Abstract
Background
Primaquine (PQ) is the only currently licensed antimalarial that prevents Plasmodium vivax (Pv) relapses. It also clears mature P. falciparum (Pf) gametocytes, thereby reducing post-treatment transmission. Randomized PQ treatment in a treatment-to-reinfection cohort in Papua New Guinean children permitted the study of Pv and Pf gametocyte carriage after radical cure and to investigate the contribution of Pv relapses.
Methods
Children received radical cure with Chloroquine, Artemether-Lumefantrine plus either PQ or placebo. Blood samples were subsequently collected in 2-to 4-weekly intervals over 8 months. Gametocytes were detected by quantitative reverse transcription-PCR targeting pvs25 and pfs25.
Results
PQ treatment reduced the incidence of Pv gametocytes by 73%, which was comparable to the effect of PQ on incidence of blood-stage infections. 92% of Pv and 79% of Pf gametocyte-positive infections were asymptomatic. Pv and to a lesser extent Pf gametocyte positivity and density were associated with high blood-stage parasite densities. Multivariate analysis revealed that the odds of gametocytes were significantly reduced in mixed-species infections compared to single-species infections for both species (ORPv = 0.39 [95% CI 0.25–0.62], ORPf = 0.33 [95% CI 0.18–0.60], p<0.001). No difference between the PQ and placebo treatment arms was observed in density of Pv gametocytes or in the proportion of Pv infections that carried gametocytes. First infections after blood-stage and placebo treatment, likely caused by a relapsing hypnozoite, were equally likely to carry gametocytes than first infections after PQ treatment, likely caused by an infective mosquito bite.
Conclusion
Pv relapses and new infections are associated with similar levels of gametocytaemia. Relapses thus contribute considerably to the Pv reservoir highlighting the importance of effective anti-hypnozoite treatment for efficient control of Pv.
Trial registration
ClinicalTrials.gov NCT02143934
Author summary
Plasmodium vivax (Pv) mainly affects Asia, Central and South America as well as Ethiopia. In Papua New Guinea (PNG) Pv prevalence is among the highest worldwide. The biggest challenge for the control of Pv infections is the formation of dormant liver stages, which have the ability to relapse and cause disease even after successful clearance of asexual stages in the blood circulation. Primaquine is the only licensed drug that is able to prevent Pv relapses. A randomized treatment-to-reinfection cohort in Papua New Guinean children permitted permitted the study of Pv and P. falciparum gametocyte carriage after radical cure with Primaquine and to investigate the contribution of Pv relapses to transmission. We found that most gametocyte carriers in this study were detected in asymptomatic infections and that relapses and new infections are associated with similar Pv gametocyte production. These are strong arguments emphasizing the importance of sensitive detection and early treatment of asymptomatic and submicroscopic Plasmodium spp. infections and of anti-hypnozoite treatment for an effective control of Pv.
Introduction
Primaquine (PQ) is the only currently licensed drug for preventing Plasmodium vivax (Pv) relapses [1], and also the only effective drug against mature gametocytes of P. falciparum (Pf) [2,3]. Since 2012, the World Health Organization recommends a single dose of PQ for treatment of Pf infections with the aim to reduce post-treatment Pf gametocyte carriage and thus the potential for onward malaria transmission [4].
Gametocyte development as well as morphology differs considerably between Pv and Pf [5]. Pv gametocytes mature rapidly and are detectable in the peripheral blood as early as two or three days following detection of blood-stage parasites by qPCR or light microscopy (LM), respectively [6,7]. In contrast, Pf gametocytes sequester for 7–10 days in the bone marrow before being released into the blood circulation [8], where they are observed by LM 10–15 days after the first detection of asexual parasites [9]. Gametocytes were observed in symptomatic Pv episodes at higher frequency compared to Pf episodes, despite 10-fold lower Pv blood-stage densities compared to Pf [10]. After drug treatment, Pv gametocytes are cleared within days after clearance of blood-stage infections in contrast to Pf gametocytes, which circulate over 3 weeks following successful blood-stage clearance [6,9,11,12]. Altogether the published data suggests that Pv infections produce proportionally higher gametocyte densities than Pf infections (at the same levels of asexual parasitaemia), and that Pv gametocytes mature more rapidly [9,12–14].
Not much is known about gametocyte production in primary Pv infections versus relapses from activated hypnozoites, mainly because in endemic settings it is impossible to distinguish both sources of infection. Our previous work in Papua New Guinea (PNG) showed that relapsing Pv infections contributed 73% of the gametocyte carriage [15]. A study in Thailand and Indonesia reported that densities by LM of Pv blood-stage parasites and gametocytes were similar in new infections and relapses [16]. Both studies indicated the need for efficient treatment of the hypnozoite reservoir for reducing Pv transmission [15,16].
A challenge in studying the investment of Pv infections in gametocytogenesis is the generally low and often submicroscopic density of asexual parasites and gametocytes. In addition, scarce Pv gametocytes can easily be misclassified by LM due to their resemblance to late trophozoites [17]. Investigating gametocyte production of Pv infections hence requires sensitive and specific molecular methods. For Pf, studying gametocytes by LM is more feasible because of the distinct crescent-shaped morphology of gametocytes and generally higher parasite densities; however also for Pf, molecular methods are crucial for studying gametocytes in low-density Pf infections. Molecular detection of gametocytes usually targets transcripts of the Pf or Pv 25 kDa ookinete surface antigen precursor (pfs25 or pvs25, respectively) [18,19], which are highly expressed in mature gametocytes [20,21]. Expression of the pfs25 transcripts is mainly female specific, hence male gametocytes are detected to a much lower extend by pfs25-based assays [22]. Female gametocytes are generally over-represented in peripheral blood samples with about 3.5 female per each male gametocyte [23,24]. It can therefore be estimated that pfs25 RT-qPCR assays detect approx. 70% of the total number of gametocytes. Both pfs25 and pvs25 quantitative reverse transcription PCR (qRT-PCR) or nucleic acid sequence-based amplification (NASBA) can detect as few as 1 Pf gametocyte or 10 Pv gametocytes per 50 μl blood and are therefore up to 50x more sensitive than LM [18,19,25].
