Abstract
Background
Beneficial aspects of solid organ transplantation, which encompass survival benefit, improved quality of life, and cost efficacy, have been clearly demonstrated. However, regional and ethnic differences require further studies to identify prognostic factors and transplant outcomes against various backgrounds. After previous efforts of a nationwide, retrospective study on the kidney transplant outcomes in Korea, a new prospective-designed version of the Korean Organ Transplantation Registry (KOTRY) was launched in 2014.
Methods
Cohorts of kidney, liver, heart, lung, and pancreas transplantation were developed. Data on demographics, comorbid conditions, laboratory tests, including tissue typing and panel reactive antibody tests, immunosuppressive regimen followed, concentration and dosage of immunosuppressants, allograft rejection type, infectious events, cardiovascular outcomes, malignancies, donor comorbidity, and outcomes of living donors are collected. Longitudinal data collection is based on a regular annual interval, and blood samples are collected before organ transplantation and again at 1 and 3 years posttransplantation. To enhance data quality, a predefined data verification system operates on a Web-based database, and transplant center users receive regular education about updates. Data are cleansed thrice a year, and feedback given to centers about outlier values and missing data. Annual auditing is conducted.
Results
Currently, 59 centers are participating in KOTRY. The estimated annual enrollment is more than 2000 cases.
Conclusions
KOTRY, as a systematic Korean transplant cohort, is expected to provide important information on Asian organ transplantation. The processes used to establish KOTRY provide a good model for launching new nationwide transplant cohort studies.
Solid organ transplantation is a lifesaving treatment option for patients with organ failure, improves quality of life, and reduces health-care cost.1-6 However, regional and ethnic differences require further studies to identify prognostic factors and transplant outcomes against various backgrounds. For instance, a major cause of death in Western kidney transplantation recipients is cardiovascular disease; however, the leading cause of death in some Asian reports was infection, and the reported incidence of cardiovascular disease was lower than expected, suggesting that the optimal management and immunosuppression protocol might differ for patients of different ethnic and regional backgrounds.7,8
According to a report from the Global Database on donation and transplantation (GODT), living donors represent the major source of transplanted organs in Asia. In the past, living liver or kidney transplantation were in the majority in Korea. However, since the legislation of transplantation law in 1999, the process of deceased donor organ transplantation has been systematized, by adopting a centralized organ allocation system, introducing an organ-procurement organization system, and implementing a public organ donation agency.9,10 All these efforts have led to a nearly eightfold increment in deceased donor organ transplantation, from 233 cases in 2000 to 1989 cases in 2015, in parallel with the increment of deceased donors from 1.09 per million population in 2000 to 9.72 per million population in 2015.
Along with the expansion of transplantation quantity, many efforts have been made to study nationwide posttransplant outcomes; these included the retrospective Korean Organ Transplantation Registry (Retro-KOTRY) study.11 The Retro-KOTRY was initiated by the Korean Society of Transplantation, and the study included 91.9% of total kidney transplantations in Korea between January 2009 and September 2012. The 1- and 3-year survival rates were 98.9% and 96.0%, and graft survival was 98.6% and 93.7%, respectively. Through that nationwide study, we were able to identify epidemiological trends, such as a rapid increase in ABO-incompatible kidney transplantation, with utilization of more spousal donors, and a more liberal or routine use of the expanded donor criteria. After previous efforts, we are now conducting a second retrospective study for the period between October 2012 and March 2014. More importantly, a new prospective-designed version of KOTRY was launched in 2014 (Figure 1). KOTRY is composed of 5 solid organ transplantation cohorts, including those of kidney, liver, heart, lung, and pancreas transplants. Herein, we present the design and protocol of KOTRY, which is expected to answer the following fundamental questions:
FIGURE 1.
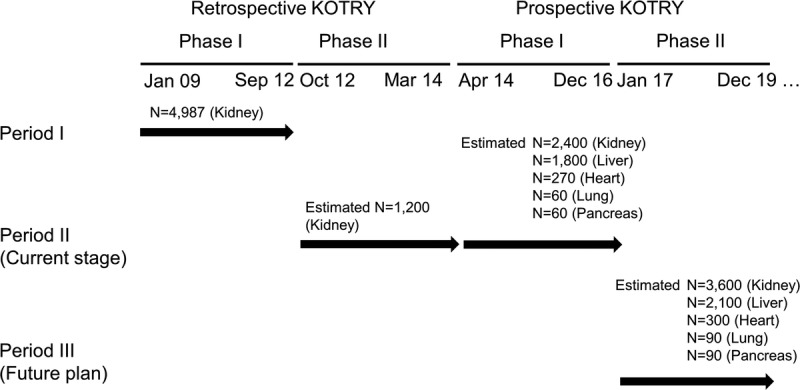
Overview of the KOTRY according to the history of registry development.
What is the primary indication for solid organ transplantation in the Korean population?
How severe is the comorbidity burden of solid organ transplantation?
What are the immediate postsurgical risks of solid organ transplantation?
What is the long-term course of solid organ transplantation?
What is the most common cause of death after solid organ transplantation?
