Supplemental digital content is available in the text.
Abstract
Background
Heart allocation systems are usually urgency-based, offering grafts to candidates at high risk of waitlist mortality. In the context of a revision of the heart allocation rules, we determined observed predictors of 1-year waitlist mortality in France, considering the competing risk of transplantation, to determine which candidate subgroups are favored or disadvantaged by the current allocation system.
Methods
Patients registered on the French heart waitlist between 2010 and 2013 were included. Cox cause-specific hazards and Fine and Gray subdistribution hazards were used to determine candidate characteristics associated with waitlist mortality and access to transplantation.
Results
Of the 2053 candidates, 7 variables were associated with 1-year waitlist mortality by the Fine and Gray method including 4 candidate characteristics related to heart failure severity (hospitalization at listing, serum natriuretic peptide level, systolic pulmonary artery pressure, and glomerular filtration rate) and 3 characteristics not associated with heart failure severity but with lower access to transplantation (blood type, age, and body mass index). Observed waitlist mortality for candidates on mechanical circulatory support was like that of others.
Conclusions
The heart allocation system strongly modifies the risk of pretransplant mortality related to heart failure severity. An in-depth competing risk analysis is therefore a more appropriate method to evaluate graft allocation systems. This knowledge should help to prioritize candidates in the context of a limited donor pool.
Heart transplantation is the preferred option for medically refractory advanced heart failure,1-4 improving survival,5 and quality of life.
Aside from rejection and complications of immunosuppressive therapies, the main limitation of transplantation is restricted access due to the low number of available grafts compared with the number of candidates registered on the waitlist.
Current allocation rules based on candidate heart severity are under review in various countries.6-8
In 2004, a new allocation system was implemented in France, allocating hearts primarily based on medical urgency (Figure 1). This high-urgency (HU) based system grants priority status to candidates on intravenous inotropes or temporary mechanical circulatory support (MCS) (HU1-status) and those on long-term MCS with device-related complications (HU2 status).9 In 2015, 56% of candidates who underwent transplantation in France had an HU status at the time of transplantation.
FIGURE 1.
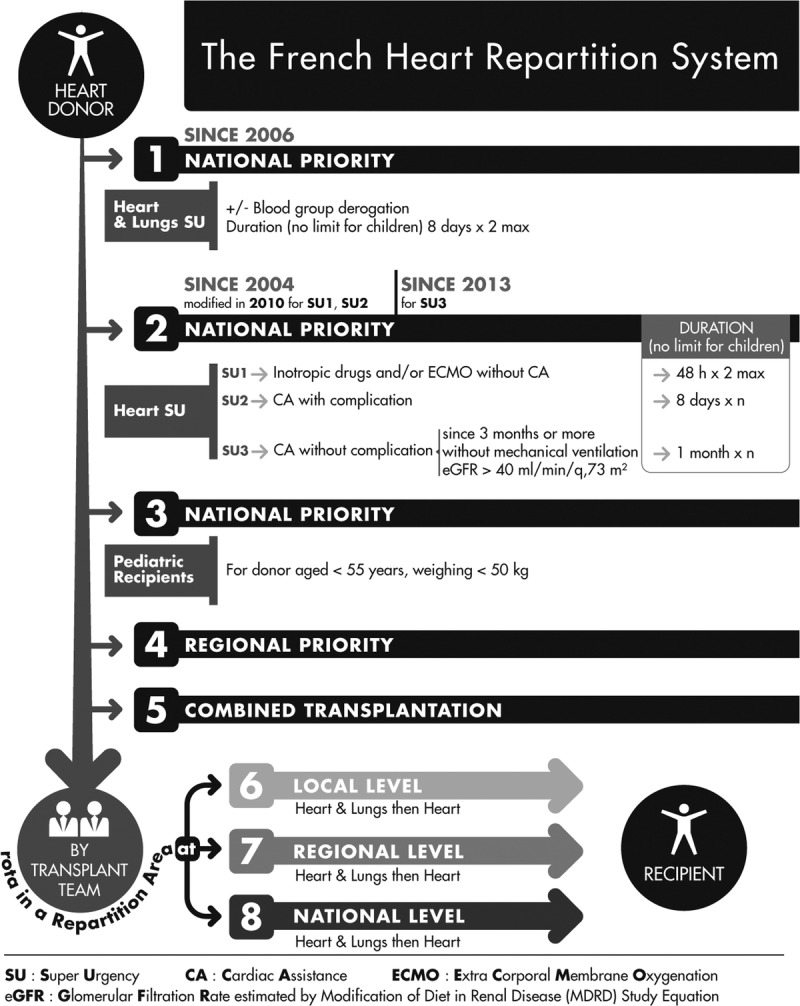