This study investigated gametocyte dynamics of Pf and Pv infections in school-aged PNG children after randomized treatment with blood-stage antimalarials plus PQ or placebo. This trial design permitted an evaluation of the contribution of hypnozoites to Pv infection parameters by comparison of two treatment arms. PQ treatment for clearance of hypnozoites reduced the risk of recurrent Pv blood-stage infection by 82% [95% CI 0.75–0.86], the risk of a Pv episode by 75% [95% CI 0.49–0.89], and the incidence of Pv gametocytes by 73% [95% CI 0.62, 0.81][15]. Another study conducted in Thailand and Indonesia revealed that density of gametocytes over time followed that of asexual parasites [16]. However, the Thai-Indonesian study depended on the presentation of patients at a health facility upon occurrence of a clinical episode and did not use molecular methods to detect submicroscopic asexual parasites or gametocytes [16]. Our initial report on the PNG cohort study is now extended to address the following questions: (i) what are risk factors for Pf and Pv gametocyte carriage? (ii) does gametocytaemia differ between Pv new infections and relapses? And (iii) does PQ treatment exert a long-term effect on Pf and Pv gametocytaemia?
Methods
Study design
The study was conducted in 2009 to 2010 in the Albinama area, East Sepik province, in PNG. A detailed study protocol has been published previously [15]. In brief, 504 children aged 5 to 10 years were randomized to two treatment arms and completed directly observed treatment (DOT) with a 3-day dose of Chloroquine (CQ), a 3-day dose of Artemether-Lumefantrine (AL) and either 20 doses of PQ (per day: 0.5 mg/kg) or placebo over four weeks. Children were screened for G6PD deficiency by using a visual colorimetric method (G6PD Assay Kit WST-Dojindo Co., Japan). Venous blood samples were collected at enrolment (prior to treatment) and 3 days after the final dose of DOT. The latter date represented day 0 of follow-up. Finger-prick samples were taken every two weeks for the first 3 months and monthly for the remaining 5 months of follow-up. Symptomatic children detected during follow-up were treated with a 3-day course of AL after confirming Plasmodium infection by rapid diagnostic test (RDT, CareStart Malaria pLDH/HRP2 Combo, AccessBio, USA).
Ethics statement
The study received ethical clearance by the PNG Institute of Medical Research (IMR) Institutional Review Board (0908), the PNG Medical Advisory Committee (09.11), the Ethikkommission beider Basel (237/11) and was registered on ClinicalTrials.gov NCT02143934. A parent or guardian of every child participant provided written informed consent for their participation.
Detection of blood-stage parasites and gametocytes
All blood samples collected were examined by LM and quantitative PCR (qPCR). Blood slides were examined by at least two independent microscopists and declared parasite negative only after examination of 200 thick-film fields [15]. Parasite DNA was extracted from 100–150 μl blood cell pellet using the FavorPrep 96-well genomic DNA extraction kit (Favorgen, Taiwan) and analyzed for Pf and Pv positivity by 18S rRNA qPCR [15,19]. All Pv and Pf qPCR positive samples were genotyped using markers Pv-msp1F3 and Pf-msp2, respectively, following previously published protocols [26,27].
RNA was extracted from all samples positive in Pf or Pv qPCR. RNA was extracted using the RNEasy 96 kit (Qiagen, Switzerland) as described previously [19] from 50μl whole blood spotted on filter papers that had been air-dried and stored in TRIzol reagent (Life Technologies, Switzerland). Gametocyte-specific transcripts were detected by pfs25 or pvs25 qRT-PCR [19] in all RNA samples for which the corresponding DNA sample had been positive by species-specific qPCR.
Statistical analysis
Children were censored on the last visit before two consecutively missed scheduled follow-up visits [15]. Comparison of LM-positive versus submicroscopic infections, and symptomatic versus asymptomatic infections was performed with 5019 samples from the follow-up period for which LM data was available. A clinical malaria episode was defined as fever (axillary temperature >37.5°C and/or fever reported in previous 2 days) and the presence of Plasmodium spp. parasites by LM. Differences in proportions were tested for statistical significance using the McNemar X2 test with continuity correction. To achieve normal distribution, qPCR densities were expressed as log10-transformed 18S rRNA genomic copies/μl blood for asexual parasites, and log10-transformed pfs25 or pvs25 transcripts/μl blood for gametocytes. Correlation between microscopic parasite counts and molecular methods was tested by Kendall’s rank sum test on log10 transformed data. Geometric means of densities were calculated. Differences in densities of asexual or sexual-stage parasites were tested for statistical significance using Welch’s Two-sample t-test.
Negative binomial regression models were used to calculate the incidence rate of Pv and Pf gametocyte positivity as previously described [15]. Gametocyte positivity during follow-up was modeled using binomial generalized estimating equations (GEE) with logit link using an exchangeable correlation matrix to account for repeated measures by child. Log10-transformed blood-stage parasite density and gametocyte density during follow-up were modeled using Gaussian GEEs with log link using an exchangeable correlation matrix. Linear fit for log10-transformed blood-stage parasite density was previously analyzed and considered adequate for both species (S1 Text). All Models were back-selected. Statistical analyses were conducted using R version 3.1.1 [28] or STATA version 14.