What is the most common cause of allograft failure?
What is the prevalence of induction and maintenance immunosuppression?
What is the prevalence of posttransplant comorbidities?
What are the genetic factors associated with the deterioration of allograft function?
What are the biomarkers that predict the deterioration of allograft function?
What are the short- and long-term courses of living donors?
METHODS AND RESULTS
Study Organization
The KOTRY consists of 59 participating centers (30 centers for kidney, 15 for liver, 4 for heart, 5 for lung, 5 for pancreas), a central coordination unit, and a medical research coordinating center (MRCC). The organizational structures include the organ-specific committee, executive committee, and steering committee. A central coordination unit leads the study process, checks enrollment status weekly, and gives feedback to the participating centers. The MRCC is in charge of data validation and statistical consultation. The Korean National Research Institute of Health (KNIH) developed and offered a global Web-based electronic data capturing system, named iCReaT. KNIH also participates in the quality assurance of the collected data, regular surveillance of study conductance process, and the management and improvement of the electronic data capturing system. Biospecimen collection, storage, and quality control are done under contract with LabGenomics, and part of the deposited biosamples is transferred to KNIH for backup and future collaboration. All of the activities are managed by the KOTRY Foundation.
Exclusion Criteria
Recipients younger than 19 years are excluded. Except simultaneous pancreas-kidney cotransplantation, those undergoing simultaneous multiorgan transplantation are excluded to ensure the homogeneity of graft-related outcome. However, sequential organ transplantations are not excluded. For liver transplantation, there is no exclusion criterion for age.
Enrollment and Informed Consent
For living donor organ transplantation, both the donor and recipient are required to register at KOTRY before transplantation. The medical records of eligible individuals are reviewed after receiving their informed consent. Blood samples are taken for DNA and serum/plasma storage before transplantation. In deceased donor organ transplantation, informed consent is taken from the recipient. Under the strengthened data protection laws of Korea, the social security identification number cannot be collected during the KOTRY. However, for outcome matching with the Korean national statistical office data or the centralized health insurance claims data, KOTRY receives an optional informed consent for the use of collected data for study of secondary outcomes. To achieve the best standardized process, we surveyed the opinions of each individual institutional review board, then submitted a standardized protocol and standardized consent format. This study was approved by the institutional review boards of all participating centers and was performed in accordance with the Declaration of Helsinki and the Declaration of Istanbul.
Study Design and Collected Variables
The KOTRY collects solid organ transplantation data to analyze epidemiological trends, graft-related outcomes, and patient mortality. In total, data on 5014 variables are collected, which are summarized in Tables 1-3. The kidney aspect involves a total of 950 variables, which are composed of 12 domains of recipient data, 3 domains of donor baseline data, and 4 domains of living donor follow-up data. The liver aspect involves 523 variables in total, which consist of 13 domains of recipient data, 4 domains of donor baseline data, and 3 domains of living donor follow-up. The heart aspect involves 886 variables with 13 domains of recipient data, and 3 domains of donor baseline data. The lung aspect involves 1495 variables with 22 domains of recipient data, and 3 domains of donor baseline data. The pancreas aspect involves 1160 variables with 16 domains of recipient data, 4 domains of donor baseline data, and 3 domains of living donor follow-up. Each domain was constructed as a single sheet in electronic case-report format (CRF) on a Web-based system (iCReaT). Longitudinal data collection is based on a regular annual interval. For the collection of early comorbidities and adverse outcome, different time points were selected according to each organ’s clinical characteristics.
TABLE 1.
KOTRY data collection formats for organ recipients: common variables in all organ transplantation
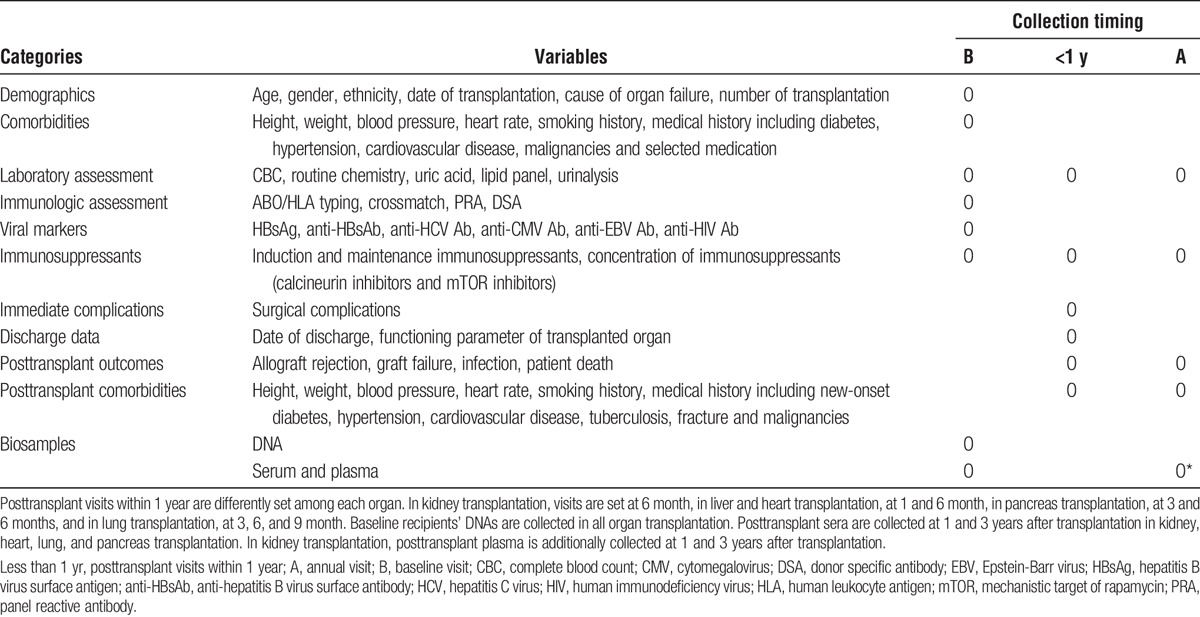
TABLE 3.
