Supplemental digital content is available in the text.
Abstract
Background
Proenkephalin (pro-ENK), a stable and reliable surrogate marker for unstable enkephalins, was found to be associated with acute kidney injury and chronic renal failure in previous studies. We aimed to investigate whether pro-ENK is linked to chronic kidney injury and poor long-term outcome in renal transplant recipients (RTR).
Methods
We included 664 stable RTR and 95 healthy kidney donors. Pro-ENK was measured in plasma with a double monoclonal sandwich immunoassay. Graft failure was defined as return to dialysis therapy or retransplantation.
Results
Median pro-ENK was 110 pmol/L (interquartile range [IQR], 85-148 pmol/L) in RTR and 48 pmol/L (IQR, 42-55 pmol/L) in kidney donors. Pro-ENK was correlated with estimated glomerular filtration rate (GFR) (rs = −0.80, P < 0.001) in RTR and with measured GFR (rs = −0.74, P < 0.001) in kidney donors. During a median follow-up of 3.1 years (IQR, 2.7-3.9 years), 45 RTR developed graft failure and 76 died. Pro-ENK was positively associated with risk (hazard ratio [HR] per standard deviation increment of the logarithm of pro-ENK; 95% confidence interval [CI]) of graft failure (HR, 4.80; 95% CI, 3.55-6.48) and mortality (HR, 1.50; 95% CI, 1.22-1.85). After adjustment of age, sex, and estimated GFR, the association of pro-ENK with graft failure remained significant (HR, 2.36; 95% CI, 1.37-4.06), whereas no significant association of pro-ENK with risk of all-cause mortality was observed (HR, 1.34; 95% CI, 0.90-2.09).
Conclusions
Plasma pro-ENK is associated with kidney function as reflected by correlations with measured GFR in both RTR and kidney donors. In addition, pro-ENK was independently associated with increased risk of graft failure in RTR. Pro-ENK may aid in identification of RTR at risk for late graft failure.
Enkephalins are well-known endogenous opioid peptides exhibiting various functions, including involvement in stress response systems, pain perception, cardiac function, bone formation, and immune responses.1-5 Enkephalin immunoreactivity is also shown to be present in skeletal muscle myofibers, intestinal smooth muscle cells, and intestinal and kidney epithelial cells.6 Given that expression of enkephalin receptors in the kidney is dense, it has been hypothesized that enkephalins may play an important biological function in renal physiology.6
Because enkephalins have a very short half-life, an assay for a stable precursor fragment of enkephalin, proenkephalin (pro-ENK) 119 to 159, has been developed and has been established as reliable surrogate marker for unstable enkephalins.7 Pro-ENK has been investigated in clinical studies in which elevated levels of pro-ENK were associated with prognosis after acute myocardial infarction,8 acute kidney injury,9 and chronic renal failure.10 Furthermore, in a recent study, pro-ENK was found to be associated with a faster decline of renal function and an increased risk of developing chronic kidney disease (CKD) in a population-based cohort.11
Because enkephalins have a role in both immunology and renal damage, we hypothesized that pro-enkephalin may be a marker for determining long-term renal prognosis in renal transplant recipients (RTR). Therefore, the aim of our present study was to investigate the association of pro-ENK with chronic kidney injury and long-term outcome in RTR.
MATERIALS AND METHODS
Study Population
All RTRs (aged ≥18 years) that were 1 year or longer posttransplantation were approached for participation during outpatient clinic visits between 2008 and 2011, as described previously.12 All RTRs were transplanted in the University Medical Center Groningen, Groningen, the Netherlands. None of the RTR underwent desensitization before transplantation, and none of them had a positive crossmatch before transplantation. Subjects had no history of drug or alcohol abuse. Written informed consent was obtained from 707 (87%) from the 817 initially invited RTR. For the present analyses, we excluded patients with missing data on pro-ENK (n = 43), leaving 664 RTR eligible for analyses. We also included 95 healthy donors, who all gave a kidney for living renal transplantation and agreed to participate. The study protocol was approved by the institutional review board, which adhered to the Declaration of Helsinki. The cohort on which the study was based is registered at clinicaltrials.gov as ‘TransplantLines Food and Nutrition Biobank and Cohort Study (TxL-FN)’ with number NCT02811835.
Data Collection
The measurement of clinical parameters has been described in detail previously.13 Information on medical history and medication use was obtained from patient records. Participants’ height and weight were measured with participants wearing indoor clothing without shoes. Body mass index (BMI) was calculated as weight (kilograms) divided by height squared (square meter). Blood pressure was measured per strict protocol as previously described.13 Information on alcohol consumption and smoking behavior was obtained by using a questionnaire. Alcohol consumption was classified as 0, 0 to less than 10, 10 to 30, or greater than 30 g/d. Smoking behavior was classified as never, former or current smoker. Diabetes was defined as use of antidiabetic medication or fasting plasma glucose of 7.0 mmol/L or greater. Physical activity was assessed with the Short QUestionnaire to ASsess Health-enhancing physical activity, which has been developed and validated by the Dutch National Institute of Public Health and Environment to assess daily life physical activity in the Dutch adult population.14 Per a strict protocol at a day before their visit to the outpatient clinic, all RTRs were asked to collect a 24-hour urine sample. Urine was collected under oil and chlorhexidine was added as an antiseptic agent. Blood was drawn in the morning after completion of the 24-hour urine collection. Dietary intake was assessed with a validated semi quantitative food frequency questionnaire developed by Wageningen University, Wageningen, the Netherlands. The food frequency questionnaire inquired about intake of 177 food items during the last month, taking seasonal variations into account.