Results
Gametocyte positivity and density in submicroscopic infections
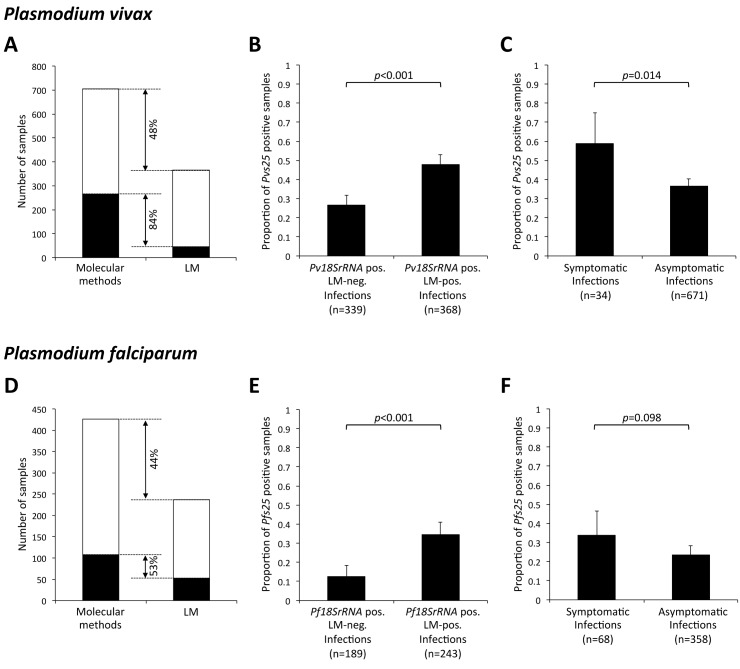
Molecular methods were superior to LM especially for detection of gametocytes but also for blood-stage parasites (Fig 1). By LM Pv gametocytes were detected in only 44 out of 366 Pv positive samples (12%), whereas by molecular detection 265 out of 705 Pv samples (38%, p<0.001) were gametocyte-positive. Pf gametocyte rates by LM were 21% (52/237) and by qRT-PCR 25% (107/426). 84% [CI95: 79–88%] and 53% [CI95: 43–63%] gametocytaemia was submicroscopic, for Pv and Pf respectively (Fig 1A and 1D). In one Pv and two Pf samples gametocytes were detected by LM but not by qRT-PCR, indicating most probably RNA degradation. For Pv infections, a late-stage trophozoite can be misread as a gametocyte, however LM slides of this study were read by three independent microscopists. Overall, gametocyte densities by LM and by molecular methods were significantly correlated in samples positive by both methods (Kendall’s tau test, pvs25: tau = 0.24, p-value = 0.037, pfs25: tau = 0.23, p-value = 0.027). Due to the low sensitivity of LM in gametocyte detection, all further results presented here derive from molecular gametocyte detection.
Fig 1. Pv (top) and Pf (bottom) gametocyte positivity among 5019 follow-up samples.
(A, D) Detection of blood stage parasites and gametocytes by LM and molecular methods, using pv18S or pf18S rRNA qPCR for detection of blood-stage parasites and pvs25 or pfs25 qRT-PCR for detection of gametocytes. Black: gametocyte positive samples. White: parasite positive samples without gametocytes. (B, E) Proportion of gametocytes positives (by molecular methods) in submicroscopic and LM-positive samples. (C, F) Proportion of gametocyte positives (by molecular methods) in symptomatic and asymptomatic infections. Error bars indicate 95% confidence intervals by X2 distribution.
Significantly more Pv infections carried qRT-PCR detectable gametocytes compared to Pf (38% vs. 25%, p<0.001). Microscopically patent infections of both species carried gametocytes more often than submicroscopic infections (Pv: 48% vs. 27%, Pf: 35% vs. 13%, p<0.001, Fig 1B and 1E). Similarly, gametocyte-specific transcript numbers were significantly higher in LM-positive than LM-negative samples for both species (S1 Fig).
Gametocyte positivity and density in symptomatic versus asymptomatic infections
During the follow-up period, 34 Pv episodes and 68 Pf clinical episodes were observed. The proportion of gametocyte carriers was 22% higher in clinical episodes compared to asymptomatic P. vivax infections (59% vs. 37%, p = 0.014, Fig 1C). For P. falciparum, a similar trend was observed but did not reach statistical significance (34% vs. 23%, p = 0.098, Fig 1F). However, due to a much higher number of asymptomatic infections than clinical episodes, the overwhelming majority of Pv and Pf gametocyte carriage (92% [CI95: 88–95%] and 79% [CI95: 69–86%]) occurred in asymptomatic children. Pv gametocyte densities showed the same trend as asexual densities in both clinical episodes and asymptomatic infections, but this was not the case for Pf (S2 Fig).
The effect of PQ treatment on gametocytaemia during follow-up
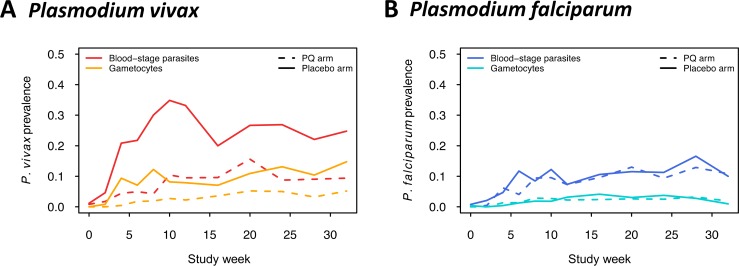
Pv gametocyte prevalence increased steadily throughout the follow-up period and was on average almost 3-fold higher in the placebo arm than in the PQ arm, similar to patterns observed in Pv blood-stage parasite prevalence (Pv gametocytes median fold difference PL>PQ: 2.9 [IQR: 2.0–3.8], Pv blood-stages median fold difference PL>PQ: 2.8 [IQR: 2.2–4.2], Fig 2A). No difference in Pf gametocyte prevalence was observed between study arms (Pf gametocytes median fold difference PL>PQ 1.0 [IQR: 0.7–1.4], Pf blood-stages median fold difference PL>PQ: 1.1 [IQR: 0.9–1.3], Fig 2B).