Schematic representation of the French heart allocation system. Preference is given to high priority level (SU) patients who are waiting for a heart-lung transplant. Priority access is given to candidates who are on circulatory support (IV inotrope, ECMO), and would be at high risk of needing a ventricular assist device (VAD) or total artificial heart (SU1), or candidates who have had an infection or complication after left ventricular assist device (SU2). Then, children have priority access to transplantation based on their specific characteristics (morphology and morbidity): the organs of a donor younger 55 years and weighing less than 50 kg will preferentially go to a child. In the absence of priorities and the need for a combined transplantation, the order of the propositions is based on a rotation that allows for local variation and practices within a hierarchy from local (same hospital or a network) to regional or national levels.
Despite this approach designed to reduce waiting list mortality, the rate of death or delisting due to worsening medical condition was as high as 25.8 per 100 candidate years in 2015.10 Furthermore, the 1-year cumulative incidence of death on the waitlist, considering access to transplantation as a competing event, was surprisingly higher in elective candidates than in those listed as HU status (10%; 95% confidence interval [CI], 8-11 for HU1; 7%; 95% CI, 4-11 for HU2; and 15%; 95%CI, 14-17 for non-HU candidates10). These results show that the current allocation system may overprioritize HU candidates relative to the others. Within this context, the French national transplantation agency initiated studies to assess the current allocation system to develop a more effective and equitable allocation system.11 One goal was to develop a waiting list mortality risk score from commonly available candidate variables not related to medical practice or assessment.12 Here, we aimed to determine which candidate subgroups are favored or disadvantaged by the current allocation system using competing risk survival analyses.
MATERIALS AND METHODS
Population and Data
We used data collected from the French national database CRISTAL, which is comprised of demographic, clinical, and biological data concerning all patients registered on the waitlist for heart transplantation. Data are collected by the transplant teams at listing, at the time of transplantation, and annually thereafter. Withdrawals from the waiting list and deaths of listed patients are notified prospectively. The quality of the recorded data is set to a high standard as CRISTAL is the primary tool for organ allocation in France.
The study population comprised all patients registered on the national waitlist for heart transplantation between January 2010 and December 2013. Patients were followed up for 1 year after their registration. Baseline clinical characteristics of the patients were those recorded at registration.
Missing values for relevant covariates (items described in Table 1) were substituted with values obtained by multiple imputations per a Markov chain Monte Carlo approach with uninformative prior information (SAS MI procedure). The SAS MIANALYZE procedure was used to combine the results of the analyses throughout the 20 imputed data sets.13
TABLE 1.