Laboratory Procedures
Urine electrolytes were directly analyzed per standard laboratory procedures. Renal function in RTR was estimated with the combined creatinine cystatin C-based Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation from 2012.15 In the 95 healthy kidney donors, glomerular filtration rate (GFR) was measured by constant low dose infusion of the radiolabeled tracer 125I-iothalamate16,17 and estimated GFR (eGFR) was estimated with the creatinine-based CKD-EPI equation.18 Urine albumin was determined by nephelometry (Dade Behring Diagnostic, Marburg, Germany). Plasma electrolytes and serum cholesterol were measured using standard laboratory procedures. Serum creatinine was assessed using a modified version of the Jaffé method (MEGA AU 510; Merck Diagnostica, Darmstadt, Germany). Class I and class II antihuman leukocyte antigen antibodies (HLAab), at time of pro-ENK sampling, were assessed by ELISA (LATM20X5, One Lambda, Canoga Park, CA).
Measurement of pro-ENK
An assay for stable pro-ENK (amino acids 119-159 of pro-ENK A) has been previously reported7 and was modified as recently described.8 In brief, 2 mouse monoclonal anti-pro-ENK antibodies were developed by immunization with pro-ENK peptide (amino acids 119-159 of pro-ENK A). One antibody (2 μg) was used to coat polystyrene tubes. The other antibody labeled with methyl-acridinium ester served as the detector antibody. Standards (pro-ENK peptide; amino acids 119-159 of pro-ENK A) and samples (50 μL) were incubated in tubes with the detector antibody (150 μL). After equilibration, tubes were washed and bound chemiluminescence was detected with a luminometer LB952T/16 (Berthold, Bad Wildbad, Germany). The lower detection limit of the assay was 5.5 pmol/L. Intra-assay and interassay coefficients of variation were, 6.4% and 9.5% at 50 pmol/L, and 4.0% and 6.5% at 150 pmol/L, respectively. The assay was provided by Sphingotec GmbH (Hennigsdorf, Germany). Pro-ENK was measured in plasma at baseline in RTR, and in plasma before (median, 138 days; interquartile range [IQR], 81-242 days), and after transplantation (median, 50 days; IQR, 49-57 days) in kidney donors.
Outcome Ascertainment
The primary endpoints of this study were all-cause mortality and death-censored transplant failure, defined as return to dialysis therapy or retransplantation. The continuous surveillance system of the outpatient program ensures up-to-date information on patient status. General practitioners or referring nephrologists were contacted in case the status of a patient was unknown. Endpoints were recorded until the end of May 2013. There was no loss to follow-up for the primary endpoints.
Statistical Analysis
Baseline characteristics are presented per tertiles of pro-ENK. Continuous data are presented as mean with SD or as median and IQR in case of skewed distribution. Categorical data are presented as percentages.
Multivariable linear regression was performed to determine whether patients’ characteristics were associated with pro-ENK concentrations. In the first model, all analyses were adjusted for age and sex, the second model was further adjusted for eGFR, and the last model included all variables. The Spearman's rank correlation coefficient was calculated for the associations of pro-ENK with urinary albumin excretion (UAE), eGFR, and measured GFR (mGFR) (only available in kidney donors). To study the prospective association between pro-ENK and risk of graft failure and all-cause mortality, Cox proportional hazards regression analysis was used to calculate hazard ratios (HRs) per 1 SD increment in log-transformed values and 95% confidence intervals (CIs). We first calculated HRs (95% CIs) for the crude model. Second, we calculated HRs (95% CIs) alternately adjusted for sex, age, BMI, smoking, high-density lipoprotein (HDL) cholesterol, triglycerides, serum glucose, antihypertensive drugs, calcineurin inhibitors, eGFR, or UAE. In the first multivariable model, we adjusted for age, sex, and eGFR. In the second multivariable model, we additionally adjusted model 1 for BMI, smoking, HDL cholesterol, triglycerides, serum glucose, antihypertensive drugs, calcineurin inhibitors, and UAE. In the third multivariable model, we additionally adjusted model 1 for time since renal transplantation, donor status, donor age, cold ischemia time, delayed graft function, HLAab class I, HLAab class II, rejection type, and cytomegalovirus infection. Nonlinearity was tested by using the likelihood ratio test, comparing nested models with linear or linear and cubic spline terms. We evaluated whether pro-ENK adds additional prognostic information for risk prediction with the use of the receiver operating characteristic (ROC) curves for pro-ENK only and for the multivariable model with and without inclusion of pro-ENK, and by testing on differences in Harrell’s C statistics and −2 Log Likelihood of prediction models with and without inclusion of pro-ENK in the model.
All P values are 2-tailed. A P value less than 0.05 was considered statistically significant. All analyses were conducted with the use of the statistical package IBM SPSS (version 22.0.1; SPSS, Chicago, IL), STATA/SE (version 13.1; StataCorp. College Station, TX, USA) and RStudio (version 0.98.1091; RStudio, Boston, MA).
