microscopy (POM) with crossed polarizers in transmission mode. At the initial state, all cells displayed typical oily streak cholesteric textures (Figure 2). No evident aggregates were observed in the POM textures of the nanocomposites, suggesting that the M-SiO2 dispersed homogeneously in the DFCLC host even when the M-SiO2 concentration was increased up to 3.0 wt% (Figure 2f). It was found that the cholesteric (N*)-isotropic (I) and I-N* phase transition decreased with the increase of nanoparticle concentration as shown in Supporting Information Figure S5. One possible explanation of these observations is related to the decreased ordering in the DFCLC matrix after doping the M-SiO2 nanoparticles in the medium, as reported before for other nanocolloidal systems.10
Figure 2.
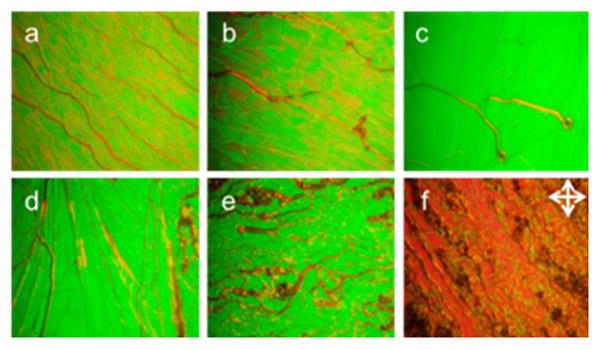
POM textures of DFCLC nanocomposites with different concentration of M-SiO2 nanoparticles at 0 wt% (a), 0.5 wt% (b), 1.0 wt% (c), 1.5 wt% (d), 2.0 wt% (e) and 3.0 wt% (f) at room temperature.
The electro-optical properties of M-SiO2/DFCLC nanocomposites were further investigated by applying an AC field at different frequencies and a constant voltage of 20 V. Figure 3 displays the POM textures of 2 wt% M-SiO2 doped DFCLC and pure DFCLC host with different frequencies at a constant voltage. As it can be observed, at the low frequency of 0.1 kHz, a homeotropic texture (Figure 3a and 3d) was formed, where the helix was unwound by a dielectric coupling between LC molecules and the vertical electric field. The inset shows the conoscopic interference pattern with a Maltese cross, confirming the existence of the molecular alignment perpendicular to the substrate, i.e., homeotropic (H) state. Upon increasing the frequency to 30 kHz, a focal conic (FC) texture was developed (Figure 3b and 3e), and the light was scattered due to the random orientation of the helical axes of the DFCLC domains. When the frequency was further increased to 70 kHz, the LC arrangement transformed into a planar state where the helical axis is perpendicular to the substrates of LC cell (Figure 3c and 3f). The transmittance and contrast ratio of DFCLC nanocomposites with different concentrations of M-SiO2 nanoparticles were evaluated through the transmission spectra detected by a fiber-optic spectrometer. The higher and lower transmission intensities were defined as I+ in the homeotropic state and I- when the light source was extinguished; the contrast ratio (CR) was defined as CR=T+/T- where T+ was assigned to the transmission intensity in the homeotropic state and T- was assigned to transmission of the FC or the planar state. As shown in Figure 4a, an improved contrast ratio for all M-SiO2 doped DFCLC nanocomposites compared with the pure DFCLC host was attained. Nevertheless, in the planar state (higher frequencies), the transmittance decreased with the increase of the M-SiO2 content (Figure 4b), which enabled a partially transparent state.
Figure 3.
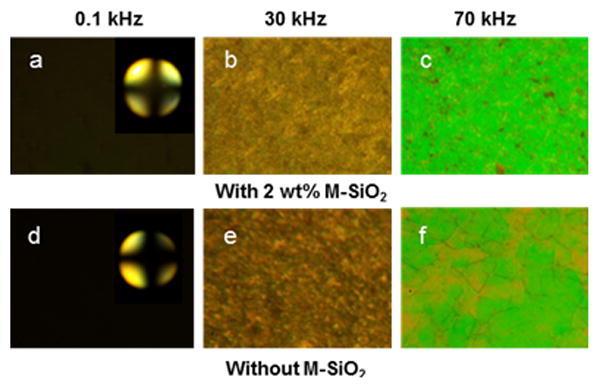
POM textures of DFCLC with 2 wt% M-SiO2 and without M-SiO2 at 0.1 kHz, 30 kHz and 70 kHz under an applied constant voltage of 20 V.
