Abstract
Background
End-stage liver disease (ESLD) is the most common cause of secondary immunoglobulin A nephropathy (IgAN). Multiple mechanisms have been proposed to explain the association between liver disease and IgAN. Although some mechanisms are expected to reverse in patients after liver transplant, the long-term renal prognosis is unclear for these patients.
Methods
This observational retrospective cohort study examined the renal outcomes of 14 patients who had IgAN with end-stage liver disease and subsequently underwent either liver transplant alone or combined liver and kidney transplant at a single tertiary care center.
Results
Of the 7 patients who underwent liver transplant alone, hematuria persisted in 2, 4 had progressive loss of kidney function with worsening proteinuria in 3 but only 1 reached end-stage renal disease 5 years posttransplant. Among 7 combined liver and kidney transplant recipients, 1 had histologic and 1 had histologic and clinical recurrence of IgAN without kidney allograft loss.
Conclusions
IgAN in patients with advanced liver disease does not necessarily resolve after liver transplant but has overall favorable renal outcomes.
End-stage liver disease (ESLD) is commonly complicated by kidney dysfunction, which in turn leads to a worse prognosis.1,2 The kidney dysfunction can be functional or structural, ranging from prerenal azotemia and hepatorenal syndrome causing acute kidney injury to immunoglobulin A (IgA) nephropathy (IgAN) and membranoproliferative glomerulonephritis causing chronic kidney disease.2 Each condition carries a different presentation, treatment, prognosis, and risk of recurrence. Therefore, when ESLD patients undergo evaluation for liver transplant, it is critical to assess their kidney function and understand the cause of any underlying kidney dysfunction. Patients who are not expected to recover their kidney function after liver transplant usually benefit from combined liver and kidney transplant (CLKT). Others may need modified immunosuppressive regimens that minimize or avoid use of calcineurin inhibitors to preserve the remaining kidney function.3-5
IgAN is a common glomerular lesion that can occur in patients with liver disease.6-8 Historically, it was categorized as a hepatic glomerulonephritis.9 It is characterized by the deposition of IgA-containing immunocomplexes (IgA-ICs) in the glomerular mesangium, resulting in variable degrees of mesangial expansion and proliferation.10 When IgAN occurs in patients with liver disease, it is usually called secondary IgAN. Liver disease is the most common cause of secondary IgAN.11 In a study of 60 patients with ESLD who underwent transjugular renal biopsy at liver transplant, 12 (25%) of 48 patients who had adequate biopsies had IgAN, and 3 (6%) other patients had IgAN along with another glomerular lesion.7 Pathogenesis of secondary IgAN in ESLD patients is not well understood. Multiple mechanisms have been proposed to explain the association between IgAN and ESLD. The main focus has been on impaired clearance of IgA and IgA-ICs by the failing liver.11-13 The prognosis for patients with this glomerular lesion is also not clear. Reports describing renal outcomes for ESLD patients with IgAN are sparse and conflicting. For example, a few reports suggested that this condition tends to remain stable and rarely progresses to end-stage renal disease (ESRD).7,14 In contrast, Pouria et al11 reported outcomes for 8 patients with liver disease and biopsy-proven IgAN, 4 of whom progressed to ESRD.
The present study examined the renal outcomes of ESLD patients with biopsy-proven IgAN who underwent liver transplant at our institution. Our goal was to inform all healthcare providers caring for these patients about the long-term prognosis for patients with this condition after liver transplant. Based on the proposed mechanism of impaired clearance of IgA by the failing liver, we hypothesized that IgAN has a benign course and may even resolve after a new functioning liver is transplanted. It should also not recur in kidney allografts in patients undergoing CLKT.
MATERIALS AND METHODS
We identified all adults who underwent liver transplant alone (n = 2354) or CLKT (n = 151) at Mayo Clinic in Rochester, MN, between January 1, 1992, and January 1, 2016. Pathology reports were searched to identify patients who underwent native or allograft kidney biopsy on or before the transplant date. Patients were included in the study if the kidney biopsy showed IgAN. Medical records review was completed for patients meeting the inclusion criteria. Demographics, comorbidities, the cause of ESLD, and the presence of other diagnoses on kidney biopsies were abstracted. Laboratory study results were collected, including serum creatinine, measured glomerular filtration rate (GFR), estimated GFR, and the presence of hematuria and proteinuria at transplant and at 12 and 60 months after transplant.