RESULTS
RTR Characteristics
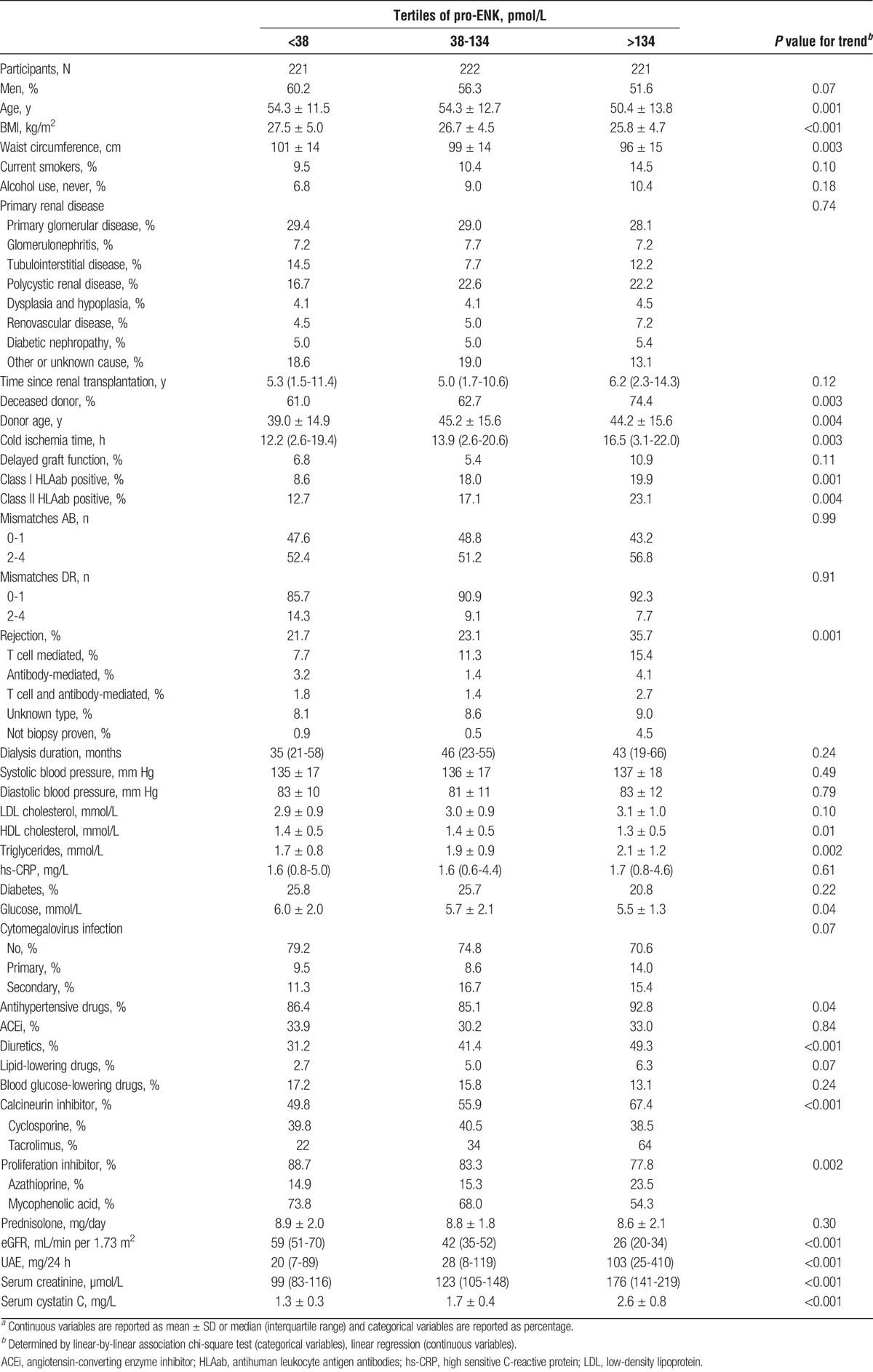
Baseline median pro-ENK was 110 pmol/L (IQR, 85-148 pmol/L), with slightly higher values in women (median, 116; IQR, 87-160 pmol/L) compared with men (median, 106; IQR, 84-148 pmol/L). Baseline characteristics are shown per tertiles of pro-ENK (Table 1). At baseline, RTRs, who had a higher pro-ENK, were more likely to be younger and to have a lower BMI and a smaller waist circumference. RTRs in the highest tertile of pro-ENK were also more likely to receive an allograft from a deceased and older donor, to have a longer cold ischemia time, to be class I and II HLAab positive, and to have a transplant rejection. Men and women in the highest tertile of pro-ENK were more likely to have a lower HDL cholesterol, higher triglycerides, a lower serum glucose, and to use less antihypertensive medication, including diuretics, less lipid-lowering drugs, and more calcineurin and proliferation inhibitors, compared with men and women in the lowest tertile of pro-ENK. Subjects with high levels of pro-ENK had lower baseline eGFR and higher UAE.
TABLE 1.
Baseline characteristics per tertiles of plasma pro-ENK of 664 RTRsa
Pro-ENK was inversely correlated with eGFR (rs = −0.80; P < 0.001; Figure 1), and creatinine clearance (rs = −0.77; P < 0.001), and positively with UAE (rs = 0.34; P < 0.001), serum creatinine (rs = 0.72; P < 0.001), and cystatin C (rs = 0.80; P < 0.001).
FIGURE 1.
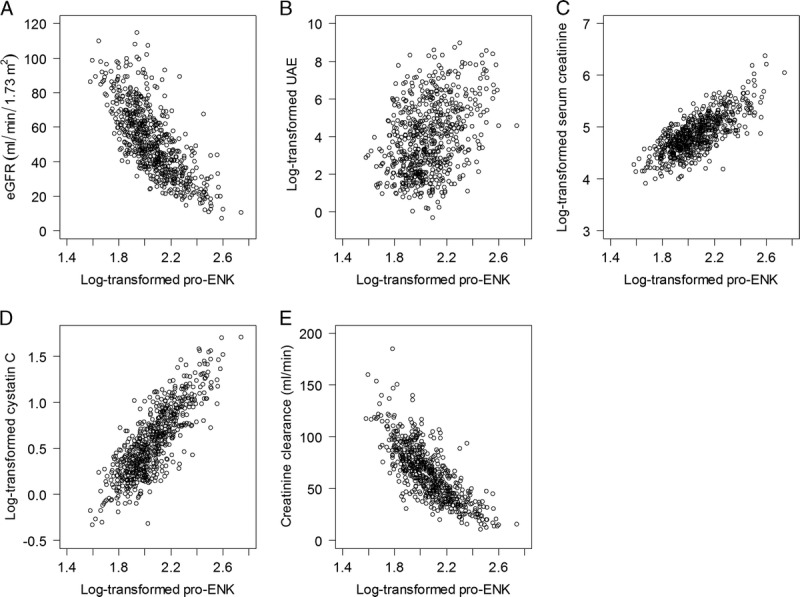
Scatterplot of the associations of plasma pro-ENK with eGFR (A), UAE (B), serum creatinine (C), cystatin C (D), and creatinine clearance (E) in 664 RTRs.
