Abstract
Background
The cytoprotective effects of hemeoxygenase-1 and its product biliverdin/bilirubin are widely acknowledged in experimental transplant medicine. However, its potentially beneficial effect during organ reperfusion is not established.
Methods
In a matched study, we compared markers of reperfusion injury (alanine aminotransferase/aspartate aminotransferase) and transplantation outcome (complication rates, liver function, and survival) between recipient groups with “normal” versus “increased” preoperative bilirubin values. Groups were matched for donor and recipient age, liver disease, year of transplantation, and recipient’s preoperative condition (modified model for end-stage liver disease score excluding bilirubin).
Results
The postoperative transaminase peak was significantly higher when comparing the “normal” to the “increased” bilirubin group (maximum aspartate aminotransferase “normal” 2013 [325-13 210] U/L vs “increased” 1360 [221-15 460] U/L, P = 0.006; maximum alanine aminotransferase “normal” 1151 [82-6595] U/L vs “increased” 820 [66-5382] U/L, P = 0.01). Grafts in the “increased” bilirubin group had faster recovery of graft function with faster decrease in international normalized ratio at days 3 and 7 posttransplantation in the “increased” vs “normal” bilirubin group. Although long-term functional parameters (international normalized ratio and bilirubin posttransplantation) as well as surgical and biliary complication rates were similar in both groups, 1-year survival rates were significantly higher in the group with increased preoperative bilirubin (graft survival, “normal” 86% vs “increased” 97%; P = 0.006).
Conclusions
Increased bilirubin levels of liver graft recipients before transplantation are associated with reduced reperfusion injury and improved survival after transplantation.
Liver grafts from expanded-criteria donors are associated with inferior outcome. However, the severe organ shortage has resulted in expansion of donor selection criteria and increasing numbers of expanded-criteria donor organs are accepted for transplantation.1-3 Liver transplantation with expanded-criteria grafts is often associated with severe ischemia and reperfusion injury (IRI), resulting in primary nonfunction or delayed graft function.4 The extent of IRI is an important determinant for the severity of postoperative hepatocyte dysfunction and cholangiopathy.5-8 Reperfusion injury also induces an inflammatory cascade that results in the long-term immunological changes and chronic graft rejection.9 Minimizing IRI is important, and various strategies have been proposed for its attenuation.10,11 The translation of such strategies into clinical practice, however, remains often unsuccessful either due to practical inapplicability or lack of a clinical effect.
Hemeoxygenase-1 (HO-1) has been proposed as a potent mediator for reducing IRI and its cytoprotective, antioxidant, anti-inflammatory, immune-modulative, and vasorelaxation effects have been studied extensively.12-15 HO-1 cleaves heme to biliverdin, carbon monoxide, and free iron. Biliverdin, in turn, is reduced to bilirubin, which is known for its antioxidative properties.16-18 Bilirubin scavenges reactive oxygen species, a process in which it is being oxidized back to biliverdin. This oxidation reaction is effective for both unconjugated and conjugated bilirubin.19 This redox cycle is believed to be—among others—one of the pivotal and inducible mechanisms of bilirubin induced cytoprotection in hepatic IRI, rejection, and inflammation.18,20 Moreover, the beneficial effects of both HO-1 and bilirubin are not limited to hepatic cytoprotection after liver transplantation. The protective effects also extend to other systems, such as in gastrointestinal, cardiovascular, and autoimmune diseases.21-24
On the other hand, bilirubin is known as a marker of cholestasis and liver dysfunction. It is 1 of the 3 variables used to calculate the model for end-stage liver disease score (MELD), which is widely used to estimate a patient’s short-term survival while awaiting liver transplantation.25 However, increased bilirubin levels before liver transplantation do not predict posttransplant outcome because the liver is replaced during surgery. In fact, high bilirubin levels during transplantation could offer a protection against IRI. Recently, our group demonstrated protective effects of increased pretransplant bilirubin levels against IRI in the setting of living donor liver transplantation.26 In contrast, the effects of high bilirubin levels for deceased donor liver transplantation remain unclear.27 The deceased donor population is generally very heterogeneous and many recipient and donor-related factors like age or steatosis confounded earlier analysis. Therefore, we evaluated the impact of preoperative bilirubin values on IRI, and early outcome after liver transplantation in our deceased donor transplant population, controlling recipient and donor variables in a matched study design.
MATERIALS AND METHODS
Study Design
The study was approved by the University Health Network’s Research Ethics Board (14-8069-BE) and conformed with the ethical guidelines of the 1975 Declaration of Helsinki. Between January 2000 and December 2013, all adult patients receiving a full size heart beating donor (HBD) graft at our institution were prospectively entered into our transplant database (n = 854). Patients suffering from acute liver failure or receiving a retransplantation were excluded from the study population.27 Patients were divided into a “normal” (n = 215) and an “increased” bilirubin group (n = 639). The cutoff value dividing both groups was 1.2 mg/dL (21 μmol/L), which is the upper limit of the normal range for total serum bilirubin28 and the 25th percentile of the preoperative total bilirubin levels in our orthotopic liver transplantationv (OLT) population group. To obtain 2 comparable groups for the influence of preoperative bilirubin values on IRI, patient matching was performed 1:1 for the following criteria: donor age (±3 years), recipient age (±3 years), recipient’s etiology of liver disease (hepatitis B virus and/or hepatitis C virus [HCV], ethyl-toxic liver cirrhosis, nonalcoholic steatohepatitis, primary sclerosing cholangitis, others, other etiologies; hepatocellular carcinoma [HCC]), recipient MELD score subtracted by the bilirubin fraction (MELD ex bilirubin = 11.2 × ln international normalized ratio [INR] + 9.57 × ln[creatinine (mg/dL)]),26 and period of transplantation (year of OLT ±3 years). The matching for a MELD score ex bilirubin (calculated by serum creatinine and INR only) is supposed to provide a similar preoperative clinical condition in both groups independent of the serum bilirubin. In case more than 1 patient from the “increased bilirubin” group fit the criteria of a patient from the “normal bilirubin” group, the patient with the closed match was selected for the analysis. Sufficient matches in both groups were found in 116 paired cases. The high number of matching failures was mainly caused by the unequal distribution of patients with a biliary etiology between both groups. Furthermore, bilirubin values often correlated with INR and creatinine values causing a discrepancy of the modified MELD score (ex bilirubin) in both groups.