Organ-specific information
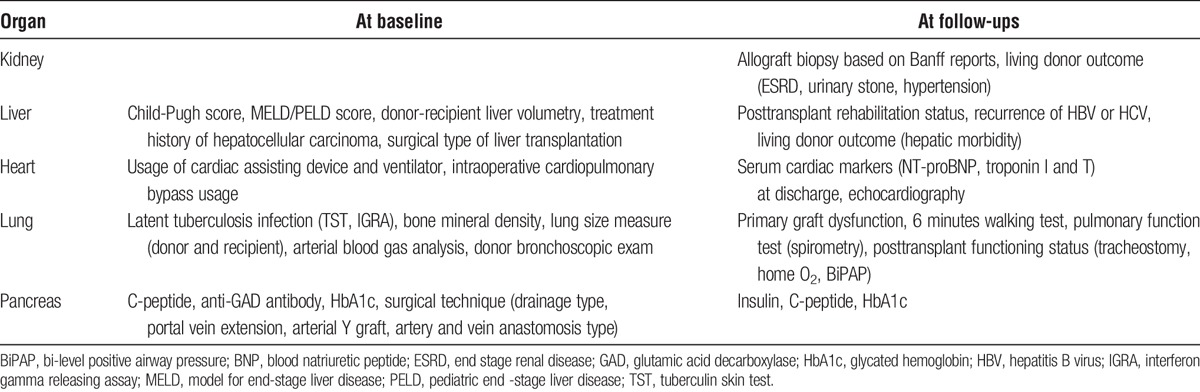
TABLE 2.
KOTRY data collection formats for organ donors: common variables in all organ transplantation
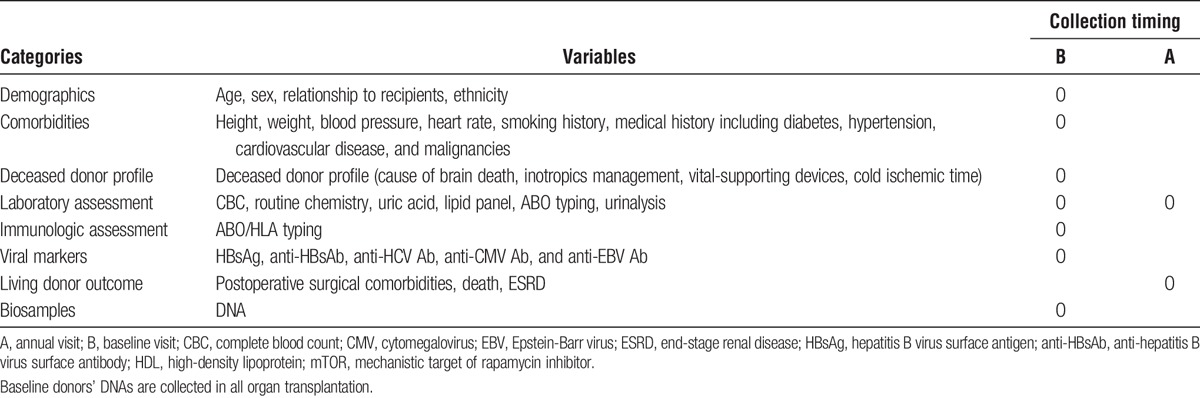
To account for the time-varying nature of posttransplantation comorbidity and to deal with repeated events, we collected posttransplantation comorbidity at every follow-up visit, which allows the analysis of comorbidity duration, and the effects of new-onset comorbidities and their duration on posttransplant outcomes. For example, the duration of transient new-onset diabetes after transplantation or repeated incidence of cardiovascular disease can be collected. Follow-up records will be tracked up to the patients’ deaths. However, graft-related variables, including rejection, graft function, and general laboratory profiles, will be tracked until graft loss. To minimize follow-up loss, newsletters regarding registration status and follow-up performance are periodically sent to each participating center and a transfer system is used. If a patient underwent transplantation in center A, and was then followed up by center B which also participated in KOTRY, the transfer system allows center B to input that patient’s data. To increase the follow-up rate of living donors, the KOTRY emphasizes the importance of follow-up of living donors to each participating center’s physicians and surgeons.