Fig 2.
Prevalence of blood-stage parasites and gametocytes of Pv (A) and Pf (B) during follow-up by treatment arm.
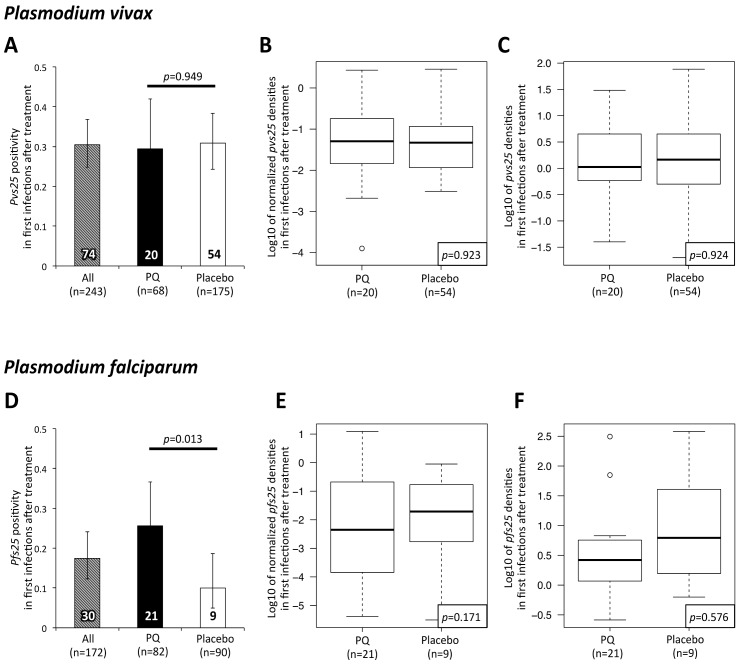
To assess in detail Pv gametocyte production in primary infections versus relapses, we compared gametocyte positivity and density in first infections after blood-stage plus placebo (first Pv infection either from relapse (80%80%) or infective bite (20%)) or blood-stage plus PQ treatment (first Pv infection always from infective bite) [15]. We also assessed subsequent Pv infections, i.e. all but the first parasite-positive sample per child, which in both arms can result from an ongoing infection, a relapsing hypnozoite or a new infection from a mosquito. First Pv re-infections after baseline treatment were equally likely to carry gametocytes in both treatment arms (PQ: 29% vs. Placebo: 31%, Fig 3A), and the same was observed for subsequent infections (PQ: 42% [CI95: 32–52%] vs. Placebo: 41% [CI95: 36–47%], p = 1). To investigate whether gametocyte densities were simply following the asexual densities or if other factors play a role, we compared absolute as well as normalized gametocyte densities. Gametocyte densities were normalized by dividing pvs25 or pfs25 transcript numbers/μl by Pv- or Pf-18S rRNA copy numbers/μl. Absolute and normalized Pv gametocyte densities did not differ between treatment arms in first infections (Fig 3B and 3C) nor in subsequent infections (S3 Fig).
Fig 3. Gametocyte positivity and density in first Pv (top) and Pf (bottom) infections after treatment with blood-stage antimalarials alone (placebo) or blood-stage antimalarials plus PQ (PQ).
(A, D). Proportion of Pv and Pf gametocyte carriers among first infections by treatment arm. Figures within the bars indicate absolute numbers of gametocyte-positive first infections following treatment. Error bars indicate 95% confidence intervals by X2 distribution. (B, E). Normalized Pv and Pf gametocyte densities in first infections by treatment arm. Densities were normalized by dividing pvs25 or pfs25 transcript numbers/μl by Pv- or Pf-18S rRNA copy numbers/μl. (C, F) Absolute Pv and Pf gametocyte densities in first infections by treatment arm. Densities are expressed as log10 of pvs25 and pfs25 transcripts/μl.
We also investigated Pf gametocyte carriage by comparing Pf gametocyte positivity and density in first Pf infections after treatment. Significantly more Pf gametocyte carriers were observed among first infections in the PQ-arm compared to the placebo arm (PQ: 26% vs. Placebo: 10%, Fig 3D). No significant difference between trial arms was observed in subsequent samples (PQ: 30% [CI95 22–39%] vs. Placebo: 31% [CI95 23–40%], p = 0.961). Pf absolute and normalized gametocyte densities did not differ significantly between the treatment arms (Fig 3E and 3F, S3 Fig).
Risk factors for gametocytes positivity and density
Pv gametocytes were detected more frequently (Table 1, OR for 1-log increase of density = 1.95, p<0.001) and in higher densities (Table 2, p<0.001) with increasing blood-stage parasite density. Apart from reducing the number of Pv positive samples during follow-up (S1 Table), PQ treatment had no further effect on Pv gametocyte positivity (Table 1).