The 2 groups were compared for differences in IRI, postoperative liver function, and complications. Subanalyses were performed within the “increased bilirubin” group for “mildly” (>1.2 mg/dL ≤ 3 mg/dL, >21 μmol/L ≤ 51 μmol/L), “moderately” (>3 mg/dL ≤ 6 mg/dL, >51 μmol/L ≤ 102 μmol/L), and “severely increased” (>6 mg/dL, > 102 μmol/L) preoperative bilirubin values.
OLT Procedure and Immunosuppression
Organ procurement and full size graft OLT was performed as previously described.29,30 Cava replacement transplantation was the procedure of choice. The immunosuppression regimen consisted primarily of calcineurin inhibitors (CNI), either cyclosporine or tacrolimus, and steroids. Antiproliferative drugs (mycophenolate, mammals target of rapamycin inhibitors or Azathioprine) were used in CNI-sparing regimens when there was concern about CNI-induced side effects. Steroids were tapered during the first 3 months after transplantation and consecutively stopped if no immunological event had occurred.
Donor, Preoperative Recipient, and Perioperative Data
Donor age, gender, and body mass index (BMI) were documented at the time of organ procurement. For the recipients, the following preoperative data were collected: age, gender, preoperative biochemical profile, calculated MELD score, and etiology of liver disease. Cold ischemia time (CIT), warm ischemia time (WIT), and type of biliary anastomosis were documented as perioperative variables. Grafts from donor older than 65 years, donor BMI greater than 35 kg/m2, or CIT greater than 12 hours were considered to be expanded criteria organs.31 Primary nonfunction of the graft was defined as death or retransplantation within 7 days after OLT in the absence of any infectious or vascular problems.32
Postoperative Outcome
Liver graft function was evaluated clinically and by biochemical markers measured daily after transplantation until discharge and afterwards at regular intervals. Reperfusion injury was determined by maximum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) within 48 hours of transplantation.6,33 To assess graft function, we evaluated INR shifts within the first week as compared with the pretransplant values (as marker of initial function), absolute INR levels 14 days after transplantation (as early marker of liver function), as well as total serum bilirubin 3 months after transplantation (as marker of liver function after bilirubin stabilization).
Patient’s postoperative intensive care unit (ICU) and hospital stay was recorded for each case. Surgical complications were documented according to the Clavien-Dindo classification.34 Scores greater than 3 were considered as relevant complications. The first year incidence of biliary complications and cellular rejection confirmed by core biopsy were identified for comparison between both groups. Additionally, graft and patient survival at 1 year was compared, and the cause of graft loss was analyzed. Graft failure was defined as death or retransplantation.
Statistical Analysis
The data were analyzed with the SPSS 20 statistical package (IBM, Chicago, IL). Data were presented as median (range). Mann-Whitney U test and Kruskal-Wallis test were used for all continuous variables for comparison of the study groups. Differences between categorical variables were tested with χ2-test. Graft and patient survivals were calculated by Kaplan-Meier method and compared by log-rank test. A P value below 0.05 was considered as significant.
Normal distribution was tested by Kolmogorov-Smirnov test. For further analysis, bilirubin and transaminases values were logarithmically transformed to approach normal distribution for these data sets, minimizing the statistically distorting effect of outliers. In a multivariate linear regression analysis, the preoperative total bilirubin (logarithmized) was analyzed together with donor BMI, as well as CIT and WIT as independent variables to evaluate their impact on the maximum AST (logarithmized) as dependent variable and indicator for IRI.
RESULTS
From January 2000 until December 2013, 854 adult patients (excluding cases of acute liver failure) received a full size HBD liver graft as their 1st transplantation at our institution. Out of this patient population, 116 matches were found between the “normal” (preoperative total serum bilirubin ≤ 1.2 mg/dL) and the “increased bilirubin” groups (in total 232 patients).
Donor Data, Recipient Demographics, and Perioperative Status
The matching variables (donor age, recipient age, etiology of liver disease, preoperative MELD ex bilirubin, transplant period) were distributed equally in both groups (Table 1). The remaining donor characteristics, gender and BMI, were similar in both groups. Recipient characteristics that were not covered by the matching criteria were also comparable in both groups.
TABLE 1.