At our institution, GFR is measured with a short iothalamate clearance test.15 For patients who did not have a measured GFR, estimated GFR based on the Modification of Diet in Renal Disease study equation was used instead.16 Hematuria was defined as more than 3 erythrocytes per high-power field on urine microscopy. Proteinuria was quantified in milligrams per day from a 24-hour urine collection. If 24-hour urine collection was not available, the ratio of protein to osmolality or protein to creatinine was used to predict 24-hour proteinuria.17
At our institution, patients who undergo CLKT have follow-up protocol kidney biopsies at 4, 12, and 24 months and at 5 and 10 years after transplant. Biopsy findings were reviewed when available to identify any recurrent IgA deposition. The dates of diagnosis of ESRD, IgA recurrence in the allograft, and death were noted when applicable. All results were reported descriptively for each patient. The study protocol was approved by the Mayo Clinic Institutional Review Board.
RESULTS
After excluding 1 patient who died soon after transplant, 14 patients were included in the analysis (0.6% of all 2505 liver transplant recipients). Of those, 7 underwent liver transplant alone and 7 (4.6% of all CLKT) underwent CLKT.
Liver Transplant Alone Recipients
Data for liver transplant alone recipients are summarized in Table 1. Median age (range) at transplant was 47 (30-59) years. Causes of ESLD included α1-antitrypsin deficiency, alcoholic liver disease, hepatitis C cirrhosis, cholangiocarcinoma, and cryptogenic cirrhosis. All patients had IgAN on kidney biopsy, and the pathologic findings are summarized in Table 2. Median GFR (range) at transplant was 66 (31-120) mL/min per 1.73 m2, and median 24-hour proteinuria was 302 (91-855) mg. All patients had microscopic hematuria before transplant. GFR and hematuria trends and proteinuria trends for liver transplant patients are summarized in Figures 1 and 2, respectively. Hematuria resolved in all but 2 recipients and proteinuria worsened over time in 3 recipients. Three patients (43%) had stable or improved GFR over the 5 years after transplant (Figure 1). In 1 patient (patient 4), acute kidney injury developed perioperatively and resulted in chronic kidney disease; the patient received sirolimus. No other patients had acute kidney injury in the perioperative time. Three (43%) other patients had progressive and sustained loss of kidney function over 5 years despite resolution of microscopic hematuria in 2 of them. In 1 patient (patient 7), ESRD developed 5 years after liver transplant. Liver allograft function was satisfactory and for recipients on calcineurin inhibitors, the level was typically maintained within goal (Tacrolimus between 4 and 6 ng/mL). None of these patients had a kidney biopsy after liver transplant, so the exact cause of chronic kidney disease was not confirmed histologically and remained unknown.
TABLE 1.
Summary of clinical data for liver transplant patients
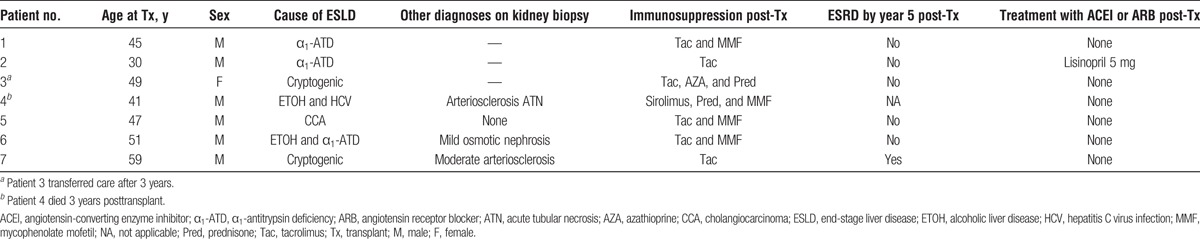
TABLE 2.