Figure 4.
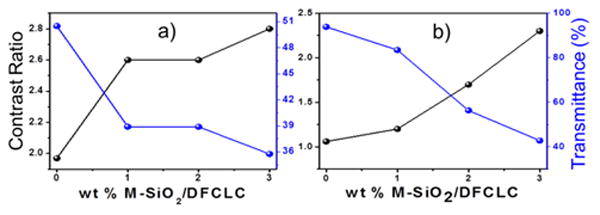
Contrast ratio (black) and transmittance (blue) curves of incident light (550 nm) with different concentrations of M-SiO2 nanoparticles in DFCLC at (a) focal conic state (30 kHz, 20V) and (b) planar state (70 kHz, 20V).
Figure 5 shows the photographs of a colorful image behind the two LC cells respectively containing 2 wt % M-SiO2 doped DFCLC (top) and the pure DFCLC host (bottom) in the H (transparent), FC (scattered) and planar states (partially transparent). The colorful image (yellow sphere, red helix and space-filling model of surfactants on black-doted blue background) behind both LC cells can be clearly observed at low frequency (Figure 5a and 5d, 0.1 kHz, H state), whereas when a higher frequency was applied (Figure 5b and 5e, 30 kHz, FC) the colorful image behind both cells was not visible due to light scattering of the FC domains. However, for the DFCLC thin film containing 2 wt% M-SiO2, the scattering is greatly enhanced and the colorful image behind the cell cannot be perceived due to the stronger scattering effect from the embedded nanoparticle system. When a higher frequency was applied, both the pure DFCLC and M-SiO2 doped DFCLC reconfigured into the planar state (Figure 5c and 5f, 70 kHz, planar state), however the M-SiO2 doped sample was partially transparent due to the influence of silica nanoparticles in the helical arrangement in the LC cell. It is noteworthy that both P and FC states were found to be stable for a long period of time even when the applied field was removed, since no noticeable change in the states, i.e., partially transparent and opaque, was observed (Figures S8 and S9).
Figure 5.
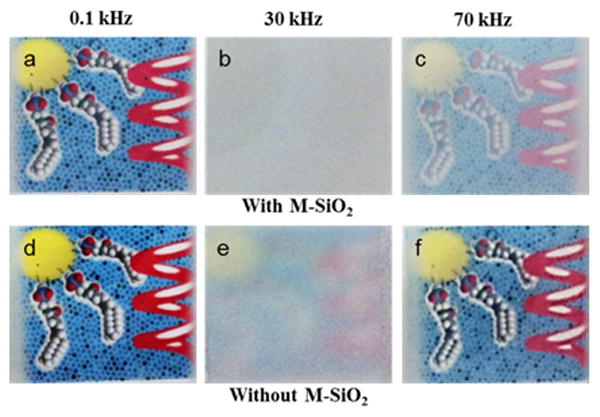
Photographs of a colorful image positioned behind the LC cells containing DFCLC with 2 wt% M-SiO2 (a, b, c) and without M-SiO2 (d, e, f) at 0.1 kHz, 30 kHz and 70 kHz respectively under an applied constant bias of 20 V.
To have a clear insight about the possible arrangement of LC molecules in the M-SiO2 doped DFCLC and the pure DFCLC, a schematic representation of the M-SiO2 doped DFCLC nanocomposite and pure DFCLC is illustrated in Figure 6. At lower frequencies, the helix is unwound and the molecules tend to align parallel to the vertical electric field due to the LC exhibiting a positive dielectric anisotropy (Δε>0) where the homeotropic state is preferred (Figure 6 top). An increase in the frequency induced the cholesteric organization but the helical axes are randomly oriented resulting in the FC state, i.e., scattered state (Figure 6 middle). When the frequency is further raised, the helical axes orient parallel to the electric field due to the LC exhibiting a negative dielectric anisotropy (Δε<0), assembling into the planar configuration (Figure 6 bottom). Doping M-SiO2 nanoparticles might result in the increase of defect density in the helix configuration and subsequently a stronger light scattering due to the refractive index mismatching among FC multi-domains; other possible explanation of the achievement of higher contrast ratio could be attributed to the dynamic turbulence of the nanoparticles in LC media when subjected to the fields at higher frequencies, where an enhance of scattering is produced by localized variations in the index of refraction at both FC and planar states.