Biosamples
DNA samples from each donor and recipient are collected before organ transplantation. In kidney, heart, lung, and pancreas transplantation, sera are collected from recipients at baseline, before transplantation, and again at 1 and 3 years after organ transplantation. Baseline samples are collected in liver transplantation recipients. From 2017, additional plasma samples from the recipients are collected before kidney transplantation, and again at 1 and 3 years postkidney transplant.
Study Outcomes
The primary outcomes are graft failure and patient death. In kidney transplantation, graft failure is defined as sustained (more than 3 months) dependency on dialysis. In liver, heart, and lung transplantation, graft failure is defined as patient death or retransplantation. Pancreas graft failure is defined as insulin dependence or death with a full or partially functioning graft.
Pathology data collected included acute or chronic rejection12 and other diagnoses, such as virus infection and calcineurin inhibitor toxicity. Definitions of the major posttransplantation outcomes are as follows: cardiovascular disease is defined as cardiovascular death, myocardial infarction, ischemic heart disease with relevant clinical evidence (accompanied by therapeutic intervention or objective findings), new-onset congestive heart failure requiring hospital admission and arrhythmia. Stroke includes nontraumatic hemorrhagic or ischemic brain disease confirmed by computed tomography or magnetic resonance image. Tuberculosis is defined as clinically active disease, as evidenced by typical chest radiography imaging, microbiological confirmation, or treatment with anti-tuberculosis drugs. Causes of death are classified into cardiovascular, sudden cardiac death, infection, malignancy, liver disease, accident, suicide, and others.
Living donor outcomes are collected for living liver or kidney transplantation cases. Death, cause of death, and surgical morbidities are collected in both liver and kidney transplantations. Newly developed diseases, including diabetes, hypertension, and urinary stones, are collected in living kidney transplantation donors.
Data Validation
Quadruple layers of data validation are available. First, a predefined automated data validation system is used at data input, to prevent simple errors. Automated data validation system checks are implemented for essential data elements, to minimize missing variables, and have predefined allowed data ranges, to reduce extreme outliers due to simple input error. Additionally, an automated data validation system guide is used to prevent entering of values inconsistent with other variables, by opening or blocking data fields in screens after a logical test of preentered data values. Second, manual data validation is performed quarterly by the MRCC by feedback to each participating center. Third, during the outcome adjudication meeting, the distribution of major outcomes is discussed, and outlier values are sent to each investigator. Finally, annual auditing are conducted for all participating centers, to survey their status, including ethical study conductance, adherence to the standardized study protocol, and direct comparison of randomly selected data with the original medical record. These processes are conducted using the Registries for Evaluating Patient Outcomes tool by the Agency for Healthcare Research Quality.13
Building Statistical Analysis Files and Response to the Data Request
A statistical analysis file is built thrice a year, after a quarterly data cleansing process. When the participating center requests their own data, the last validated statistical analysis file is sent to the requesting center. We aim at a feedback time of 4 hours, in parallel with the standard operating protocol of Scientific Registry of Transplant Recipients.14 To request all centers’ data, items of the requested variable are released as a deidentified set (at patient- and center-level) after approval of the organ committee in KOTRY, after review of the study proposal. Each center has access to the main database located in KNIH and can download their own data set; however, this is not recommended due to network traffic and incompleteness of data validation. Currently, KOTRY focuses on the ease of data cleansing through an attached online automatic plotting system, and on giving more informative feedback to the participating center, and finally on enforcing information technology-security issues.