Summary of native kidney biopsy findings for liver transplant alone recipientsa
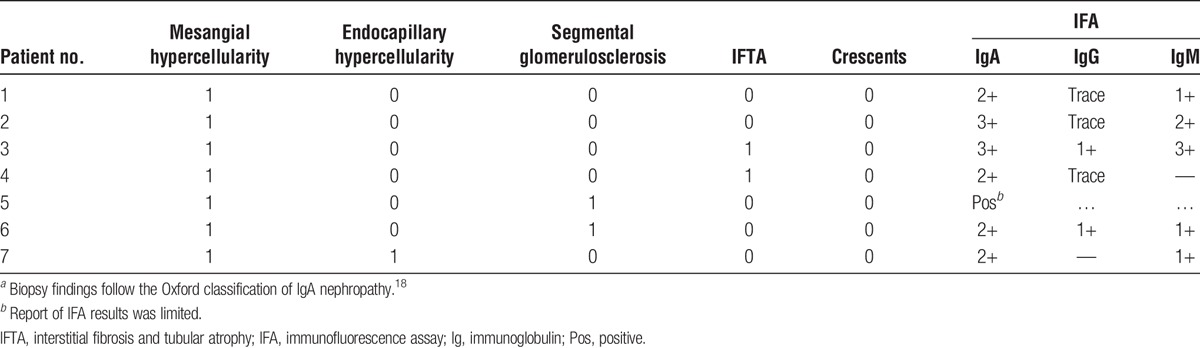
FIGURE 1.
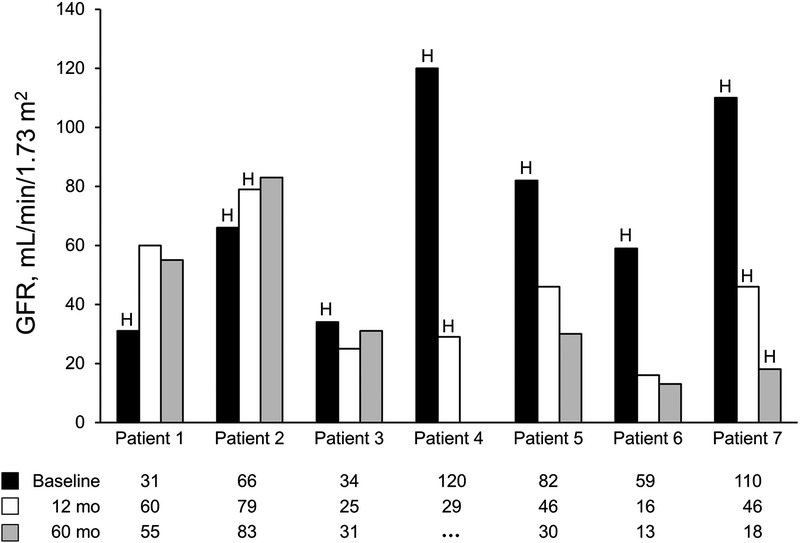
GFR and hematuria trends for liver transplant patients. Patient 4 had acute kidney injury peritransplant and died 3 years posttransplant. The letter H indicates hematuria.
FIGURE 2.
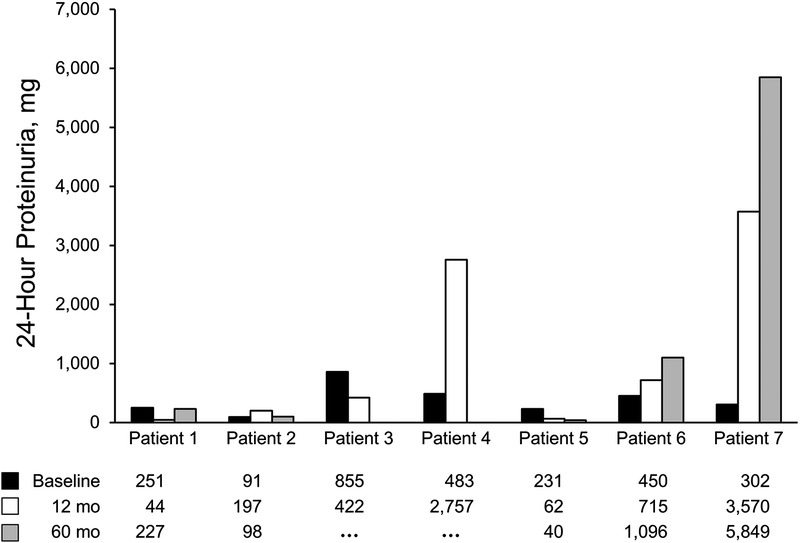
Proteinuria trends for liver transplant patients. Patient 3 transferred care after 3 years. Patient 4 died 3 years posttransplant.