Figure 6.
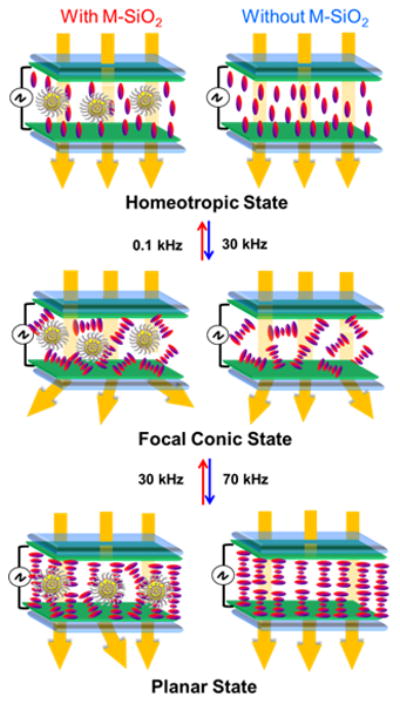
(a) Schematic illustration of M-SiO2 doped DFCLC nanocomposite (left) and pure DFCLC (right) in the LC cell at three different states driven by different frequencies but under constant voltage.
In summary, we have designed and functionalized silica nanoparticles with a covalently-attached mesogenic monolayer through a silane coupling agent. The resulting M-SiO2 nanoparticles were soluble in organic solvents and characterized by 1H NMR, TGA, UV-Vis, TEM, and EDX techniques. The organo-soluble M-SiO2 nanoparticles were doped in a dual frequency cholesteric LC medium with different concentrations (0.5 wt%∼3.0 wt%) exhibiting superior dispersion. From the electro-optical measurements, it was found that doping silica nanoparticles was able to greatly improve the contrast ratio of the nanocomposites compared with the pure DFCLC, which might result from the refractive index mismatching among FC multi-domains, and the dynamic turbulence of the nanoparticles in LC cell when subjected to the fields at higher frequencies. The optimal contrast ratio for both FC and planar states was achieved in 2 wt% M-SiO2 doped DFCLC nanocomposite. Importantly, three stable states, i.e., transparent, opaque, and partially transparent were accomplished by doping M-SiO2 nanoparticles into the DFCLC under different frequencies at low applied voltage, which indicates that is possible to make devices with tunable transparency with such functional materials. This work disclosed here to enhance scattering and reversibly control molecular orientation driven by frequency through hybrid nanoparticles offers an impetus in developing dynamic functional architectures and devices such as smart windows. Moreover, the synthetic route demonstrated here could provide an easy access to synthesize diverse surface-functionalized SiO2 nanoparticles with desired properties, which could improve the properties of other liquid crystalline systems through nanoparticle-LC synergetic interaction.
Supplementary Material
Acknowledgments
We thank the support from the AFOSR, AFRL, NIH (through grant R01EY020641) and the State of Ohio TVSF fund (through grant TECG201604), the CONACYT Scholarship 211982 to K.G. and China Scholarship Council to Z.Z. is gratefully acknowledged. The TEM data were obtained at the (cryo) TEM facility at the LCI supported by the Ohio Research Scholars Program Research Cluster on Surfaces in Advanced Materials.