Donor and recipient characteristics
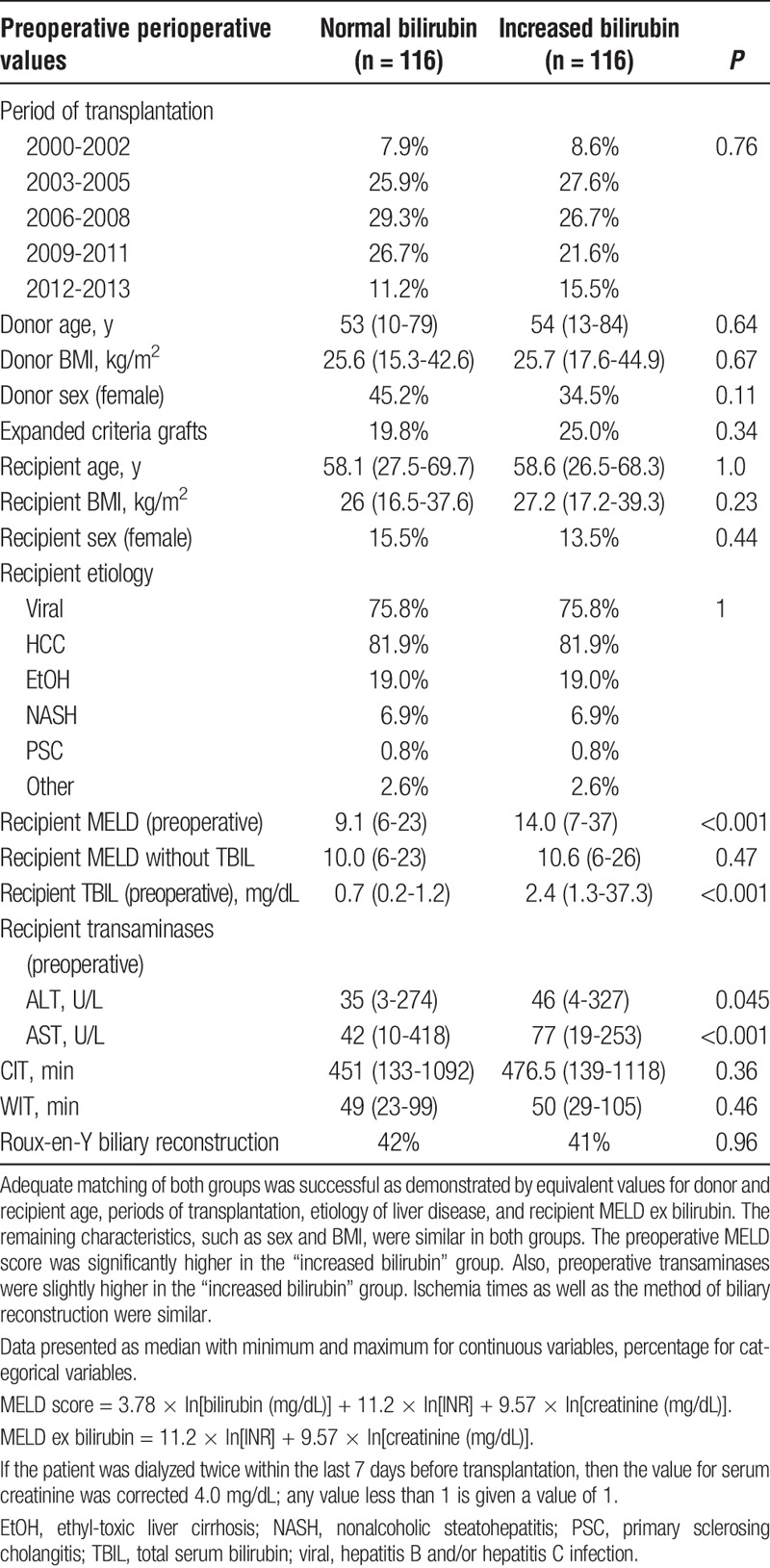
As expected, the preoperative medical MELD score was significantly higher in the “increased bilirubin” group (“normal” 9 [6-23] vs “increased” 14 [7-37], P < 0.001). The preoperative transaminases were significantly higher in the “increased bilirubin” group (ALT, “normal” 35 [3-274] U/L vs “increased” 46 [4-327] U/L, P = 0.045; AST: “normal” 42 [10-418] U/L vs “increased” 77 [19-253], P < 0.001).
WIT and CIT during transplantation were comparable in both groups. Also, the frequency of Roux-en-Y anastomosis compared with end-to-end bile duct anastomosis was comparable in both groups.
In the “normal bilirubin” group, 19.8% of transplanted organs met our criteria for expanded criteria grafts versus 25.0% in the “increased bilirubin” group (P = 0.34).
Ischemia-Reperfusion Injury and Liver Function
The postoperative transaminase peak was significantly higher when comparing the “normal” to the “increased bilirubin” group (maximum AST “normal” 2013 [325-13 210] U/L vs “increased” 1360 [221-15 460] U/L, P = 0.006; maximum ALT “normal” 1151 [82-6595] U/L vs “increased” 820 [66-5382] U/L, P = 0.01; see Figure 1).
FIGURE 1.