Association of pro-ENK With Selected Variables
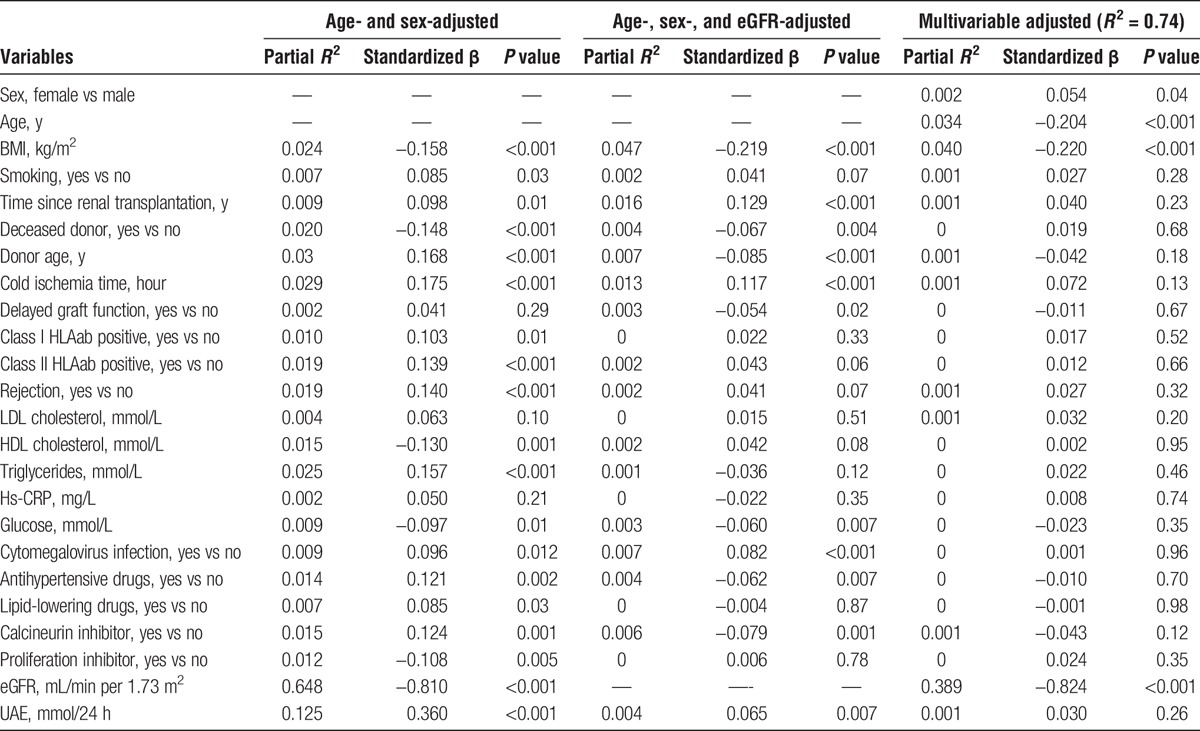
Table 2 shows the associations between log-transformed pro-ENK levels and variables of interest adjusted for (1) age and sex; (2) age, sex, and eGFR; and (3) multiple variables of interest. In the analyses adjusted for age and sex, pro-ENK levels were positively and significantly associated with current smoking, time since renal transplantation, donor age, cold ischemia time, positive class I and II HLAab, triglycerides, cytomegalovirus infection, antihypertensive drug use, use of lipid-lowering drugs and calcineurin inhibitors, and UAE. Significant inverse associations with pro-ENK, independent of age and sex, were observed for BMI, donor status, HDL cholesterol, serum glucose, use of proliferation inhibitors, and eGFR. After further adjustment for eGFR, time since renal transplantation, cold ischemia time, cytomegalovirus infection, and UAE were positively associated with pro-ENK levels, whereas BMI, donor status, donor age, delayed graft function, serum glucose, antihypertensive drug use, and use of calcineurin inhibitors were inversely associated with pro-ENK. When adding all the variables in the multivariable model, pro-ENK was significantly positively associated with female sex, whereas inverse associations were observed with age, BMI, and eGFR. The multivariable model had an adjusted R2 of 0.74, with eGFR being the most important contributing determinant (partial R2 = 0.39), indicating that a large percentage of circulating pro-ENK levels may be explained by eGFR.
TABLE 2.
Multivariable linear regression analysis with log-transformed plasma pro-ENK as dependent variable in 664 RTRs
Pro-ENK in Kidney Donors
We included 95 kidney donors (Table S1, SDC, http://links.lww.com/TXD/A46). Mean age of the healthy donors was 52.4 ± 9.7 years, and 40% of donors were male. Median mGFR before donation was 112 mL/min (IQR, 98-133 mL/min), with a median pro-ENK concentration of 48 pmol/L (IQR, 42-55 pmol/L), whereas the median mGFR postdonation was 72 mL/min (IQR, 64-83 mL/min), with a median pro-ENK concentration of 74 pmol/L (IQR, 63-82 pmol/L). Combining data from pre and postdonation, pro-ENK was inversely correlated with mGFR (rs = −0.74, P < 0.001; Figure S1, SDC, http://links.lww.com/TXD/A46), eGFR (rs = −0.68, P < 0.001), and creatinine clearance (rs = −0.62, P < 0.001), and positively correlated with serum creatinine (rs = 0.60, P < 0.001), but not correlated with UAE (rs = −0.03, P = 0.64). The percent change in pro-ENK (between predonation and postdonation) was also inversely associated with the percent change in mGFR (rs = −0.25, P = 0.01), whereas the percent change of pro-ENK was not associated with the percent change in UAE (rs < 0.001, P = 0.99).