Table 1. Multivariable predictors of Pv and Pf gametocyte positivity during follow-up.
| Pv gametocyte positive | Pf gametocyte positive | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Blood-stage density (by qPCR), per 10x increase | 1.95 | 1.47 | 2.58 | <0.001 | 1.23 | 0.99 | 1.51 | 0.059 |
| PQ treatment | 1.03 | 0.68 | 1.58 | 0.878 | 1.21 | 0.78 | 1.90 | 0.395 |
| Mixed Pf/Pv (by qPCR) | 0.39 | 0.25 | 0.62 | <0.001 | 0.33 | 0.18 | 0.60 | <0.001 |
| First infection | 0.64 | 0.42 | 0.98 | 0.040 | 0.45 | 0.25 | 0.81 | 0.007 |
| Days after DOT (ref:0–60) | ||||||||
| 61–120 | 0.59 | 0.39 | 0.89 | 1.31 | 0.62 | 2.76 | ||
| 121–180 | 1.48 | 0.91 | 2.42 | <0.001 | 1.32 | 0.63 | 2.75 | 0.266 |
| >180 | 2.54 | 1.47 | 4.39 | 0.73 | 0.32 | 1.67 | ||
| Constant | 0.36 | 0.22 | 0.59 | <0.001 | 0.29 | 0.11 | 0.77 | <0.013 |
OR, odds ratio
DOT, directly observed treatment.
ORs were obtained using binomial generalized estimating equations with logit-link allowing for repeated visits by back-selection from the full model. The full model included fever, infection status at enrolment by qPCR (Pf or Pv positive), LLIN use (less than 100%), sex, village of residence, hemoglobin at baseline (>9 g/dl), age. No significant interaction of PQ treatment with days post DOT was detected.
Table 2. Multivariable predictors of Pv and Pf gametocyte density during follow-up.
| Pv gametocyte density | ||||
|---|---|---|---|---|
| exp(β) | 95% CI | p-value | ||
| Blood-stage density (by qPCR) per 10x increase | 1.37 | 1.20 | 1.56 | <0.001 |
| PQ treatment | 0.97 | 0.80 | 1.18 | 0.765 |
| Mixed Pf/Pv (by qPCR) | 0.73 | 0.59 | 0.90 | 0.003 |
| Age | 0.94 | 0.89 | 0.99 | 0.017 |
| Days after DOT (ref: 0–60) | ||||
| 61–120 | 1.08 | 0.86 | 1.35 | |
| 121–180 | 1.15 | 0.90 | 1.47 | 0.163 |
| >180 | 1.30 | 1.03 | 1.64 | |
| Constant | 1.11 | 0.69 | 1.81 | 0.661 |
β, regression coefficient.
Coefficients were obtained using Gaussian generalized estimating equations with log-link by allowing for repeated visits and by back-selection from the full model. The full model included fever, infection status at enrolment by qPCR (Pf or Pv positive), LLIN use (less than 100%), sex, village of residence, hemoglobin at baseline (>9 g/dl), first infection. No predictors were associated with Pf gametocyte densities (S1 Text). No significant interaction of PQ treatment with days post DOT was detected.
In Pv positive samples, the odds of Pv gametocytes were 60% reduced and gametocyte densities were 30% lower in mixed Pf/Pv infections compared to single-species Pv infections (Table 1, p<0.001; Table 2 p = 0.003). The odds for Pv gametocyte carriage increased significantly over the whole follow-up period (Table 1 and Fig 2, p<0.001), and a 36% reduction on the odds of being gametocyte positive was observed in first Pv infections compared to subsequent infections (Table 1, p = 0.040). No other factors were associated with the odds for Pv gametocyte carriage during follow-up (Table 1). Pv gametocyte density, but not positivity, decreased with age (Table 2, p = 0.017) following the age trend in asexual parasites (Table 3, [29]).
Table 3. Multivariate predictors Pv and Pf blood-stage parasite density during follow-up.
| Pv blood-stage density | Pf blood-stage density | |||||||
|---|---|---|---|---|---|---|---|---|
| exp(β) | 95% CI | p-value | exp(β) | 95% CI | p-value | |||
| PQ treatment | 1.04 | 0.93 | 1.15 | 0.505 | 0.85 | 0.68 | 1.06 | 0.143 |
| Mixed Pf/Pv (by qPCR) | - | - | - | - | 0.79 | 0.63 | 1.00 | 0.048 |
| Fever | - | - | - | - | 2.41 | 1.82 | 3.19 | <0.001 |
| Age | 0.96 | 0.92 | 0.99 | 0.014 | - | - | - | - |
| Days after DOT (ref: 0–60) | ||||||||
| 61–120 | 0.94 | 0.83 | 1.07 | 0.93 | 0.69 | 1.25 | ||
| 121–180 | 0.72 | 0.62 | 0.83 | <0.001 | 0.89 | 0.62 | 1.25 | <0.001 |
| >180 | 0.51 | 0.44 | 0.59 | 0.59 | 0.43 | 0.80 | ||
| Constant | 5.13 | 3.83 | 6.87 | <0.001 | 16.37 | 12.37 | 21.66 | <0.001 |
β, regression coefficient.
Coefficients were obtained using Gaussian generalized estimating equations with log-link by allowing for repeated visits and by back-selection from the full model. The full model included fever, infection status at enrolment by qPCR (f. or v. positive), LLIN use (less than 100%), sex, village of residence, hemoglobin at baseline (>9 g/dl), first infection. Non-associated predictors were shown by “-”in the respective line. No significant interaction of PQ treatment with days post DOT was detected.
As for Pv gametocytes, the odds for Pf gametocytes were 70% reduced in mixed Pf/Pv infections compared to single-species Pf infections (Table 1, p<0.001). As an effect of delayed Pf gametocyte maturation, gametocyte positivity was 55% lower in first Pf infections compared to subsequent infections (Table 1, p = 0.007). Other risk factors for Pf gametocytes were investigated, but none of the parameters tested was significant. The Pf gametocyte positivity was slightly higher in samples with high asexual densities, yet this association did not reach the 5% significance level (Table 1, OR for 1-log increase of density = 1.23, p = 0.059). In contrast to Pv, Pf gametocyte densities were not associated with any of the factors assessed (S1 Text). Fever was strongly associated with increasing blood-stage Pf parasitaemia (Table 3, OR = 2.41, p<0.001), but had no effect on gametocyte density.