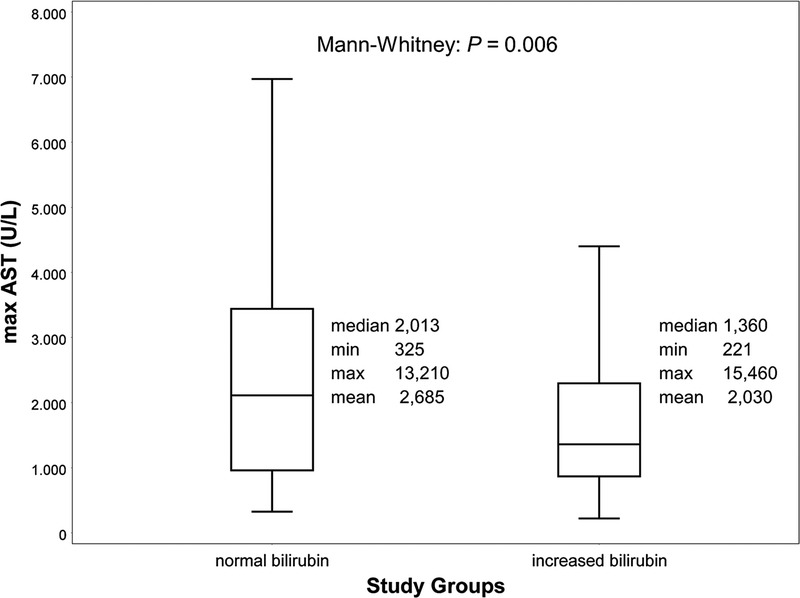
Peak AST after transplantation. Box-plot (median with 25th and 75th percentile, whiskers represent inner fences, outliers were excluded from plot for clearer visualization) for maximal aspartate aminotransferase (AST) values within 2 days after transplantation. The postoperative rise in transaminases was significantly lower when comparing the “increased” (>1.2 mg/dL, >21 μmol/l) to the “normal bilirubin” (≤1.2 mg/dL, ≤21 μmol/L) group.
In a multivariate linear regression analysis, we investigated if the preoperative bilirubin value had an independent influence on the peak of transaminases. Preoperative bilirubin values and peak AST were logarithmically transformed to achieve a normal distribution. AST was used as a surrogate marker of IRI (dependent variable), whereas CIT, WIT, donor BMI, and preoperative bilirubin values were investigated as independent variables. Preoperative bilirubin had a significantly attenuating impact on peak AST values (β, −0.15; P = 0.04; 95% confidence interval [CI], −0.299 to −0.008), whereas both CIT and donor BMI significantly increased AST levels (CIT β, 0.18; P = 0.01; 95% CI, 0.0-0.002; BMI β, 0.27; P < 0.001; 95% CI, 0.025-0.088). Prolonged WIT appeared to increase IRI as well, but did not reach significance (β, 0.13; P = 0.08; 95% CI, −0.001 to 0.022).
Grafts in the “elevated bilirubin” grouped picked up liver function faster than those from the “normal bilirubin” group. Within the first 24 hours, INR values had increased by a median of only 0.21 (−4.1 to 2.9) in the “increase bilirubin” group versus 0.31 (−0.3 to 2.5) in the “normal bilirubin” group (P = 0.04). By day 3, median INR values in the “normal bilirubin” group were still increased when compared to the pretransplant values (0.24 [−0.71 to 2.1]), whereas the median INR values in the “increased bilirubin” group had recovered the baseline value (−0.01 [−3.95 to 3.1], P < 0.001). This faster adjustment of the INR in the “increased bilirubin” group was even more pronounced at day 7 (“normal” −0.03 [−0.87 to 0.28] vs “increased” −0.24 [−5.27 to 0.99], P < 0.001; see Figure 2A). Fourteen days after transplantation, when INR values had adjusted to stable level as early marker of liver function, absolute INR values were similar in both “normal” and “increased bilirubin” groups (“normal” 1.2 [0.9-2.2] vs “increased” 1.2 [1.0-3.6], P = 0.27; see Figure 2B). The total bilirubin levels 90 days after transplantation as later marker of liver function were comparable in both groups (“normal” 0.5 [0.1-6.2] mg/dL vs “increased” 0.6 [0.1-12.8] mg/dL, P = 0.07; see Figure 2C).