Association of pro-ENK With Risk of Graft Failure and All-Cause Mortality in RTR
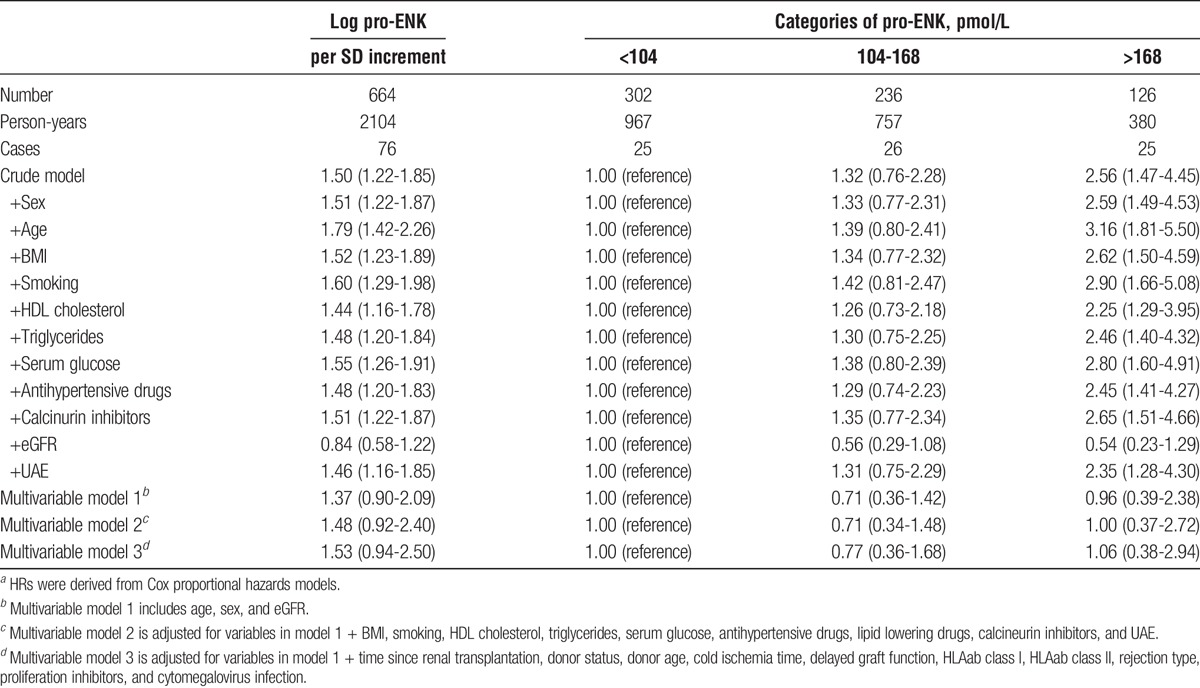
Median follow-up was 3.1 years (IQR, 2.6-3.9 years), During follow-up, 45 RTR developed graft failure and 76 died. Causes of graft failure are presented in Table S2, SDC, http://links.lww.com/TXD/A46. Median follow-up was 3.1 (2.7-3.9) years among RTR not developing graft failure, and 1.9 (0.7-2.4) years among RTR developing graft failure. In the crude analysis without adjusting for covariates, higher pro-ENK was associated with increased risk (HR per 1-SD increment of log-transformed pro-ENK; 95% CI) of developing graft failure (4.80; 3.55-6.48; Table 3; Figure 2A, log-rank P < 0.001). After adjustment for age, sex, and eGFR, the association with risk of graft failure remained (HR, 2.36; 95% CI, 1.37-4.06; Table 3, Figure 3A). Further adjustment for potential confounders did not materially affect the association of pro-ENK with increased risk of developing graft failure (multivariable model 2: 2.15; 1.08-4.29; multivariable model 3: 2.34; 1.20-4.56; Table 3). The ROC curves (Figure 4) showed areas under the curve of 0.87 (95% CI, 0.82-0.93), 0.89 (95% CI, 0.84-0.94) and 0.89 (95% CI, 0.84-0.94), respectively for predicting risk of graft failure. When analyzing the additional prognostic information of pro-ENK for the prediction of graft failure by comparing Harrell’s C statistics of prediction models with and without inclusion of pro-ENK, no significant difference was observed between both models (P for comparison of multivariable model 1 with and without inclusion of pro-ENK = 0.84; Table S3, SDC). However, when investigating differences in the −2 Log Likelihood of models with and without inclusion of pro-ENK, the −2 Log Likelihood significantly improved with pro-ENK included in the model (P for comparison of multivariable model 1 with and without inclusion of pro-ENK = 0.002; Table S3, SDC, http://links.lww.com/TXD/A46), indicating that the models including pro-ENK had a higher predictive capacity.
TABLE 3.