Analysis of only subsequent infections showed similar results to the analysis of the entire follow-up period (S2 Table). Considering subsequent infections only, Pv gametocytes were reduced by 47% [13–68%] and Pf gametocytes were reduced by 63% [26–82%] in mixed-species infections compared to single-species infections (Pv p-value: 0.013, Pf p-value: 0.006, S2 Table). It was not possible to analyse the effect of mixed-species co-infection on gametocyte carriage in first positive samples following treatment due to very low sample size for either species.
Discussion
This study represents a first detailed investigation of the contribution of Pv relapses to the infectious reservoir. The transmission potential attributable to relapses was estimated by comparing gametocyte positivity and density in children that had received either PQ or placebo treatment. A major finding was that Pv gametocytes were detected in equal proportions and equal density in Pv positive samples of both trial arms. In the PQ arm, the majority of Pv infections derived from new mosquito bites, while in the placebo arm 80% of infections were caused by relapsing hypnozoites [15]. Gametocyte densities as well as the proportion of gametocyte carriers concurred in both arms, thus indicating that new and relapsing infections produce gametocytes at equal rates. Similar conclusions were drawn from a study in south-east Asia, where Pv gametocyte densities and positivity had closely mirrored parasitaemia in both, clinical primary and recurrent infections [16]. Gametocyte production in relapses thus seems indistinguishable from that in new infections. This finding highlights the importance of anti-hypnozoite drugs to prevent relapses for an effective interruption of Pv transmission.
Sample storage in this cohort was not optimal for RNA preservation. Blood was spotted onto Whatman 3MM filter paper in the field, and stored at room temperature for up to 5 weeks until transferred into TRIzol reagent. This procedure was suboptimal compared to sampling in RNA-stabilizing reagents [19]. A more recent cross-sectional study in PNG employed sampling in RNAprotect Cell Reagent (Qiagen, Switzerland) and found gametocytes in 78% and 60% of Pf and Pv qPCR-positive samples in children aged 6–9 years [25]. Almost universal Pv gametocyte prevalence (95%) was found in Brazilian samples stored in liquid nitrogen [30]. The relatively low gametocyte positivity in this cohort was indicative of poor RNA quality, which likely resulted in a substantial underestimation of gametocyte rates. The gametocyte rate in the present study thus reflects a minimum prevalence. Because RNA quality and sample volume did not vary within the study, the comparative analyses of treatment arms and risk factors remain unaffected, even if these results need to be regarded as referring to infections with moderately high gametocyte densities.
The vast majority (>80%) of gametocyte carriers were asymptomatic for both species, and over 20% of Pv and over 30% of Pf gametocyte positive samples were submicroscopic. Although gametocyte densities were lower in submicroscopic infections compared to LM-positive infections for both species, they may nonetheless be potentially infective to mosquitoes. Mosquito feeding experiments have demonstrated that submicroscopic infections can infect mosquitoes, albeit at lower rates than microscopically patent infections, and thus contribute to onward transmission [31–35]. Our results highlight the importance of treating all malaria infections in the community, as asymptomatic individuals will not report themselves to health facilities and thus generally remain untreated and infectious for longer periods.
Co-infections with both species are common in PNG [36,37] including in this cohort, and interactions between co-infecting species in mixed infections have been investigated previously. However, these former studies focused on the asexual stages of Pv and Pf [38,39] or risk for clinical diseases [29,38,40] and did not address transmission stages. Gametocytes in the host are influenced by a complex interplay of parasite factors (such as stress response) and host factors (such as immunity), and this complexity is enhanced by a second co-infecting Plasmodium species. Our finding of significantly reduced gametocytes in mixed-species infections compared to single-species infections is a first indication of species interaction affecting the transmission stages. Confirmation of our results is required in other studies investigating Plasmodium species interactions with specific focus on the transmission stages.
In the first post-treatment Pf infections gametocytes were more frequently detected in the PQ arm than in the placebo arm. This is likely explained by the slower acquisition of new Pv infections in PQ-treated individuals, compared to a fast relapse rate in individuals retaining hypnozoites in the liver. Indeed, in the placebo arm 52% of first Pf infections carried a Pv co-infection as opposed to only 21% in the PQ arm. Accordingly, the multivariate analysis showed reduced odds of Pf as well as Pv gametocytes in mixed-species infections compared to single-species infections. In addition, a Pf co-infection reduced Pv gametocyte densities by half. A study in 0.5 to 5 year old PNG children with uncomplicated malaria confirmed that Pv gametocytaemia in Pf/Pv mixed infections was reduced compared to Pv single infections [41]. Moreover, a community study in PNG showed a lower proportion of Pf gametocyte carriers in Pf/Pv mixed infections compared to Pf single-species infections [25]. Similar findings had been reported from Thailand [42]. More longitudinal studies designed specifically to address gametocyte dynamics in mono- and mixed-species infections are needed to confirm potential cross-species interaction and its effect on sexual stage development.
Conclusion
Onset and rate of Pv gametocyte production did not differ between relapses and primary infections. This is a strong argument for treatment policies and elimination strategies that support PQ treatment of all Pv infections. The vast majority of gametocyte carriers in this study were detected in asymptomatic infections, which suggests that sensitive detection and early treatment of asymptomatic and submicroscopic Plasmodium spp. infections may be crucial for an effective control of transmission. PQ treatment prevented relapses and thus reduced Pv gametocyte carriage by 73%. These and other Plasmodium species interactions that can substantially affect gametocyte production warrant further investigation.