FIGURE 2.
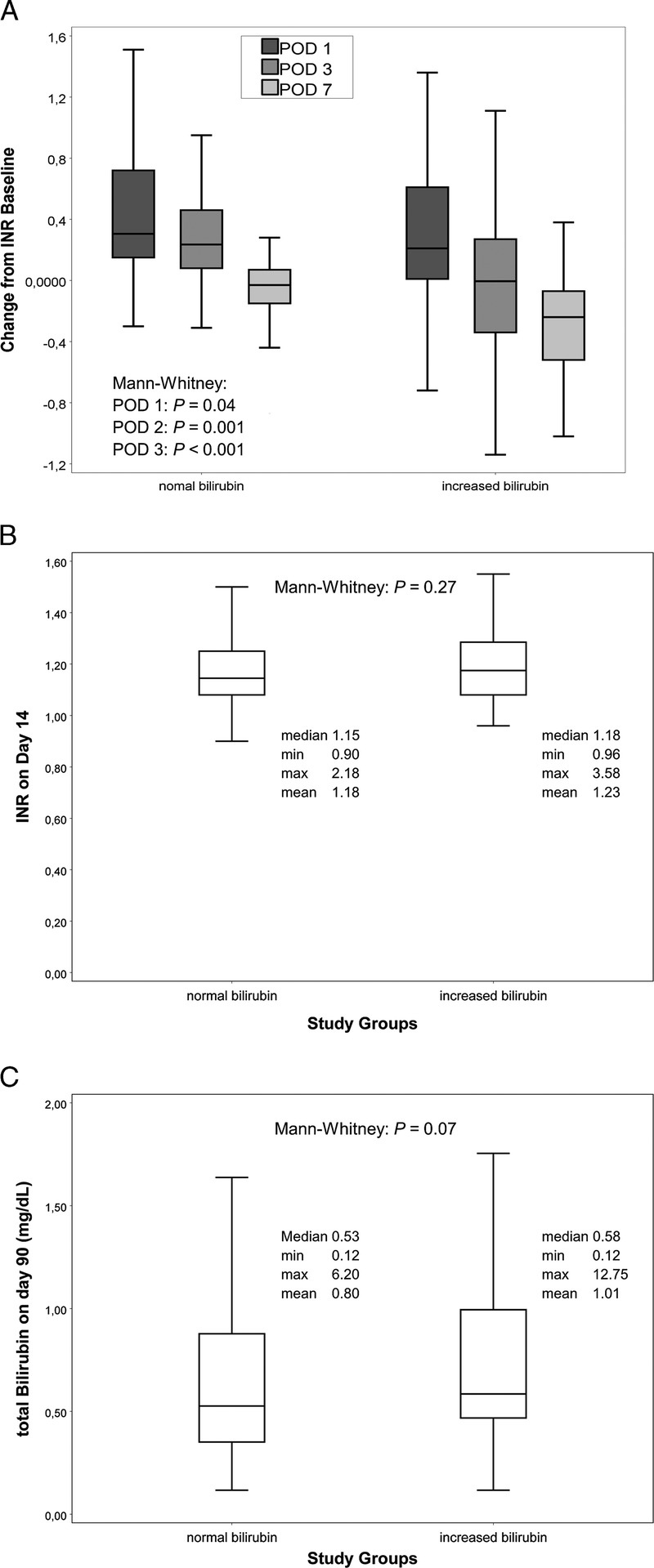
Postoperative Liver Function. Box-plot (median with 25th and 75th percentile, whiskers represent inner fences, outliers were excluded from plot for clearer visualization). A, Changes from pretransplant INR 1, 3, and 7 days after transplantation—liver grafts picked up function more quickly as demonstrated by a more rapid drop of INR values after transplantation. INR 14 days after transplantation (B) and total bilirubin 90 days after transplantation (C), both groups have equivalent marker of postoperative liver function.
Subgroup Analysis, Dose Dependency of Bilirubin Effect
To evaluate a potential dose dependency of the protective bilirubin effect, we further divided the “increased bilirubin” group. Analyzing “mildly,” “moderately,” and “severely increased” subgroups, the IRI appeared to be lowest in the first 2 groups. However, the “severely increased” group was rather small (n = 13) and the subgroups were otherwise not matched to each other. Accordingly, a dose-dependent, protective effect of bilirubin was not verifiable. Furthermore, no functional differences (INR at 14 days, bilirubin at 30 days) were observed in between the subgroups.
Postoperative Outcome
Postoperative ICU stay (“normal” 1 [0-115] vs “increased” 2 [0-53], P = 0.09) as well as length of hospital stay (“normal” 10.5 [4-196] vs. “increased” 12 [5-182], P = 0.1) were similar in both groups (Table 2). However, 1-year graft (“normal” 86% vs “increased” 97%, P = 0.006; see Figure 3) and patient survival (“normal” 87% vs “increased” 97%, P = 0.01) was significantly better in the “increased bilirubin” group. After the first year, survival curves decreased parallel resulting in a 3-year graft survival of 75% in the “normal bilirubin” group versus 85% in the “increased bilirubin” group (P = 0.06, see Figure 3). The patient survival behaved accordingly (“normal” 76% vs “increased” 85%, P = 0.08).
TABLE 2.
Complications and survival rates
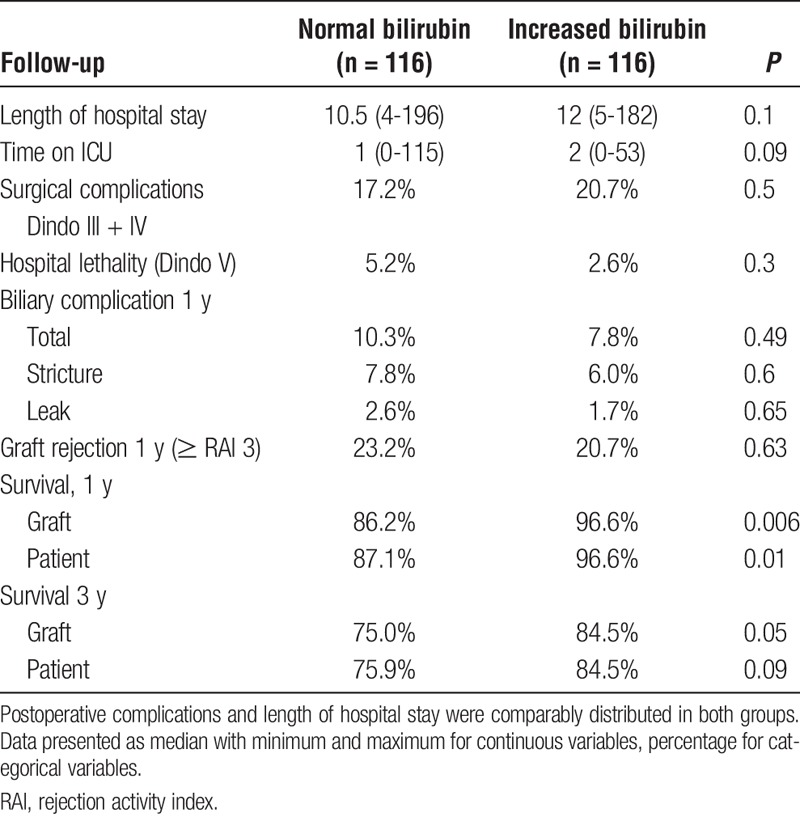
FIGURE 3.