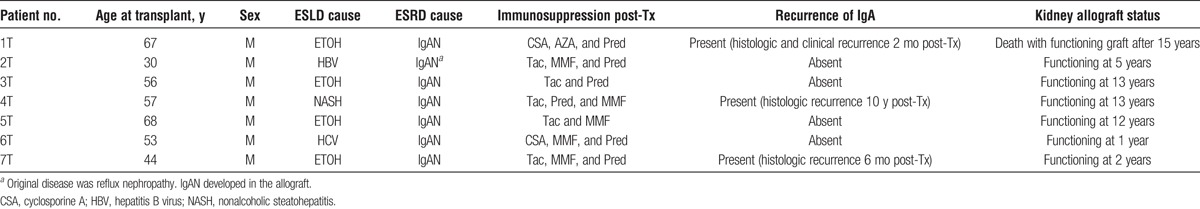
CLKT Recipients
Data for the patients who underwent CLKT are summarized in Table 3. All the patients had ESRD due to IgAN (1 had previous kidney transplant for reflux nephropathy, and IgAN developed in the allograft). The median age (range) at CLKT was 56 (30-68) years. One patient (patient 7T) had a histologic recurrence of IgA on protocol biopsy 5 months posttransplant but did not have any clinical features of IgAN. His kidney function remained stable afterward. One patient (patient 4T) had a histologic recurrence of IgA on the 10-year protocol biopsy that also showed chronic glomerulopathy. At that time, liver allograft function was normal. One patient (patient 1T) had histologic and clinical recurrence of IgAN 2 months posttransplant associated with mild cellular rejection. He presented with microscopic hematuria and peak 24-hour proteinuria of 2400 mg. After he was treated with methylprednisolone and antithymocyte globulin, his proteinuria resolved. Later, after enalapril was added to his regimen, his kidney function stabilized.
TABLE 3.
Summary of clinical data for CLKT recipients
DISCUSSION
In this select cohort of ESLD patients with biopsy-proven IgAN, kidney outcomes were overall favorable after liver transplant. However, in 3 patients (43%), GFR decreased by more than 40% by year 5 posttransplant, and in 1 patient, the disease progressed to ESRD, although the exact cause of ESRD cannot be attributed to IgA without kidney biopsy. CLKT did not prevent recurrence of IgAN in kidney allografts. Two patients (29%) had evidence of recurrent IgA in the allograft within 6 months posttransplant; 1 of these patients had clinical features of IgAN. Despite that, overall kidney allograft survival was excellent with no graft lost from recurrent IgA at last follow-up.
Chronic liver disease is commonly associated with secondary IgAN. In fact, among all patients with secondary IgAN, liver disease is the most common cause.12 Secondary IgAN is a pathologic diagnosis with findings like what is observed with primary IgAN. The main characteristic is the presence of mesangial deposits that are predominantly IgA. These deposits can be associated with variable degrees of mesangial expansion and hypercellularity. Although immunofluorescence studies are predominantly positive for mesangial IgA staining, IgG, IgM, or C3 can also be present to a lesser degree.10,12,14 In studies that performed immunofluorescence assay on kidney tissue from ESLD patients, 30% to 90% of the samples had predominantly IgA staining.14 However, not all patients with mesangial IgA staining have other features of IgAN. For example, in 1 study in which ESLD patients underwent renal biopsy at liver transplant, 5 patients had mesangial IgA staining, but only 2 had other features of IgAN.6
In our cohort, all patients had clinical features of IgAN, which reflects the criteria we used to include them in this study. All patients had mesangial expansion and proliferation, but the degree of tubulointerstitial disease varied from minimal to mild.