Statistical Considerations
Descriptive data analysis will be conducted for baseline characteristics. To study outcomes, time-to-event analysis will be primarily used. Life-table methods or Kaplan-Meier curves will be used to represent allograft or patients’ survival, and time to major outcomes (cardiovascular disease, cancer, infection, acute rejection, and so on). For the competing nature of outcome events (eg, patient death vs cancer occurrence), competing risk models will be adopted for regression modeling.15 The multilevel characteristics of data were adjusted using a shared-frailty model,16 in which adjustment should be made for time-dependent confounders or different data hierarchies. To encompass the wide variability of allograft functional decline, the Bayesian smoother will be used.17 Longitudinal allograft functional changes and associated factors will be analyzed using a mixed linear model. Because the format of follow-up data is a repeated panel structure, the marginal structural model with time-varying confounder adjustment can be applied.18
Statistical Power
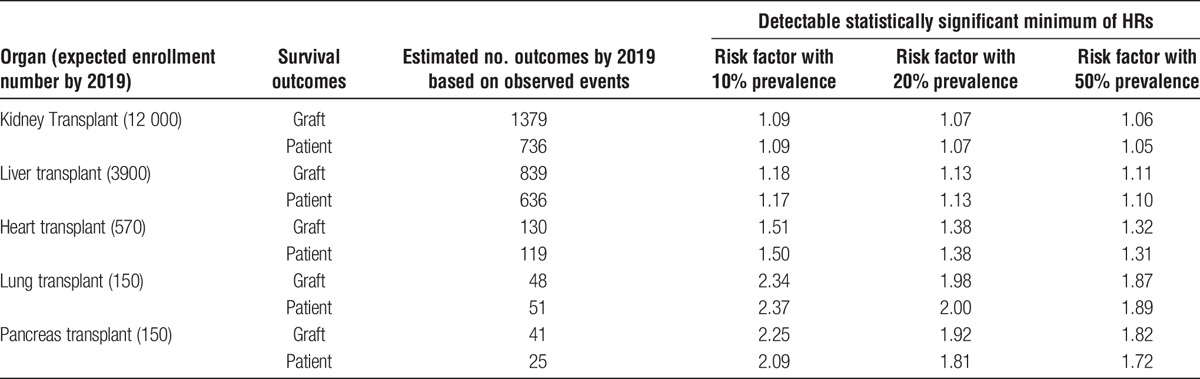
From 2017, new annual enrollments are estimated as 1200 for kidney, 700 for liver, 100 for heart, and 30 for lung and pancreas transplantation, respectively. In kidney transplantation, the previous Retro-KOTRY collected the data of 4987 kidney recipients, and the effort is ongoing to collect the missing information (approximately 1200 kidney recipient’s data) from the end of the previous Retro-KOTRY enrollment and the launch of the prospective KOTRY-kidney (Figure 1). With the assumption of attaining the patient enrollment plan, Table 4 shows the minimum hazard ratios (HRs) detectable at a given prevalence level of risk factors by 2019, using exponential models based on the 20-year patient and graft survival for solid organ transplants from the Organ Procurement and Transplantation Network.19 The KOTRY-kidney cohort is estimated to detect a relative risk of 1.05 and 1.06 for graft survival and patient survival, respectively, with a 50% prevalent risk factor, at 5% alpha error and 20% beta error in an analysis using a Cox regression model (Table 4). Similarly, the KOTRY-liver, heart, lung, and pancreas cohorts will be able to detect HRs of 1.11, 1.32, 1.87, and 1.82, respectively, for graft survival.
TABLE 4.
Minimum detectable increase in relative risk of graft survival, patient survival and acute rejection
Representativeness
In 2015, the total numbers of organ transplant centers and KOTRY-participating centers were as follows: for kidney, 30 of 66 centers participated in KOTRY; for liver, 15 of 44; for heart, 4 of 13; for lung, 5 of 7; for pancreas, 5 of 9. As large-volume centers joined KOTRY, the numbers of organ transplantations performed in KOTRY-participating centers were predominantly as follows: for kidney, 1565 (82.8%) of 1891; for liver, 1073 (77.1%) of 1392; for heart, 127 (87.6%) of 145; for lung, 61 (95.3%) of 64; for pancreas, 51 (86.4%) of 59.
DISCUSSION
The KOTRY is a prospective nationwide cohort. KOTRY will enroll new solid organ transplant patients and donors, collect detailed epidemiological data, and conduct rigorous data validation with the goal of providing the infrastructure for standardized posttransplant outcome research.20 KOTRY will collect detailed information regarding organ transplantation; however, every effort will be made to keep the variables concise and precise. Asia is a rapidly changing region, not only in terms of its fast-growing economy, but also in terms of lifestyle, disease prevalence, and an aging population structure.21,22 In Korea, changes are also found in the transplantation field. Deceased donor kidney transplantation comprised 20% of total kidney transplantation in 2000; however, it reached 45% in 2015. ABO-incompatible kidney transplantation has expanded rapidly,11 accounting for almost one third of living kidney transplantations (2016 interim data of KOTRY). The role of the registry in managing this changing epidemiology is invaluable.
A number of successful models for a solid organ transplant registry exists.23 The Collaborative Transplant Study (CTS) is a global transplant registry, contributing to the international reference source of transplant-related outcome study. CTS is based on voluntary international communication, and has contributed to the discovery of the clinical importance of HLA mismatching, and the study of rare-disease outcomes.24 The Scientific Registry of Transplant Recipients has presented good examples of data linkage analysis with an administrative database, allowing a change in the organ allocation system based on scientific evidence.14 Recent studies on living donor outcomes are also based on data matching, based on the United States Renal Data System reports, and are used for the establishment of further guidance.25,26 The Australia and New Zealand Dialysis and Transplant (ANZDATA) registry is the national registry of Australia and New Zealand for dialysis and kidney transplantation.27 In addition to many published articles, ANZDATA is also involved in quality assurance issues in countries, and gives feedback to the participating center.28,29 International Registry for Heart and Lung Transplantation is largest worldwide repository of heart and lung transplant activity.30 The Swiss Transplant Cohort Study is a recently-launched cohort, which has very similar characteristics to KOTRY.31 The Global Database on donation and transplantation is a database for the infrastructure and legislation for global transplantation,32 which does not collect patient-level data.