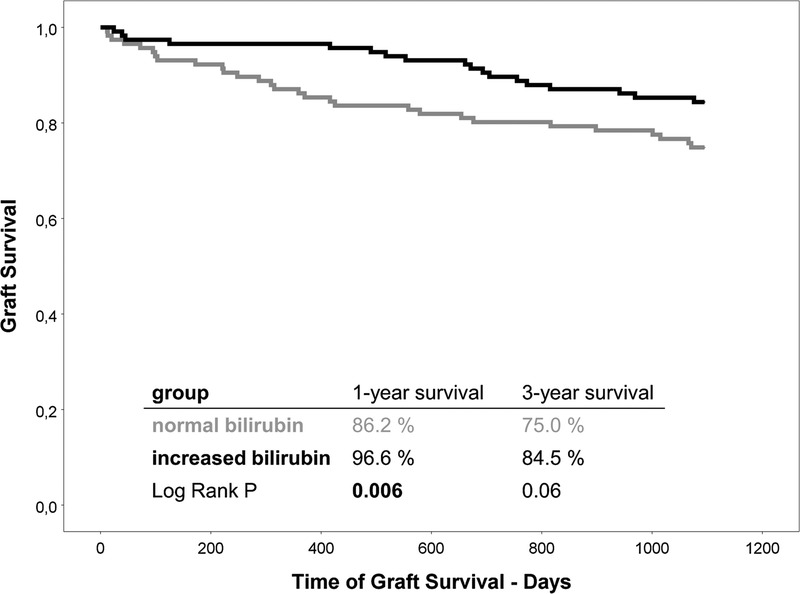
Liver graft survival after transplantation. Kaplan-Meier graphs for graft survival 3 years after transplantation. Survival after 1 year was significantly better in the “increased bilirubin” group. Afterward, survival curves of both groups decrease in a parallel manner.
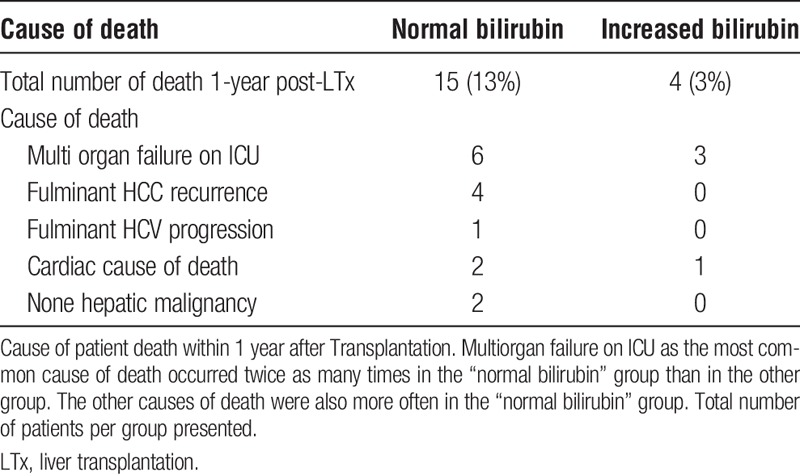
None of the patients lost their graft due to primary nonfunction or rejection. Only 1 patient was retransplanted within the first year after transplantation (patient from the “normal bilirubin” group due to a thrombosis of the hepatic artery). The most common cause of graft failure within 1 year was death due to multiorgan failure after a prolonged ICU stay (6 patients in “normal” and 3 patients in “increased bilirubin” group). Four patients of the “normal bilirubin” group died within 1 year of fulminant cancer (HCC) recurrence and 1 because of hepatitis C progression. Cardiac causes accounted for 2 deaths in the “normal” and 1 death in the “increased” bilirubin group. Two more patients from the “normal” group died of aggressive, nonhepatic malignant diseases that were diagnosed for the first time after transplantation (Table 3).
TABLE 3.
Cause of death
The rate of severe surgical complications (Clavien-Dindo score 3b-5) was similar in both groups (“normal” 17.2% vs “increased” 20.7%, P = 0.5). The in-hospital mortality (Clavien-Dindo score 5) was lower in the “increased” group, however, without a significant difference (“normal” 5.2% vs. “increased” 2.6%, χ2-test P = 0.3). Biliary complications within the first year were also similarly distributed between both groups (“normal” 10.3% vs “increased” 7.8%, P = 0.49). Bile duct strictures occurred in 7.8% of the “normal bilirubin” patients, compared to 6.0% in the “increased bilirubin” group (P = 0.60). The rate of bile leaks within the first year after transplantation was 2.6% in the “normal bilirubin” and 1.7% in the “increased bilirubin” group (P = 0.65). Episodes of biopsy proven cellular rejection within the 1st posttransplantation year occurred in 23.2% of “normal” and 20.7% of “increased bilirubin” patients (P = 0.63).
DISCUSSION
In this study, we analyzed the effects of preoperative bilirubin levels on outcome after deceased donor liver transplantation. Two groups of recipients with either normal or increased preoperative bilirubin values were matched, minimizing confounding differences due to preoperative patient condition, disease etiology, donor age, and period of transplantation. As indicator of IRI, we demonstrated significantly increased peak transaminases after OLT in the “normal bilirubin” group when compared with recipients with increased bilirubin values before transplantation. Although stabilized marker of liver function after the initial posttransplant phase and surgical complication rates were similar in both groups, “increased bilirubin” levels were associated with a significantly better 1-year graft survival rate, suggesting a clinically relevant protective effect of increased perioperative bilirubin values. Additionally, liver grafts appeared to pick up liver function more quickly in “increased bilirubin” recipients, as demonstrated by the more pronounced early posttransplant INR changes.