From our cohort findings, it is difficult to comment on the prevalence of IgAN among ESLD patients undergoing liver transplant because all included patients underwent kidney biopsy for a reason. The true prevalence is likely higher for multiple reasons. First, not all ESLD patients undergo kidney biopsy, even when they have clear evidence of kidney disease. With the increased risk of bleeding and the low likelihood of changing management, many nephrologists do not perform kidney biopsies on such patients. Second, having normal kidney function does not rule out the presence of pathologic changes. A few studies reported the presence of glomerular lesions even in patients with normal kidney function,7,9 although the meaning of this finding is unclear.
Clinically, patients with secondary IgAN present similarly to patients with primary IgAN. Patients can be asymptomatic, and the most common abnormality is microscopic hematuria. Fewer patients may have nephrotic syndrome and progressive kidney disease, likely indicating primary IgAN.11 In our cohort, all patients had microscopic hematuria at transplant. Median 24-hour proteinuria was about 300 mg, and none of the patients had nephrotic proteinuria (Figure 2). It is important to pay close attention to urinary proteinuria and urine microscopic findings during evaluation of ESLD patients. This laboratory data may help clarify the main cause of kidney dysfunction because ESLD patients are prone to many conditions that can result in kidney disease. Some of these conditions are reversible and others are chronic and progressive. For example, the use of diuretics to control ascites and the need for recurrent paracentesis procedures in the setting of low systemic arterial pressure put ESLD patients at risk of prerenal kidney injury. This prerenal state can progress to acute tubular necrosis. Liver failure and concomitant viral hepatitis increase risk of glomerular diseases including membranoproliferative glomerulonephritis, cryoglobulinemia and membranous nephropathy. Treatment with nephrotoxic medications and exposure to contrast during imaging may result in tubulointerstitial injury. Some of these conditions can be present concomitantly, making the diagnostic process more challenging. In the posttransplant period, recurrence of primary disease, calcineurin inhibitors toxicity, and opportunistic infections can result in kidney disease as well.
The pathogenesis of IgAN in liver disease is not completely understood. ESLD patients have increased circulating levels of IgA-IC. Woodroffe et al19 showed that 100% of liver cirrhosis patients had circulating immunocomplexes, and 80% of the complexes were IgA-ICs. Unlike in primary IgAN patients who had an intermittent appearance of immunocomplexes, these complexes were constantly present in liver cirrhosis patients. These circulating immunocomplexes have been shown to be deposited in other organs, such as skin and jejunal capillaries.20 Multiple mechanisms have been proposed for the increase in circulating IgA-ICs. First, an increase in exposure to antigen resulting from compromised gastrointestinal mucosal integrity and shunting across a failing liver may lead to formation of more IgA-ICs.11 Second, the clearance of IgA and IgA-ICs appears to be decreased in patients with cirrhosis of the liver.13,21 This decrease could be related to a loss of hepatocytes, an altered position of Fc receptors on hepatocytes, and changes in the IgA molecule itself.13,21-23 Third, it is plausible that shunting of blood into systemic circulation and bypassing the liver could reduce the clearance of these immunocomplexes by the liver. A more detailed review of these mechanisms has been written by Pouria et al.11 IgA molecules were shown to have abnormal glycosylation in patients with alcoholic liver disease, like in primary IgAN, and in the same study, soluble CD89-IgA (which is a feature of primary IgAN) was also present in patients with alcoholic cirrhosis.24 This may be an additional mechanism of IgAN in this subgroup of patients and may explain the stronger association between alcoholic liver disease and IgAN.