Candidate characteristics according to 1-year postregistration status (N = 2053 registered on the cardiac waitlist between 2010 and 2013)
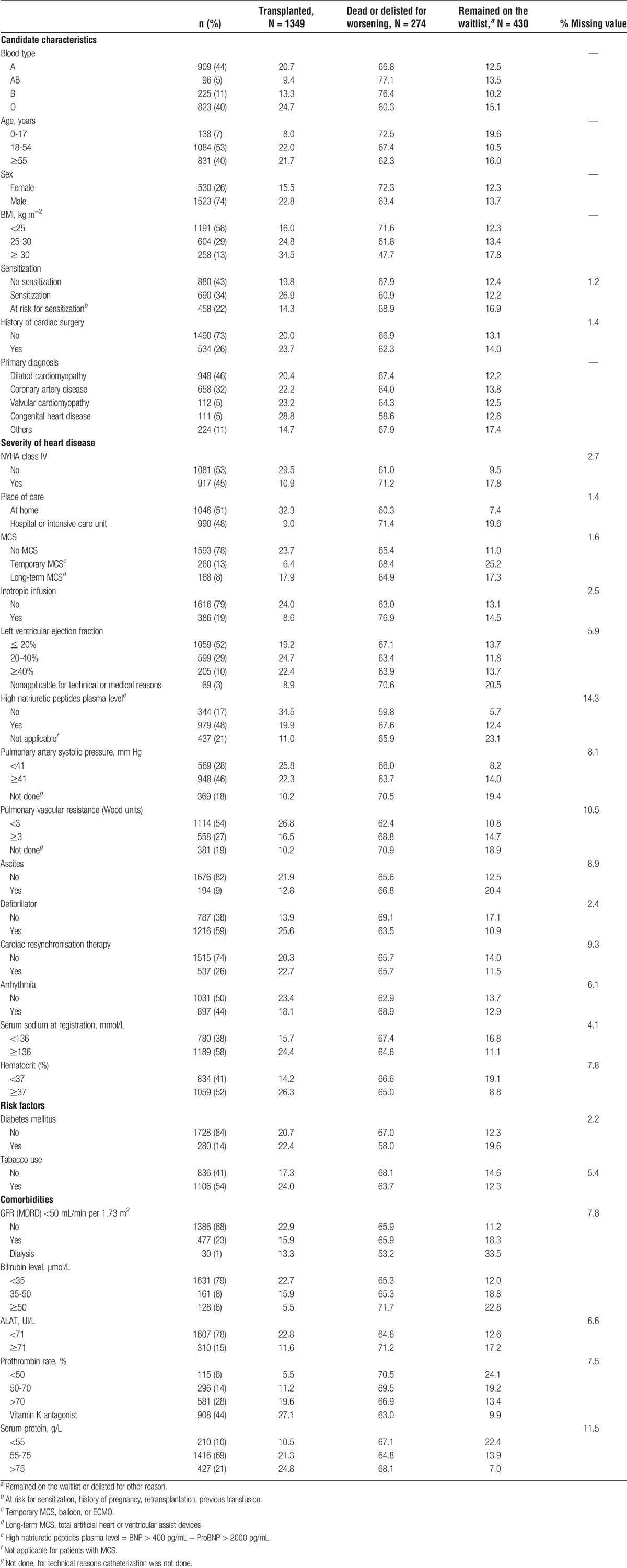
Variables with more than 20% missing data were excluded from the analysis (date of primary diagnosis, heart rate, left ventricular end diastolic diameter, factor V, and INR values).
Statistical Analysis
We first categorized the patients per their 1-year waiting list outcome: (1) transplanted patient group, (2) died on the waiting list or delisted for worsening medical condition patient group, (3) event-free patient group, consisting of patients remaining on the waiting list or those who were delisted for reasons other than transplantation or worsening medical condition. Categorization of continuous variables was done per medical relevance, proportional hazards assumption, and graphic analysis of the relationship between continuous variable, modeled as restricted cubic splines, and outcomes. They are finally presented as categorical variables, reported as frequencies (%).
Time-to-event was defined as the time from registration to delisting (transplantation, death and delisting for worsening medical condition, or withdrawal from the waitlist for other reasons) or end of follow-up for patients who remained on the waitlist.