Our results demonstrate in a transplantation setting that the cytoprotective effect of bilirubin might be clinically relevant. Bilirubin has been known to be a powerful physiological anti-oxidant for a long time.16-18,20 Furthermore, several animal studies have suggested beneficial effects of bilirubin on hepatic reperfusion injury. In a rodent model, Kato et al35 could demonstrate that a bilirubin rinse of liver grafts before transplantation is a simple strategy to ameliorate oxidative stress and hepatobiliary dysfunction. Similarly, Fondevila et al36 demonstrated a cytoprotective effect of biliverdin in a rodent liver transplantation model. Different to Kato et al, this group did not treat the liver graft before transplantation, but rather during and after reperfusion in the recipient rat. Similar effects have been described in animal studies for IRI after other solid organ transplantation.20 Despite these favorable outcomes, a previous retrospective analysis of a deceased donor population by Manzinate et al27 failed to reproduce the results of these animal studies in a clinical setting. In this group of liver transplant recipients, no correlation was found between preoperative bilirubin and postoperative IRI as well as graft survival. However, especially the heterogeneity of the donor group likely confounded these findings. In contrast, in a study investigating kidney transplantation, Lee et al37 were able to demonstrate a protective effect of postoperatively increased serum bilirubin levels on graft survival and rates of acute rejection after kidney transplantation. In a previous study, we analyzed the protective effects of increased preoperative bilirubin values on IRI after living donor liver transplantation representing a homogeneously healthy donor population.26 We found significantly lower transaminase peak after transplantation in recipients with preoperatively increased bilirubin values. However, because of the limited preservation injury during live donation liver transplantation, we were not able to demonstrate a clinical benefit. Now, in a group of HBD graft recipients, about 20% of all transplanted organs met expanded criteria graft standards, increasing the relevance of IRI in this population.
In our study population, primary non function as the maximal manifestation of IRI did not occur. However, patient survival was mainly compromised by hepatic dysfunction and failure after prolonged ICU treatment and HCC as well as HCV recurrence. Recently, the extent of IRI has been suggested to be a major risk factor for an aggressive recurrence of both HCC and HCV.38-40 Thus, an association between decreased IRI due to bilirubin cytoprotection and reduced numbers of patient mortality for fulminant HCC and HCV recurrence appears possible.
Our results indicate that serum bilirubin levels have a relevant effect on IRI after human OLT. Several authors have suggested using this protective effect by applying recombinant bilirubin/biliverdin perioperatively.20 However, a clinical application has not been established, and treatment protocols have not been developed thus far. Further prospective clinical studies are mandatory to develop strategies to implement bilirubin administration and HO-1 induction during and after transplantation. In addition, the indication of bilirubin-lowering treatment strategies like biliary stenting in patients awaiting an OLT might have to be reconsidered, bearing in mind the protective effect during the transplantation.
Our study had several limitations. To minimize the heterogeneity of our HBD graft recipients, we used a matched study design. During the matching process, many cases had to be excluded because no adequate match was available. Thus, the study groups had a rather small sample size. Also, additional donor risk factors influencing reperfusion injury (eg, use of vasoactive drugs, long-standing hypotension, length of ICU stay, laboratory values, and so on) were not taken into account in our analysis. The validity of our results was still conclusive because of the coherent posttransplant treatment regimen we used for all patients and because of the balanced matching result of our study groups. However, the sample size was too small to effectively analyze the dose dependency of the bilirubin effect. Subanalyses of certain subgroups were also not possible because of the low case numbers, for example, analyzing a potentially protective effect of bilirubin and HO-1 on HCV and HCC recurrence after transplantation. Finally, we assume that the protective effect of bilirubin in reperfusion injury as described in many experimental cell culture and animal studies has had a positive impact on posttransplant outcome. Our retrospective clinical analysis however lacks mechanistic evaluation of this effect (eg, quantification of oxidative stress).
Although 1-year graft and patient survival is significantly higher in the increased bilirubin group, the cause of death in 40% of patients in the normal bilirubin group was related to recurrence of HCC or other malignancies which may not be directly related to the protective effect of high bilirubin. Further studies with larger number of patients are necessary to elucidate these aspects.
Our matching strategy was based on modified MELD score using only INR and creatinine values (excluding bilirubin). The modified MELD score allows to adequately evaluate and quantify the preoperative degree of liver failure and patient condition. Using other scoring system such as Child Pugh score for matching would have only added the potential value of serum albumin as another marker of liver synthetic function in addition to INR.
In conclusion, high bilirubin levels of OLT recipients before transplantation are associated with reduced IRI and improved survival after transplantation. Inducible HO-1, as the likely mechanism of protection of bilirubin, represents a clinical relevant target for future studies.
Footnotes
Published online 5 July, 2017.
The authors declare no funding or conflicts of interest.
V.N.S. participated in research design, performance of the research, data analysis, and writing the article. N.G. participated in the performance of the research and data analysis. J.M.K. participated in the performance of the research and data analysis. M.M. participated in research design and data analysis. M.S. participated in research design and writing the article. N.S.participated in research design, writing the article, and performance of the research.