The KOTRY is designed to play an academically oriented role, rather than act as an administrative registry. KOTRY includes the cause of death and the reasons of graft loss, to enable studying of the underlying mechanisms. KOTRY also collects detailed profiles on immunosuppression, to produce relevant data about optimized immunosuppression. The KOTRY attempts to collect longitudinal data: various long-term metabolic outcomes are collected, and the time-dependent change of covariates is also gathered, enabling more sophisticated analyses, including competing risk analysis, multilevel modeling, or marginal structural analysis.
As a mother cohort, KOTRY has many strengths. A large sample size is the most important feature as a mother cohort, because it offers a higher probability of obtaining a matched control. To ensure faster accumulation of data, KOTRY plans to merge previous Retro-KOTRY data into the prospective KOTRY kidney data in the future. Preciseness is another strength of KOTRY: a well-defined phenotype is more relevant than an administrative database or a claims database. The KOTRY focuses on width rather than depth, to encompass as many centers as possible; however, it also has details on selected features, such as biopsy reports describing the Banff staging system, infection sheets reporting causative organisms, and a detailed description of malignancy, including the location and type. With such details, KOTRY can efficiently provide a matched control for any future study. Playing a role as a study platform is another goal of KOTRY, and KOTRY is expected to provide the basis for future studies.
These characteristics are also strengths of a biosample cohort. All samples in KOTRY are centralized in terms of quality assessment and storage. The samples can be used as a whole for a validation study of potent candidate biomarkers, or be used only in selected cases for explorative studies in a nested case-control study design. All analyzed data will be stored in the KOTRY biomarker database, which can serve as a systems biology database, such as a phenotype-genotype database. Additional stepwise approaches are also possible, because KOTRY stores various types of samples to be prepared for multiomics studies.33,34 However, some issues, such as controlling for batch effect and early depletion of common phenotype samples, are challenges that KOTRY needs to overcome. Because biosamples are very limited resources, the steering committee of KOTRY has decided not to use a certain quota of biosamples in the preparation for long-term outcome analysis.
To summarize, the strengths of KOTRY are as follows: (1) It provides a systematic prospective solid organ transplant cohort in the Asian population. (2) It offers good phenotype characterization based on a detailed clinical history. (3) It validates data rigorously. (4) It offers statistical power from a large cohort, which enables the study of rare outcomes. (5) It is expected to serve as a mother cohort for future specific subcohorts. (6) It acts as a centralized collection, storage, and quality control facility for biosamples. (7) It collects living donor outcomes.
The authors expect that in practice, KOTRY will be the representative standard cohort for producing parametric indices regarding organ transplantation in Korea and for providing standard control data for other specified studies. Serving as a common data warehouse may allow participating researchers to be more involved in data validation of their own. Furthermore, as a systems biology database, KOTRY can deposit genotype data and biomarker data along with detailed phenotype data. As a forum of group discussion, KOTRY can produce study questions that are priorities in Korean and Asian transplantation. Finally, KOTRY can play a public role by providing an annual report to the general population and can perform a quality assurance role in the long term. To translate these hopes into reality, continuous participation and pertinent efforts are needed, and much remains to be done.
In conclusion, the KOTRY is a nationwide solid organ transplantation cohort registry in Korea. To serve as a valuable national and international resource, continuous efforts are needed. The KOTRY, as a systematic Korean transplant cohort, is expected to provide important information on Asian organ transplantation. The processes used to establish the KOTRY represent a good model for launching of new nationwide transplant cohort studies.
Supplementary Material
ACKNOWLEDGMENTS
The authors appreciate the support and cooperation of the staffs of participating centers. The development of KOTRY was impossible without their efforts. The authors also appreciate invaluable advices from Gerhard Opelz, Caner Süsal, Bernd Döhller (CTS), Jeremy Chapman (University of Sydney), Stephen McDonald (ANZDATA), Robert Merion, Alan Leichtman (University of Michigan), and Peter Reese (University of Pennsylvania).