Supporting information
(DOCX)
A. and C. Pv and Pf gametocyte densities were expressed as log10 of pvs25 and pfs25 transcripts/μl. B. and D. Pv and Pf parasite densities were expressed as log10 of pv18S rRNA and pf18S rRNA gene copies/μl. C. and E. Pv and Pf normalized gametocyte densities. Densities were normalized by division of pvs25 and pfs25 transcripts/μl by pv18S rRNA or Pf18S rRNA genomic copies/μl, respectively.
(DOCX)
A. and C. Pv and Pf gametocyte densities were expressed as log10 of pvs25 and pfs25 transcripts/μl. B. and D. Pv and Pf parasite densities were expressed as log10 of pv18S rRNA and pf18S rRNA gene copies/μl. C. and E. Pv and Pf normalized gametocyte densities. Densities were normalized by division of pvs25 and pfs25 transcripts/μl by pv18S rRNA or Pf18S rRNA genomic copies/μl, respectively.
(DOCX)
A. and C. Proportion of P. vivax and P. falciparum gametocyte carriers among subsequent infections by treatment arm. Figures within the bars indicate absolute numbers of gametocyte-positive subsequent infections following treatment. Error bars indicate 95% confidence intervals by X2 distribution. B. and E. Normalized P. vivax and P. falciparum gametocyte densities in subsequent infections by treatment arm. Normalization was done by dividing pvs25 or pfs25 transcript numbers/μl by Pv- or Pf-18S rRNA copy numbers/μl. C. and F. Absolute P. vivax and P. falciparum gametocyte densities in subsequent infections by treatment arm. Densities are expressed as log10 of pvs25 and pfs25 transcripts/μl.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We gratefully acknowledge the study participants and their parents or guardians, and the field team in PNG. We specially thank Anna Rosanas-Urgell and Alice Ura from PNGIMR for preserving RNA of samples.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the International Centers of Excellence in Malaria Research (Award Number: U19 AI089686-03). IF was supported by the Swiss National Science Foundation (Award Numbers: 310030_134889 and 310030_159580). SK was supported by the National Health and Medical Research Council (NHMRC, Award Number: 1052760). LJR was supported by the NHMRC (Award Number: 1016443). IM was supported by the NHMRC (Award Numbers: 1043345 and 1021544). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11: 280 doi: 10.1186/1475-2875-11-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis. 2014;14: 130–139. doi: 10.1016/S1473-3099(13)70268-8 [DOI] [PubMed] [Google Scholar]
- 3.Shekalaghe SA, Drakeley C, Gosling R, Ndaro A, van Meegeren M, Enevold A, et al. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PloS One. 2007;2: e1023 doi: 10.1371/journal.pone.0001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of September 2012 meeting. Malar J. 2012;11: 424 doi: 10.1186/1475-2875-11-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24: 377–410. doi: 10.1128/CMR.00051-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis. 2013;208: 1688–1694. doi: 10.1093/infdis/jit394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenzie FE, Jeffery GM, Collins WE. Gametocytemia and fever in human malaria infections. J Parasitol. 2007;93: 627–633. doi: 10.1645/GE-1052R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cisteró P, et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood. 2014;123: 959–966. doi: 10.1182/blood-2013-08-520767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malariatherapy data. Trans R Soc Trop Med Hyg. 2001;95: 497–501. [DOI] [PubMed] [Google Scholar]
- 10.Mckenzie FE, Wongsrichanalai C, Magill AJ, Forney JR, Permpanich B, Lucas C, et al. Gametocytemia in Plasmodium vivax and Plasmodium falciparum infections. J Parasitol. 2006;92: 1281–1285. doi: 10.1645/GE-911R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9: 136 doi: 10.1186/1475-2875-9-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pukrittayakamee S, Imwong M, Singhasivanon P, Stepniewska K, Day NJ, White NJ. Effects of Different Antimalarial Drugs on Gametocyte Carriage in P. Vivax Malaria. Am J Trop Med Hyg. 2008;79: 378–384. [PubMed] [Google Scholar]
- 13.Nacher M, Silachamroon U, Singhasivanon P, Wilairatana P, Phumratanaprapin W, Fontanet A, et al. Risk factors for Plasmodium vivax gametocyte carriage in Thailand. Am J Trop Med Hyg. 2004;71: 693–695. [PubMed] [Google Scholar]
- 14.Taylor LH, Read AF. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol Today Pers Ed. 1997;13: 135–140. [DOI] [PubMed] [Google Scholar]
- 15.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CSN, et al. Strategies for Understanding and Reducing the Plasmodium vivax and Plasmodium ovale Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model. PLoS Med. 2015;12: e1001891 doi: 10.1371/journal.pmed.1001891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, et al. Gametocyte dynamics and the role of drugs in reducing the transmission potential of Plasmodium vivax. J Infect Dis. 2013;208: 801–812. doi: 10.1093/infdis/jit261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Basic Malaria Microscopy—Part I. Learner’s guide. 2010.