REFERENCES
- 1.Saidi RF. Utilization of expanded criteria donors in liver transplantation. Int J Organ Transplant Med. 2013;4:46–59. [PMC free article] [PubMed] [Google Scholar]
- 2.Feng S, Lai JC. Expanded criteria donors. Clin Liver Dis. 2014;18:633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 4.Elias-Miro M, Jimenez-Castro MB, Rodes J, et al. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555–568. [DOI] [PubMed] [Google Scholar]
- 5.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury—a fresh look. Exp Mol Pathol. 2003;74:86–93. [DOI] [PubMed] [Google Scholar]
- 6.Chen XB, Xu MQ. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int. 2014;13:125–137. [DOI] [PubMed] [Google Scholar]
- 7.Mourad MM, Algarni A, Liossis C, et al. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. 2014;20:6159–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cursio R, Gugenheim J. Ischemia-reperfusion injury and ischemic-type biliary lesions following liver transplantation. J Transplant. 2012;2012:164329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Land W, Messmer K, Events E. The Impact of ischemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplant Rev. 1996;10:108–127. [Google Scholar]
- 10.Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Golen RF, Reiniers MJ, Olthof PB, et al. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol. 2013;28:394–400. [DOI] [PubMed] [Google Scholar]
- 12.Origassa CS, Camara NO. Cytoprotective role of heme oxygenase-1 and heme degradation derived end products in liver injury. World J Hepatol. 2013;5:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards JA, Wigmore SJ, Devey LR. Heme oxygenase system in hepatic ischemia-reperfusion injury. World J Gastroenterol. 2010;16:6068–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sass G, Barikbin R, Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Z Gastroenterol. 2012;50:34–40. [DOI] [PubMed] [Google Scholar]
- 15.Wang CF, Wang ZY, Li JY. Dual protective role of HO-1 in transplanted liver grafts: a review of experimental and clinical studies. World J Gastroenterol. 2011;17:3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. [DOI] [PubMed] [Google Scholar]
- 17.Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9–14. [DOI] [PubMed] [Google Scholar]
- 18.Baranano DE, Rao M, Ferris CD, et al. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stocker R, Peterhans E. Antioxidant properties of conjugated bilirubin and biliverdin: biologically relevant scavenging of hypochlorous acid. Free Radic Res Commun. 1989;6:57–66. [DOI] [PubMed] [Google Scholar]
- 20.Ollinger R, Wang H, Yamashita K, et al. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007;9:2175–2185. [DOI] [PubMed] [Google Scholar]
- 21.Chang M, Xue J, Sharma V, et al. Protective role of hemeoxygenase-1 in gastrointestinal diseases. Cell Mol Life Sci. 2015;72:1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czibik G, Derumeaux G, Sawaki D, et al. Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Basic Res Cardiol. 2014;109:450. [DOI] [PubMed] [Google Scholar]
- 23.Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113:1776–1782. [DOI] [PubMed] [Google Scholar]
- 24.Vitek L. Impact of serum bilirubin on human diseases. Pediatrics. 2005;115:1411–1412. [DOI] [PubMed] [Google Scholar]
- 25.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 26.Spetzler VN, Goldaracena N, Kaths JM, et al. High preoperative bilirubin values protect against reperfusion injury after live donor liver transplantation. Transpl Int. 2015;28:1317–1325. [DOI] [PubMed] [Google Scholar]
- 27.Manzinate F, McDaid J, Devey L, et al. Pretransplant bilirubin concentration does not correlate with early reperfusion injury following liver transplantation. Transplantation. 2007;83:103–104. [DOI] [PubMed] [Google Scholar]
- 28.Whebi M, Staros EB. Bilirubin. Medscape Drugs & Diseases website. http://emedicine.medscape.com/article/2074068-overview. Updated January 14, 2015. Accessed August 8, 2015.
- 29.Lladó L, Figueras J. Techniques of orthotopic liver transplantation. HPB (Oxford). 2004;6:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormack L, Selzner M, Clavien P-A. The transplant operation. In: Killenberg PG, Clavien P-A, editors. Medical care of the liver transplant patient. 3rd ed Malden, MA: Blackwell Publishing Ltd; 2006:229–241. [Google Scholar]
- 31.Durand F, Renz JF, Alkofer B, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008;14:1694–1707. [DOI] [PubMed] [Google Scholar]
- 32.Ploeg RJ, D'Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation—a multivariate analysis. Transplantation. 1993;55:807–813. [DOI] [PubMed] [Google Scholar]
- 33.Rosen HR, Martin P, Goss J, et al. Significance of early aminotransferase elevation after liver transplantation. Transplantation. 1998;65:68–72. [DOI] [PubMed] [Google Scholar]
- 34.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 35.Kato Y, Shimazu M, Kondo M, et al. Bilirubin rinse: a simple protectant against the rat liver graft injury mimicking heme oxygenase-1 preconditioning. Hepatology. 2003;38:364–373. [DOI] [PubMed] [Google Scholar]
- 36.Fondevila C, Shen XD, Tsuchiyashi S, et al. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. [DOI] [PubMed] [Google Scholar]
- 37.Lee JP, Kim DH, Yang SH, et al. Serum bilirubin affects graft outcomes through UDP-glucuronosyltransferase sequence variation in kidney transplantation. PLoS One. 2014;9:e93633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornberg A, Witt U, Kornberg J, et al. Extended ischemia times promote risk of HCC recurrence in liver transplant patients. Dig Dis Sci. 2015;60:2832–2839. [DOI] [PubMed] [Google Scholar]
- 39.Nagai S, Yoshida A, Facciuto M, et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015;61:895–904. [DOI] [PubMed] [Google Scholar]
- 40.Ciria R, Pleguezuelo M, Khorsandi SE, et al. Strategies to reduce hepatitis C virus recurrence after liver transplantation. World J Hepatol. 2013;5:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]