The prognosis for patients with IgAN in liver disease is under debate. One proposal is that IgAN in ESLD patients has a benign and limited course. Calmus et al7 reported that outcomes from 5 patients with IgAN undergoing liver transplant were like the outcomes from patients with normal kidney biopsy findings. However, not all studies have reported similar findings. Pouria et al11 reported 8 biopsy-proven cases of hepatic IgAN. Over 4 years, half the patients had ESRD and 25% had progressive CKD. It is plausible that many of the proposed pathogenic mechanisms leading to IgAN reverse after liver transplant, leading to IgAN stabilization or improvement (or both).25 The resolution of hematuria, improvement in proteinuria, and stabilization of GFR are all signs of resolving disease. In our subgroup of patients undergoing liver transplant alone, 4 (57%) patients had resolution of hematuria by the first year posttransplant. However, this did not correlate with other markers of kidney disease. Patient 6, for example, had an increase in proteinuria and a decrease in GFR despite resolution of the hematuria, but in patient 2, GFR and proteinuria remained stable despite persistent hematuria. In this cohort, the degree of proteinuria correlated better than hematuria with progression of kidney disease. This finding is like what is reported with primary IgAN. Patient 6 progressed to ESRD even though he had a normal GFR at transplant. Although we do not have follow-up biopsies to confirm the persistence of IgAN, the ongoing glomerular hematuria suggests that IgAN at least contributed to this progression. These findings argue against the notion that IgAN has a benign course in ESLD patients and could reflect our inability to differentiate primary and secondary IgAN if we consider secondary IgAN to be benign. It is also possible that the presence of IgAN at time of liver transplant increased the likelihood of other risk factors (eg, calcineurin inhibitors toxicity) to cause progressive kidney disease. Each of the 3 patients whose disease progressed had low-risk features at transplant: near-normal GFR, 24-hour proteinuria less than 1 g, normal blood pressure, and no significant tubulointerstitial disease.
Patients undergoing CLKT provide a unique opportunity to understand the pathogenesis of IgAN. In the presence of a new healthy liver, the risk of recurrence of secondary IgAN should be minimal or absent. Because these patients undergo protocol kidney biopsy at our institution, we had the opportunity to detect both histologic and clinical recurrence. Two patients (29%) had histologic recurrence within the first 6 months, and 1 patient (patient 1T) had signs of clinical recurrence as well. Both patients had satisfactory liver allograft function at time of IgA recurrence. These data suggest the presence of another pathogenic mechanism for IgAN besides impaired IgA clearance by the failing liver. Another explanation is heterogeneity of IgAN in ESLD patients, with some having primary IgAN and others having true secondary IgAN.
This study has important strengths. We included only patients who had biopsy-proven IgAN rather than relying on clinical presentation. The follow-up time exceeded 5 years for most patients, allowing reasonable time to detect the progression of IgAN. We also had the opportunity to review protocol biopsies for CLKT patients to detect early and asymptomatic recurrence of IgAN.
Our study has limitations. First, because we did not have follow-up biopsies for patients with native kidney IgAN, we could not evaluate what happened to the mesangial deposits after liver transplant. Second, we did not have histologic data for patients who had progressive kidney disease after liver transplant. These patients may have had another reason for the worsening kidney function, including calcineurin inhibitor toxicity. Third, patients who underwent CLKT may represent a population with skewed data because they have more severe IgAN at baseline and thus are more likely to have primary IgAN rather than secondary disease. This may explain the early recurrence in our small cohort.
In conclusion, IgAN in patients with advanced liver disease does not necessarily resolve after liver transplant. Some patients have progressive kidney disease after liver transplant alone and others have recurrence of IgAN in the kidney allograft after CLKT but their overall outcome is satisfactory. This suggests IgAN heterogeneity in this group of patients and possibly the presence of more than 1 pathogenic mechanism. These possibilities must be considered when evaluating patients with ESLD and IgAN rather than if all patients have benign secondary IgAN. Further research is needed to understand the pathogenesis of IgAN in ESLD and to differentiate primary IgAN from secondary IgAN.
Footnotes
Published online 11 July, 2017.
The authors declare no conflicts of interest.
M.S.H. participated in research design, data collection, analysis and writing the article. Z.M.E.-Z. participated in research design, data analysis and writing the article.