- 18.Schneider P, Schoone G, Schallig H, Verhage D, Telgt D, Eling W, et al. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol. 2004;137: 35–41. doi: 10.1016/j.molbiopara.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 19.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for detection of Plasmodium species gametocytes. PloS One. 2013;8: e76316 doi: 10.1371/journal.pone.0076316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, et al. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci U S A. 2008;105: 16290–16295. doi: 10.1073/pnas.0807404105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, et al. The Plasmodium falciparum sexual development transcriptome: A microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143: 67–79. doi: 10.1016/j.molbiopara.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Lasonder E, Rijpma SR, van Schaijk BCL, Hoeijmakers WAM, Kensche PR, Gresnigt MS, et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016;44: 6087–6101. doi: 10.1093/nar/gkw536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert V, Read AF, Essong J, Tchuinkam T, Mulder B, Verhave JP, et al. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans R Soc Trop Med Hyg. 1996;90: 621–624. [DOI] [PubMed] [Google Scholar]
- 24.Sowunmi A, Gbotosho GO, Happi CT, Folarin OA, Balogun ST. Population structure of Plasmodium falciparum gametocyte sex ratios in malarious children in an endemic area. Parasitol Int. 2009;58: 438–443. doi: 10.1016/j.parint.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, et al. Blood-Stage Parasitaemia and Age Determine Plasmodium falciparum and P. vivax Gametocytaemia in Papua New Guinea. PloS One. 2015;10: e0126747 doi: 10.1371/journal.pone.0126747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koepfli C, Ross A, Kiniboro B, Smith TA, Zimmerman PA, Siba P, et al. Multiplicity and diversity of Plasmodium vivax infections in a highly endemic region in Papua New Guinea. PLoS Negl Trop Dis. 2011;5: e1424 doi: 10.1371/journal.pntd.0001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoepflin S, Valsangiacomo F, Lin E, Kiniboro B, Mueller I, Felger I. Comparison of Plasmodium falciparum allelic frequency distribution in different endemic settings by high-resolution genotyping. Malar J. 2009;8: 250 doi: 10.1186/1475-2875-8-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: 2014; Available: http://www.R-project.org/. [Google Scholar]
- 29.Hofmann NE, Karl S, Wampfler R, Betuela I, Felger I, Mueller I, et al. Heterogeneity in malaria transmission: defining the relationship between molecular force of infection and incidence of P. falciparum and P. vivax episodes. Submitt Parasite Epidemiol Control. submitted;
- 30.Lima NF, Bastos MS, Ferreira MU. Plasmodium vivax: reverse transcriptase real-time PCR for gametocyte detection and quantitation in clinical samples. Exp Parasitol. 2012;132: 348–354. doi: 10.1016/j.exppara.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves FP, Gil LHS, Marrelli MT, Ribolla PEM, Camargo EP, Da Silva LHP. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42: 777–779. [DOI] [PubMed] [Google Scholar]
- 32.Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PloS One. 2012;7: e42821 doi: 10.1371/journal.pone.0042821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife. 2013;2: e00626 doi: 10.7554/eLife.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouédraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PloS One. 2009;4: e8410 doi: 10.1371/journal.pone.0008410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallejo AF, García J, Amado-Garavito AB, Arévalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J. 2016;15: 48 doi: 10.1186/s12936-016-1104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, et al. Random distribution of mixed species malaria infections in Papua New Guinea. Am J Trop Med Hyg. 2000;62: 225–231. [DOI] [PubMed] [Google Scholar]
- 37.Mueller I, Widmer S, Michel D, Maraga S, McNamara DT, Kiniboro B, et al. High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar J. 2009;8: 41 doi: 10.1186/1475-2875-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce MC, Donnelly CA, Alpers MP, Galinski MR, Barnwell JW, Walliker D, et al. Cross-species interactions between malaria parasites in humans. Science. 2000;287: 845–848. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman PA, Mehlotra RK, Kasehagen LJ, Kazura JW. Why do we need to know more about mixed Plasmodium species infections in humans? Trends Parasitol. 2004;20: 440–447. doi: 10.1016/j.pt.2004.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith T, Genton B, Baea K, Gibson N, Narara A, Alpers MP. Prospective risk of morbidity in relation to malaria infection in an area of high endemicity of multiple species of Plasmodium. Am J Trop Med Hyg. 2001;64: 262–267. [DOI] [PubMed] [Google Scholar]
- 41.Karl S, Laman M, Moore BR, Benjamin JM, Salib M, Lorry L, et al. Risk factors for Plasmodium falciparum and Plasmodium vivax gametocyte carriage in Papua New Guinean children with uncomplicated malaria. Acta Trop. 2016;160: 1–8. doi: 10.1016/j.actatropica.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 42.Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, ter Kuile F, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60: 1019–1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
A. and C. Pv and Pf gametocyte densities were expressed as log10 of pvs25 and pfs25 transcripts/μl. B. and D. Pv and Pf parasite densities were expressed as log10 of pv18S rRNA and pf18S rRNA gene copies/μl. C. and E. Pv and Pf normalized gametocyte densities. Densities were normalized by division of pvs25 and pfs25 transcripts/μl by pv18S rRNA or Pf18S rRNA genomic copies/μl, respectively.
(DOCX)
A. and C. Pv and Pf gametocyte densities were expressed as log10 of pvs25 and pfs25 transcripts/μl. B. and D. Pv and Pf parasite densities were expressed as log10 of pv18S rRNA and pf18S rRNA gene copies/μl. C. and E. Pv and Pf normalized gametocyte densities. Densities were normalized by division of pvs25 and pfs25 transcripts/μl by pv18S rRNA or Pf18S rRNA genomic copies/μl, respectively.
(DOCX)
A. and C. Proportion of P. vivax and P. falciparum gametocyte carriers among subsequent infections by treatment arm. Figures within the bars indicate absolute numbers of gametocyte-positive subsequent infections following treatment. Error bars indicate 95% confidence intervals by X2 distribution. B. and E. Normalized P. vivax and P. falciparum gametocyte densities in subsequent infections by treatment arm. Normalization was done by dividing pvs25 or pfs25 transcript numbers/μl by Pv- or Pf-18S rRNA copy numbers/μl. C. and F. Absolute P. vivax and P. falciparum gametocyte densities in subsequent infections by treatment arm. Densities are expressed as log10 of pvs25 and pfs25 transcripts/μl.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.