Association between plasma pro-ENK and risk of graft failure in 664 RTRs.a
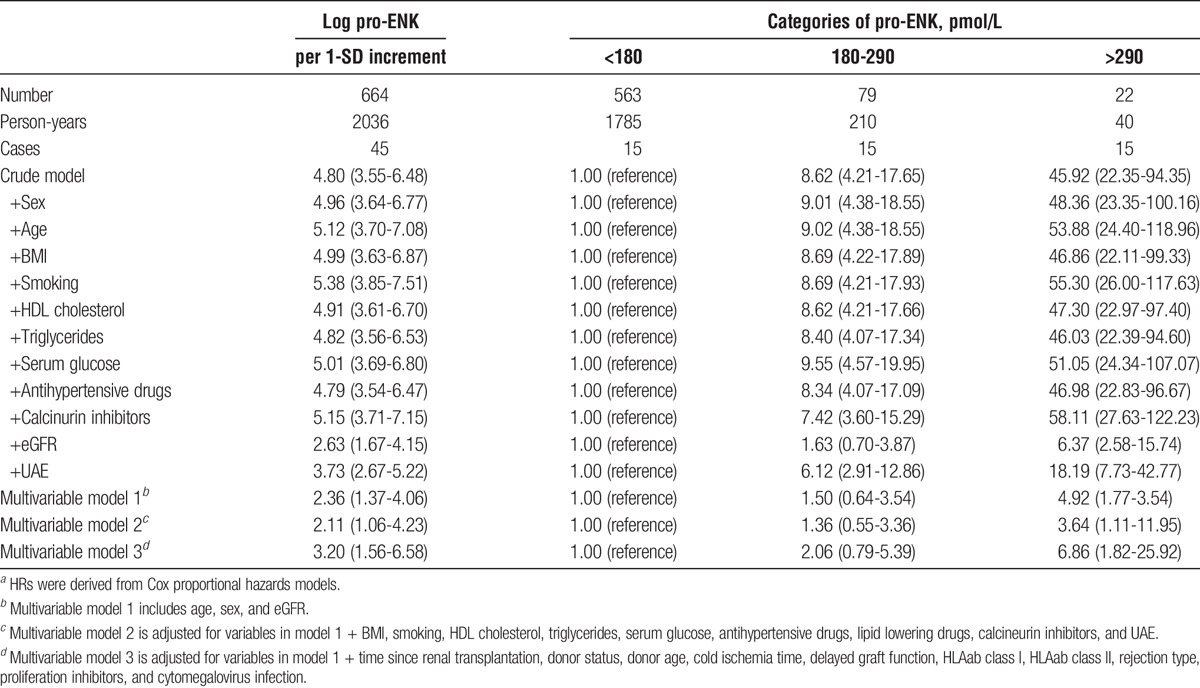
FIGURE 2.
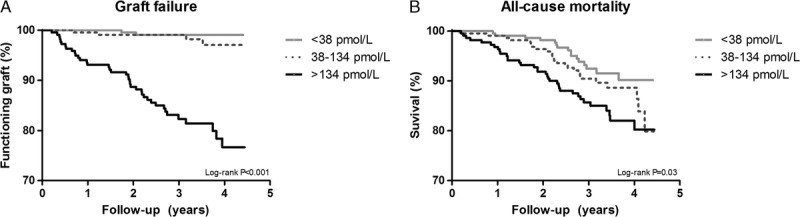
Kaplan Meier curves for graft failure (A) and all-cause mortality (B) per tertiles of plasma pro-ENK in 664 RTRs.
FIGURE 3.
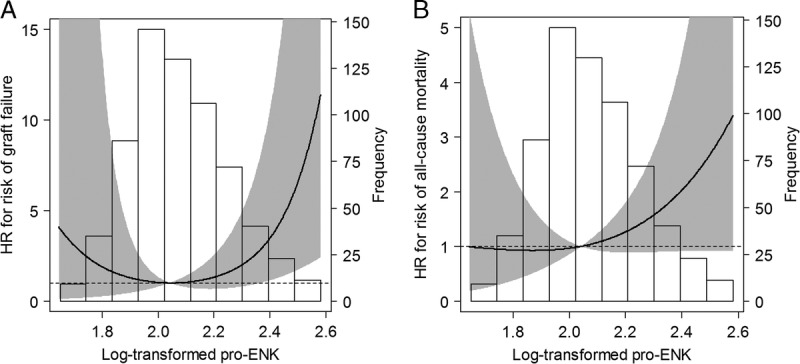
Associations of plasma pro-ENK with risk of graft failure (A) and risk of all-cause mortality (B) in 664 RTRs. Data were fit by Cox proportional hazards regression models based on restricted cubic splines with 3 knots and adjusted for age, sex, and eGFR. The grey areas indicate the 95% CIs. The spline curve is truncated at the 0.5th and 99.5th percentile of the distribution curve. Reference standard for the log-transformed pro-ENK was 2.04. P values for nonlinear association are P = 0.10 for panel A, and P = 0.36 for panel B.
FIGURE 4.
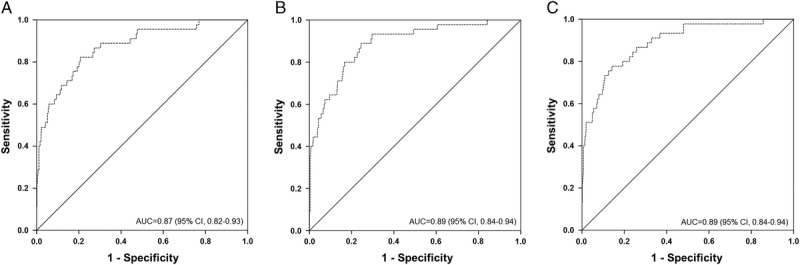
ROC curves of the association of pro-ENK with risk of graft failure in 664 RTRs. Dashed lines indicate the empirical curve, solid diagonal lines indicate no discrimination. Panel A represents the AUC for pro-ENK only. Panel B represents the AUC for age, sex, and eGFR. Panel C represents the AUC for age, sex, eGFR, and pro-ENK. AUC, area under the curve.
In crude analysis, higher pro-ENK was associated with increased risk of all-cause mortality (HR, 1.50; 95% CI, 1.22-1.85, Table 4, Figure 2B). However, after adjustment for age, sex, and eGFR, this association was not significant anymore (1.37; 0.90-2.09; Table 4; Figure 3B). Further adjustment for other variables did not materially change the association (multivariable model 2: 1.45; 0.83-2.53; multivariable model 3: 1.49; 0.92-2.40; Table 4).