Footnotes
Supporting Information: Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.a) Kitzerow HS, Bahr C, editors. Chirality in Liquid Crystals. Springer; New York, USA: 2001. [Google Scholar]; b Zheng Z, Li Y, Bisoyi HK, Wang L, Bunning TJ, Li Q. Nature. 2016;531:352–356. doi: 10.1038/nature17141. [DOI] [PubMed] [Google Scholar]; Wang L, Li Q. Adv Funct Mater. 2016;26:10–28. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bisoyi HK, Li Q. Angew Chem Int Ed. 2016;55:2994–3010. doi: 10.1002/anie.201505520. [DOI] [PubMed] [Google Scholar]
- 2.a) Xu M, Yang DK. Appl Phys Lett. 1997;70:720–722. [Google Scholar]; Hsiao YC, Tang CY, Lee W. Opt Express. 2011;19:9744–9749. doi: 10.1364/OE.19.009744. [DOI] [PubMed] [Google Scholar]; Kumar P, Kang SW, Lee SH. Opt Mater Express. 2012;2:1121–1134. [Google Scholar]; Ma J, Shi L, Yang DK. Appl Phys Express. 2010;3:021702. [Google Scholar]; e) Cheng KT, Lee PY, Qasim MM, Liu CK, Cheng WF, Wilkinson TD. ACS Appl Mater Interfaces. 2016;8:10483–10493. doi: 10.1021/acsami.5b12854. [DOI] [PubMed] [Google Scholar]
- 3.a) Bisoyi HK, Kumar S. Chem Soc Rev. 2011;40:306–319. doi: 10.1039/b901793n. [DOI] [PubMed] [Google Scholar]; Wang L, Gutierrez-Cuevas KG, Bisoyi HK, Xiang J, Gautam G, Zola RS, Kumar S, Lavrentovich OD, Urbas A, Li Q. Chem Commun. 2015;51:15039–15042. doi: 10.1039/c5cc06146f. [DOI] [PubMed] [Google Scholar]; Wang L, Gutierrez-Cuevas KG, Urbas A, Li Q. Adv Opt Mater. 2016;4:247–251. [Google Scholar]; Kumar R, Raina KK. Liq Cryst. 2015;42:119–126. [Google Scholar]
- 4.a) Kreuzer M, Tschudi T. Appl Phys Lett. 1993;62:1712–1714. [Google Scholar]; Jákli A, Almásy L, Borbély S, Rosta L. Eur Phys J B. 1999;10:509–513. [Google Scholar]; Diorio NJ, Jr, Fisch MR, West JW. Liq Cryst. 2002;29:589–596. [Google Scholar]
- 5.Payne JC, Thomas EL. Adv Funct Mater. 2007;17:2717–2721. [Google Scholar]
- 6.a) Li W, Zhu M, Ding X, Li B, Huang W, Cao H, Yang Z, Yang H. J Appl Polym Sci. 2009;111:1449–1453. [Google Scholar]; Kumar P, Raina KK. Opt Mater. 2012;34:1878–1884. [Google Scholar]; Hsu CJ, Kuo CC, Hsieh CD, Huang CY. Opt Express. 2014;22:18513–18518. doi: 10.1364/OE.22.018513. [DOI] [PubMed] [Google Scholar]
- 7.a) Rachet V, Lahlil K, Bérard M, Gacoin T, Boilot JP. J Am Chem Soc. 2007;129:9274–9275. doi: 10.1021/ja0735608. [DOI] [PubMed] [Google Scholar]; Glushchenko A, Kresse H, Reshetnyak V, Reznikov Y, Yaroshchuk O. Liq Cryst. 1997;23:241–246. [Google Scholar]; c) Busbee JD, Juhl AT, Natarajan LV, Tongdilia VP, Bunning TJ, Vaia RA, Braun PV. Adv Mater. 2009;21:3659–3662. [Google Scholar]
- 8.a) Xue C, Xiang J, Nemati H, Bisoyi HK, Gutierrez-Cuevas KG, Wang L, Gao M, Zhou S, Yang DK, Lavrentovich OD, Urbas A, Li Q. ChemPhysChem. 2015;16:1852–1856. doi: 10.1002/cphc.201500194. [DOI] [PubMed] [Google Scholar]; Lewandowski W, Wójcik M, Górecka E. ChemPhysChem. 2014;15:1283–1295. doi: 10.1002/cphc.201301194. [DOI] [PubMed] [Google Scholar]
- 9.a) Nakahara Y, Takeuchi T, Yokoyama S, Kimura K. Surf Interface Anal. 2011;43:809–815. [Google Scholar]; Heintz AS, Fink MJ, Mitchell BS. Appl Organometal Chem. 2010;24:236–240. [Google Scholar]
- 10.a) Gorkunov MV, Osipov MA. Soft Matter. 2011;7:4348–4356. [Google Scholar]; Gutierrez-Cuevas KG, Wang L, Xue C, Singh G, Kumar S, Urbas A, Li Q. Chem Commun. 2015;51:9845–9848. doi: 10.1039/c5cc02127h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