The KOTRY Participating Centers and Group Members:
• Clinical Center (Kidney)
○ Asan Medical Center: Duck Jong Han, Young Hoon Kim, Sung Shin
○ BHS Hanseo Hospital: Jin Min Kong, Hyuk Yong Kwon, Sung Hyun Son
○ Bong Seng Memorial Hospital: Joong Kyung Kim, Seong Min Kim, Joon Seok Oh
○ CHA Medical Center: Jung Jun Lee
○ Chonnam National University Hospital: Sang Young Chung, Soo Jin Na Choi
○ Chonbuk National University Hospital: Sung Kwang Park, Sik Lee
○ Chungnam National University Hospital: Kang Wook Lee, Dae Eun Choi
○ Ewha Womans University Mokdong Hospital: Seung-Jung Kim
○ Gachon University Gil Medical Center: Yeon Ho Park, Han Ro
○ Hallym University Kangdong sacred Heart Hospital: Ji Eun Oh, Hee Jung Jeon
○ Hanyang University Medical Center: Oh Jung Kwon
○ Inje University Busan Paik Hospital: Sun Woo Kang, Tae Hee Kim, Yeong Hoon Kim
○ Inje University Ilsan Paik Hospital: Young-Nam Roh
○ Keimyung University Dongsan Medical Center: Seungyeup Han
○ Korea University Anam Hospital: Cheol Woong Jung, Myung-Gyu Kim
○ Konkuk University Hospital: Jung Hwan Park
○ Kyung Hee University Hospital at Gangdong: Sang-Ho Lee
○ Kyung Hee University Hospital: Kyung-Hwan Jeong
○ Kyungpook National University Hospital: Chan-Duck Kim, Jang-Hee Cho
○ Maryknoll Medical Center: Dong Ryeol Lee, Jeongmyung Ahn
○ Pusan National University Hospital: Sang Heon Song, Harin Rhee
○ Pusan National University Yangsan Hospital: Dong Won Lee
○ Samsung Medical Center: Sung Joo Kim, Jae Berm Park, Hyo-Jun Park
○ Seoul National University Hospital: Curie Ahn and Jaeseok Yang
○ Seoul National University Bundang Hospital: Dong Wan Chae
○ Soon Chun Hyang University Hospital, Seoul: Jin Seok Jeon
○ The Catholic Univ. of Korea Seoul ST. Mary’s Hospital: Chul Woo Yang, Byung Ha Chung
○ Ulsan University Hospital, Ulsan: Hong Rae Cho, Sang Jun Park
○ Yeungnam University Hospital: Jong-Won Park
○ Yonsei University Severance Hospital: Kyu Ha Huh, Juhan Lee, Myoung Soo Kim, Beom Seok Kim, Seung Hwan Song
• Clinical Center (Liver)
○ Ajou University Hospital: Bong-Wan Kim
○ Asan Medical Center: Shin Hwang, Gi-Won Song
○ Chonbuk National University Hospital: Hee Chul Yu
○ Daegu Catholic University Medical Center: Dong Lak Choi, Joo Dong Kim
○ Ewha Womans University Mokdong Hospital: Geun Hong, Huisong Lee
○ Keimyung University Dongsan Medical Center: Koo Jeong Kang, Tae-Seok Kim
○ Konyang University Hospital: In Seok Choi
○ Korea University Anam Hospital: Dong-Sik Kim, Young-Dong Yu
○ Pusan National University Yangsan Hospital: Chong Woo Chu
○ Samsung Medical Center: Jong Man Kim, Choon Hyuck David Kwon
○ Seoul National University Hospital: Kwang-Woong Lee, Nam-Joon Yi, Hyeyoung Kim
○ Seoul National University Bundang Hospital: Ho-Seong Han, YoungRok Choi
○ The Catholic Univ. of Korea Seoul ST. Mary’s Hospital: Young-Kyoung You
○ Ulsan University Hospital, Ulsan: Yang Won Nah, Hyung-Woo Park
○ Yonsei University Severance Hospital: Dong Jin Joo, Myoung Soo Kim, Jae Geun Lee
• Clinical Center (Heart)
○ Asan Medical Center: Jae-Joong Kim, Sung-Ho Jung
○ Seoul National University Hospital: Hae-Young Lee, Hyun-Jai Cho
○ Samsung Medical Center: Eun-Seok Jeon, Jin-Oh Choi, Ga Yeon Lee
○ Yonsei University Severance Hospital: Seok-Min Kang, Jaewon Oh
• Clinical Center (Lung)
○ Asan Medical Center: Seung-Il Park, Tae Sun Shim, Sang-Bum Hong
○ Samsung Medical Center: Kyeongman Jeon, Byeong-Ho Jeong
○ Seoul National University Hospital: Young Tae Kim, Sun Mi Choi, Hyun Joo Lee
○ Pusan National University Yangsan Hospital: Hye Ju Yeo, Woo Hyun Cho
○ Yonsei University Severance Hospital: Hyo Cha Paik, Song Yee Kim, Jin Gu Lee
• Clinical Center (Pancreas)
○ Asan Medical Center: Young Hoon Kim
○ Hallym University Kangdong sacred Heart Hospital: Samuel Lee
○ Samsung Medical Center: Jae Berm Park, Kyo Won Lee
○ Seoul National University Hospital: Sang-Il Min
○ Yonsei University Severance Hospital: Myoung Soo Kim, Seung Hwan Song, Jae Geun Lee
• Statistical Collaborating Centers
○ MRCC: Joongyub Lee, Myoungjin Jan, Heejung Ahn
○ The KNIH, Centers for Disease Control and Prevention: Hyun-Young Park, Hae-Rim Choi
Footnotes
Published online 7 June, 2017.
This work was supported by a fund from the Research of Korean Centers for Disease Control and Prevention (2014ER630102).
The authors declare no conflicts of interest.
J.Y., and J.C.J. equally contributed to this work.
J.Y., J.C.J., and J.L. wrote the article. H.Y.P. and C.A. performed critical review of the article. J.Y., J.C.J, J.L., Y.H.K., H.C.P., J-J.K., H.Y.P., M.S.K., and C.A. contributed to study design, patient enrollment, study conductance, and data analysis. The KOTRY study group contributed to patient enrollment and study conductance.