TABLE 4.
Association between plasma pro-ENK and risk of all-cause mortality in 664 RTRs.a
DISCUSSION
In this prospective cohort study of RTR, we investigated the association of pro-ENK with chronic kidney injury and long-term outcome. Our results show that plasma pro-ENK is associated with kidney function as reflected by correlations with eGFR and UAE in RTR and with mGFR in healthy kidney donors. Furthermore, our results demonstrate that high pro-ENK is associated with increased risk of graft failure independent of eGFR, UAE, and other potential determinants of graft failure. Pro-ENK was not independently associated with risk of all-cause mortality.
Our data are consistent with the observations of Smith et al, Zoccali et al, and Marino et al9,10,19,20 that plasma enkephalins are markedly elevated in patients with chronic or acute renal failure compared to healthy subjects. We found pro-ENK concentrations to be lower in healthy kidney donors compared to RTR, both before and after donation. After donation, pro-ENK concentrations increased in the healthy donors, whereas their kidney function decreased after donation. The correlations of pro-ENK with eGFR in RTR and with mGFR in healthy donors were very strong (rs = −0.80 and rs = −0.74, respectively), and are in line with prior studies which also included patients with acute or chronic renal failure.9,10,19,20
Next to kidney function as the major determinant of pro-ENK observed in previous studies as well as in the present study, also age, sex, and BMI were significantly associated with pro-ENK after multivariable adjustment. These findings are consistent with previous literature.8,11,21 In a diabetic population, in healthy men and women, and in patients with acute myocardial infarction positive associations of pro-ENK were observed with age, female sex, and an inverse associations were observed for pro-ENK with BMI, crude8,11 or multivariable adjusted with inclusion of albuminuria as marker of kidney function.21
This study is the first to prospectively examine the potential association of pro-ENK with risk of graft failure and all-cause mortality in RTR. In a recent study of Schulz al,11 the association of pro-ENK with changes in renal function per year and risk of developing CKD was examined in 2568 participants of the Malmö Diet and Cancer study. They observed that high levels of plasma pro-ENK were associated with deterioration of kidney function and incidence of CKD. In a study of Shah et al, preoperative pro-ENK was associated with postoperative AKI in patients undergoing cardiac surgery.20 They hypothesized that the association reflects common cellular mechanisms, including inflammation, which causes AKI and pro-ENK release into the circulation. However, in a study of Marino et al, pro-ENK was associated with presence and severity of AKI in hospitalized patients with sepsis, independent from inflammation, as opposed to the marker neutrophil gelatinase-associated lipocalin.9
In the study of Marino et al,9 most patients without AKI or at risk had pro-ENK levels below the 99th percentile of the normal range (80 pmol/L). In our present study, higher risk of graft failure was only observed for RTR with the highest pro-ENK concentrations (>134 pmol/L, Figure 2), which are increased concentrations compared to the median pro-ENK concentrations in the healthy kidney donors (48 pmol/L).
The strong correlations of pro-ENK with eGFR and mGFR suggest that pro-ENK is a good marker for renal function. However, in the present study we also observed an association of pro-ENK with risk of developing graft failure in RTR, independent of eGFR. These results could indicate an independent and added value of pro-ENK in the identification of RTR at risk for graft failure. Arguably, the association observed could also be the result of residual confounding with respect to pro-ENK as a marker for renal function in addition to eGFR as a marker for renal function. Importantly, in the genome wide association study performed by Schulz et al,11 they identified genetic variation at the PENK locus that associated with higher pro-ENK levels and with longitudinal deterioration of kidney function and higher incidence CKD, suggesting a causal relationship between pro-ENK and CKD. When comparing prediction models by means of Harrell’s C statistics and ROC curves for models with and without inclusion of pro-ENK, we found no significant difference in predictive value for the model with inclusion of pro-ENK compared to the model without inclusion of pro-ENK. Like ROC curves for cross-sectional analyses, their time-to-event equivalent of Harrell’s C-statistics, is based on ranks rather than on continuous data, making them very insensitive for detection of differences.22,23 To avoid injudicious discarding of otherwise promising markers, it is therefore currently recommended to also compare prediction models by means of more sensitive methods, like the −2 Log Likelihood.22,23 Using this method, we indeed found a significant difference between both prediction models, indicating that pro-ENK adds prognostic value.
Because of the small molecular weight of pro-ENK (4586.60 g/mol), it is very likely that it is mainly cleared by the kidney. The association of enkephalins with renal disease therefore likely implies impaired clearance or increased production of enkephalins in renal disease. Besides inflammation, various other physiological functions have been shown to be modulated by enkephalins, including processes of cell growth, differentiation, and apoptosis.4,6,24,25 In an experimental study in conscious Sprague-Dawley rats, activation of the delta opioid system by infusion of a delta opioid receptor agonist stimulated kidney function.26 We could therefore speculate that an increment in pro-ENK concentrations could reflect counteracting the decreasing functionality of the kidney by promoting kidney function. However, further experimental studies blocking or stimulating the opioids receptors are needed to investigate the potential role of pro-ENK in this relationship.
A limitation of this study is that pro-ENK was measured only once at baseline and, therefore, we could not consider changes over time in pro-ENK concentrations. However, when the intraindividual variability of variables is considered, this results in strengthening of associations.27,28 Therefore, our use of a single pro-ENK measurement at baseline rather than multiple ones, will likely provide an underestimation of the true effect. Second, we only had data on eGFR as measure of kidney function in RTR. This is a less precise measure for renal function compared to mGFR. Third, we did not have complete data on specific cause of chronic allograft dysfunction (ie, antibody-mediated rejection of transplant glomerulopathy) since it is not standard to perform a biopsy to discern cause of graft failure mostly because it is then judged to not have any clinical consequences or too late in course of decline of renal function to allow for obtaining conclusive material. Fourth, we did not have information about whether the HLAab were donor-specific. Finally, our results of an association between pro-ENK and risk of graft failure may not be readily generalizable to newly transplanted patients, because of the relatively mature stage of sampling of the RTR (all RTR >1 year after transplantation, with the majority >5 years).