REFERENCES
- 1.Fede G, D'Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56:810–818. [DOI] [PubMed] [Google Scholar]
- 2.Sampaio MS, Martin P, Bunnapradist S. Renal dysfunction in end-stage liver disease and post-liver transplant. Clin Liver Dis. 2014;18:543–560. [DOI] [PubMed] [Google Scholar]
- 3.Farkas SA, Schnitzbauer AA, Kirchner G, et al. Calcineurin inhibitor minimization protocols in liver transplantation. Transpl Int. 2009;22:49–60. [DOI] [PubMed] [Google Scholar]
- 4.Saner FH, Cicinnati VR, Sotiropoulos G, et al. Strategies to prevent or reduce acute and chronic kidney injury in liver transplantation. Liver Int. 2012;32:179–188. [DOI] [PubMed] [Google Scholar]
- 5.Trotter J, Kahn B. Renal dysfunction and the liver transplant recipient; novel strategies for determination of reversibility and renal protective therapies pretransplant and posttransplant. Curr Opin Organ Transplant. 2012;17:225–229. [DOI] [PubMed] [Google Scholar]
- 6.Axelsen RA, Crawford DH, Endre ZH, et al. Renal glomerular lesions in unselected patients with cirrhosis undergoing orthotopic liver transplantation. Pathology. 1995;27:237–246. [DOI] [PubMed] [Google Scholar]
- 7.Calmus Y, Conti F, Cluzel P, et al. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol. 2012;57:572–576. [DOI] [PubMed] [Google Scholar]
- 8.Pillebout E, Nochy D, Hill G, et al. Renal histopathological lesions after orthotopic liver transplantation (OLT). Am J Transplant. 2005;5:1120–1129. [DOI] [PubMed] [Google Scholar]
- 9.Nochy D, Druet P, Bariety J. IgA nephropathy in chronic liver disease. Contrib Nephrol. 1984;40:268–275. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. [DOI] [PubMed] [Google Scholar]
- 11.Pouria S, Barratt J. Secondary IgA nephropathy. Semin Nephrol. 2008;28:27–37. [DOI] [PubMed] [Google Scholar]
- 12.Pouria S, Feehally J. Glomerular IgA deposition in liver disease. Nephrol Dial Transplant. 1999;14:2279–2282. [DOI] [PubMed] [Google Scholar]
- 13.Roccatello D, Picciotto G, Torchio M, et al. Removal systems of immunoglobulin A and immunoglobulin A containing complexes in IgA nephropathy and cirrhosis patients. The role of asialoglycoprotein receptors. Lab Invest. 1993;69:714–723. [PubMed] [Google Scholar]
- 14.Newell GC. Cirrhotic glomerulonephritis: incidence, morphology, clinical features, and pathogenesis. Am J Kidney Dis. 1987;9:183–190. [DOI] [PubMed] [Google Scholar]
- 15.Wilson DM, Bergert JH, Larson TS, et al. GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis. 1997;30:646–652. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease study group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DM, Anderson RL. Protein-osmolality ratio for the quantitative assessment of proteinuria from a random urinalysis sample. Am J Clin Pathol. 1993;100:419–424. [DOI] [PubMed] [Google Scholar]
- 18.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. [DOI] [PubMed] [Google Scholar]
- 19.Woodroffe AJ, Gormly AA, McKenzie PE, et al. Immunologic studies in IgA nephropathy. Kidney Int. 1980;18:366–374. [DOI] [PubMed] [Google Scholar]
- 20.Kater L, Jöbsis AC, de la Faille-Kuyper EH, et al. Alcoholic hepatic disease. Specificity of IgA deposits in liver. Am J Clin Pathol. 1979;71:51–57. [DOI] [PubMed] [Google Scholar]
- 21.Delacroix DL, Elkom KB, Geubel AP, et al. Changes in size, subclass, and metabolic properties of serum immunoglobulin A in liver diseases and in other diseases with high serum immunoglobulin A. J Clin Invest. 1983;71:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess JB, Baenziger JU, Brown WR. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology. 1992;15:702–706. [DOI] [PubMed] [Google Scholar]
- 23.Kutteh WH, Prince SJ, Phillips JO, et al. Properties of immunoglobulin A in serum of individuals with liver diseases and in hepatic bile. Gastroenterology. 1982;82:184–193. [PubMed] [Google Scholar]
- 24.Tissandie E, Morelle W, Berthelot L, et al. Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: common mechanisms for distinct diseases. Kidney Int. 2011;80:1352–1363. [DOI] [PubMed] [Google Scholar]
- 25.Ghabra N, Piraino B, Greenberg A, et al. Resolution of cirrhotic glomerulonephritis following successful liver transplantation. Clin Nephrol. 1991;35:6–9. [PubMed] [Google Scholar]