Contributor Information
Collaborators: on behalf of the KOTRY study group
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 2.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. [DOI] [PubMed] [Google Scholar]
- 3.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252–259. [DOI] [PubMed] [Google Scholar]
- 4.Aubert O, Kamar N, Vernerey D, et al. Long term outcomes of transplantation using kidneys from expanded criteria donors: prospective, population based cohort study. BMJ. 2015;351:h3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salonen T, Reina T, Oksa H, et al. Cost analysis of renal replacement therapies in Finland. Am J Kidney Dis. 2003;42:1228–1238. [DOI] [PubMed] [Google Scholar]
- 6.von der Lippe N, Waldum B, Brekke FB, et al. From dialysis to transplantation: a 5-year longitudinal study on self-reported quality of life. BMC Nephrol. 2014;15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong JC, Ro H, Hwang YH, et al. Cardiovascular diseases after kidney transplantation in Korea. J Korean Med Sci. 2010;25:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noppakun K, Ingsathit A, Pongskul C, et al. A 25-year experience of kidney transplantation in Thailand: report from the Thai Transplant Registry. Nephrology (Carlton). 2015;20:177–183. [DOI] [PubMed] [Google Scholar]
- 9.Min SI, Ahn C, Han DJ, et al. To achieve national self-sufficiency: recent progresses in deceased donation in Korea. Transplantation. 2015;99:765–770. [DOI] [PubMed] [Google Scholar]
- 10.Min SI, Ha J. Recent progresses in organ donation and transplantation in Korea. Transplantation. 2015;99:2431–2433. [DOI] [PubMed] [Google Scholar]
- 11.Ahn C, Koo TY, Jeong JC, et al. Initial report of the Korean Organ Transplant Registry: the first report of national kidney transplantation data. Transplant Proc. 2014;46:425–430. [DOI] [PubMed] [Google Scholar]
- 12.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 13.Gliklich RE, Dreyer NA, Leavy MB. Registries for evaluating patient outcomes: a user's guide. 3rd ed Rockville, United States: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 14.Leppke S, Leighton T, Zaun D, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando). 2013;27:50–56. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.Ratcliffe SJ, Guo W, Ten Have TR. Joint modeling of longitudinal and survival data via a common frailty. Biometrics. 2004;60:892–899. [DOI] [PubMed] [Google Scholar]
- 17.Ciprian M, Crainiceanu DR, Wand MP. Bayesian analysis for penalized spline regression using WinBUGS. J Stat Softw. 2005;14. [Google Scholar]
- 18.Cole SR, Hernan MA, Margolick JB, et al. Marginal structural models for estimating the effect of highly active antiretroviral therapy initiation on CD4 cell count. Am J Epidemiol. 2005;162:471–478. [DOI] [PubMed] [Google Scholar]
- 19.R. Gruessner TJ, M. Porubsky A, Rana A, et al. Comparison of 20-year patient and graft survival for all types of solid organ transplants (Txs) [ATC abstract A771]. Am J Transplant. 2013;13. [Google Scholar]
- 20.Tong A, Budde K, Gill J, et al. Standardized outcomes in nephrology-transplantation: a global initiative to develop a core outcome set for trials in kidney transplantation. Transplant Direct. 2016;2:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman J. Transplantation practice has been particularly suited to national, regional, specialist and global registries. Transplant Rev (Orlando). 2013;27:37. [DOI] [PubMed] [Google Scholar]
- 24.Opelz G, Dohler B, Ruhenstroth A, et al. The Collaborative Transplant Study registry. Transplant Rev (Orlando). 2013;27:43–45. [DOI] [PubMed] [Google Scholar]
- 25.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Sang Y, Levey AS, et al. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald SP, Russ GR. Australian registries-ANZDATA and ANZOD. Transplant Rev (Orlando). 2013;27:46–49. [DOI] [PubMed] [Google Scholar]
- 28.Gray NA, Mahadevan K, Campbell VK, et al. Data quality of the Australia and New Zealand dialysis and transplant registry: a pilot audit. Nephrology (Carlton). 2013;18:665–670. [DOI] [PubMed] [Google Scholar]
- 29.McDonald SP. Australia and New Zealand dialysis and transplant registry. Kidney Int Suppl (2011). 2015;5:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehlik J, Edwards LB, Rowe A, et al. ISHLT International Registry for Heart And Lung Transplantation—three decades of scientific contributions. Transplant Rev (Orlando). 2013;27:38–42. [DOI] [PubMed] [Google Scholar]
- 31.Koller MT, van Delden C, Muller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol. 2013;28:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahillo B, Carmona M, Álvarez M, et al. Global Database on Donation and Transplantation: goals, methods and critical issues (www.transplant-observatory.org). Transplant Rev (Orlando). 2013;27:57–60. [DOI] [PubMed] [Google Scholar]
- 33.Lipska BS, Ranchin B, Iatropoulos P, et al. Genotype-phenotype associations in WT1 glomerulopathy. Kidney Int. 2014;85:1169–1178. [DOI] [PubMed] [Google Scholar]
- 34.Gadegbeku CA, Gipson DS, Holzman LB, et al. Design of the nephrotic syndrome study network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