Strengths of this study are the prospective design, the relatively large cohort of a specific patient group consisting of well-characterized, stable RTR and the complete follow-up for both endpoints.
In conclusion, plasma pro-ENK is associated with kidney function as reflected by correlations with eGFR and UAE in RTR and with mGFR in healthy kidney donors. In addition, pro-ENK was independently associated with increased risk of graft failure in RTR. Pro-ENK may aid in early identification of RTR at risk for late graft failure who could benefit from closer monitoring.
Supplementary Material
Footnotes
Published online 7 June, 2017.
Funding Generation of the cohort was made possible by a grant from the Dutch Top Institute Food and Nutrition.
Conflicts of interest A.B. has shares in and is CEO of sphingotec GmbH, the company providing and having patent rights on the pro-ENK assay. O.H. and J.S. are employed by sphingotec GmbH.
L.M.K., O.H., J.S., A.B., R.Ad.B., and S.J.L.B. participated in the concept and design of the study. E.vd.B. and S.J.L.B. participated in the acquisition of the data. L.M.K. and O.H. participated in the statistical analyses and writing of the first draft of the article. J.S., A.B., R.T.G., M.M.J., R.Ad.B., and S.J.L.B. participated in providing intellectual content of critical importance to the work described. All authors approved to final the version of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. [DOI] [PubMed] [Google Scholar]
- 2.Hua H, Lu C, Li W, et al. Comparison of stimulating effect on subpopulations of lymphocytes in human peripheral blood by methionine enkephalin with IL-2 and IFN-γ. Hum Vaccin Immunother. 2012;8:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuong C, Van Uum SH, O'Dell LE, et al. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ovadia H, Magenheim Y, Behar O, et al. Molecular characterization of immune derived proenkephalin mRNA and the involvement of the adrenergic system in its expression in rat lymphoid cells. J Neuroimmunol. 1996;68:77–83. [DOI] [PubMed] [Google Scholar]
- 5.Drolet G, Dumont EC, Gosselin I, et al. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. [DOI] [PubMed] [Google Scholar]
- 6.Denning GM, Ackermann LW, Barna TJ, et al. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008;29:83–92. [DOI] [PubMed] [Google Scholar]
- 7.Ernst A, Kohrle J, Bergmann A. Proenkephalin A 119–159, a stable proenkephalin A precursor fragment identified in human circulation. Peptides. 2006;27:1835–1840. [DOI] [PubMed] [Google Scholar]
- 8.Ng LL, Sandhu JK, Narayan H, et al. Proenkephalin and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2014;63:280–289. [DOI] [PubMed] [Google Scholar]
- 9.Marino R, Struck J, Hartmann O, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol. 2015;28:717–724. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Ciccarelli M, Mallamaci F, et al. Plasma met-enkephalin and leu-enkephalin in chronic renal failure. Nephrol Dial Transplant. 1987;1:219–222. [PubMed] [Google Scholar]
- 11.Schulz CA, Christensson A, Ericson U, et al. High level of fasting plasma proenkephalin-A predicts deterioration of kidney function and incidence of CKD. J Am Soc Nephrol. 2017;28:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg E, Engberink MF, Brink EJ, et al. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol. 2012;7:1811–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Berg E, Pasch A, Westendorp WH, et al. Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol. 2014;25:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendel-Vos GC, Schuit AJ, Saris WH, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56:1163–1169. [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apperloo AJ, de Zeeuw D, Donker AJ, et al. Precision of glomerular filtration rate determinations for long-term slope calculations is improved by simultaneous infusion of 125I-iothalamate and 131I-hippuran. J Am Soc Nephrol. 1996;7:567–572. [DOI] [PubMed] [Google Scholar]
- 17.Visser FW, Muntinga JH, Dierckx RA, et al. Feasibility and impact of the measurement of extracellular fluid volume simultaneous with GFR by 125I-iothalamate. Clin J Am Soc Nephrol. 2008;3:1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith R, Grossman A, Gaillard R, et al. Studies on circulating met-enkephalin and beta-endorphin: normal subjects and patients with renal and adrenal disease. Clin Endocrinol (Oxf). 1981;15:291–300. [DOI] [PubMed] [Google Scholar]
- 20.Shah KS, Taub P, Patel M, et al. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin Nephrol. 2015;83:29–35. [DOI] [PubMed] [Google Scholar]
- 21.van Hateren KJ, Landman GW, Arnold JF, et al. Serum proenkephalin A levels and mortality after long-term follow-up in patients with type 2 diabetes mellitus (ZODIAC-32). PLoS One. 2015;10:e0133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE. Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- 23.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. [DOI] [PubMed] [Google Scholar]
- 24.McTavish N, Copeland LA, Saville MK, et al. Proenkephalin assists stress-activated apoptosis through transcriptional repression of NF-kappaB- and p53-regulated gene targets. Cell Death Differ. 2007;14:1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awad H, Abas M, Elgharably H, et al. Endogenous opioids in wound-site neutrophils of sternotomy patients. PLoS One. 2012;7:e47569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sezen SF, Kenigs VA, Kapusta DR. Renal excretory responses produced by the delta opioid agonist, BW373U86, in conscious rats. J Pharmacol Exp Ther. 1998;287:238–245. [PubMed] [Google Scholar]
- 27.Koenig W, Sund M, Frohlich M, et al. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA augsburg studies, 1984 and 1987. Am J Epidemiol. 2003;158:357–364. [DOI] [PubMed] [Google Scholar]
- 28.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