Death on the waiting list and transplantation are competing events because when a patient undergoes a transplantation, he is no longer at risk of dying while on the waiting list. Conversely, someone who dies on the waiting list is no longer eligible for transplantation. A cause-specific hazards ratio (csHR) determined by a Cox model and a subdistribution hazard ratio (sHR) determined by a Fine and Gray model14 were used to identify factors associated with 1-year waiting list mortality and access to transplantation in univariate and multivariable analyses.15 The csHR for death gives an estimation of the risk of death independent of the access to transplantation, whereas the sHR for 1-year waiting list mortality gives an estimation of the resulting risk of death considering access to transplantation.
Selection of numerous potential predictors was made in 2 steps: we initially included all factors associated with access to transplantation or death on the waitlist with a P value less than 0.20 by univariate analysis, using cause-specific proportional and subdistribution hazard models. Then, we constructed final models for sHR and CsHR, step by step, selecting factors with a significance of P less than 0.05.
Results obtained from the 2 sets of models (csHR and sHR) are presented considering, in 1 case, death as the event and transplantation as the competing event, and in the other, transplantation as the event and death as the competing event.16
Differences between those 2 methods can be interpreted as follows for candidates with more severe disease who have priority. If they are prioritized via the allocation system, their access to transplantation, independent of their high risk of death, is elevated, and the csHR for transplantation is high. Because they have better access to transplantation than others, the resulting risk of death on the waiting list (estimated by sHR for death) of candidates with more severe disease is lower than their inherent risk of death.
Proportional assumption was verified by the score test and graphical tools based on scaled Schoendfeld residuals.
We used the Expected Brier Score to assess the predictive power of the models obtained.17
All analyses were performed using SAS Guide V7.1. The validity of the final model was verified using the cmprsk and pec R packages from R 2.13.1 software.
RESULTS
Patients
Of the 2053 patients registered on the waitlist for heart transplantation between January 2010 and December 2013, 1349 underwent transplantation within 1 year of listing, 233 died, and 41 were delisted for a worsening medical condition. The event-free group included 369 patients remaining on the waitlist and 61 patients delisted for reasons other than a worsening medical condition (50 improved patients, 3 cancellations, and 8 patients who declined transplantation).
Table 1 shows the characteristics of the 2053 candidates: 26% were female, 7% were pediatric cases (<18 years), 40% were older than 55 years, 73% had no history of cardiac surgery, and 43% were not at risk to be sensitized. The primary transplant indication was dilated cardiomyopathy (46%), followed by ischemic cardiomyopathy (32%). Valvular and congenital heart diseases accounted for 10% of the indications. Half of the patients waited for transplantation at home and half at hospital or in an intensive care unit (ICU). Forty-eight percent of candidates had high BNP/NT-proBNP plasma levels (BNP > 400 pg/mL − NT-proBNP > 2000 pg/mL). Among the candidates, 45% were in NYHA class IV, 13% were supported by extracorporeal membrane oxygenation (ECMO) or an intra-aortic balloon pump, and 8% had long-term MCS (total artificial heart or left ventricular assist device). Inotropic infusions were used in 19% of patients and 23% received resynchronization therapy, whereas 59% had a defibrillator. Fourteen percent of candidates had diabetes mellitus and 13% a body mass index (BMI) higher than 30 kg∙m−2. Some patients presented comorbidities such as a glomerular filtration rate (GFR) less than 50 mL/min per 1.73 m2 (23%), bilirubin level of 50 μmol/L or greater (6%), or alanine aminotransferase of 71 UI/L or greater (15%). Serum sodium at registration was less than 136 mmol/L in 38% of patients and 44% were receiving a vitamin K antagonist. The hematocrit was less than 37% in 41% of patients.
Survival Analysis
Results of the univariate analysis are summarized in Table S1, SDC (http://links.lww.com/TXD/A50). Fifteen variables, independently associated with death on the waitlist or access to transplantation, were considered in the final multivariable analysis (Table 2).
TABLE 2.
Multivariable cause specific and subdistribution regression models for transplantation and death or removal for worsening medical condition while on the waitlist in the presence of competing risk
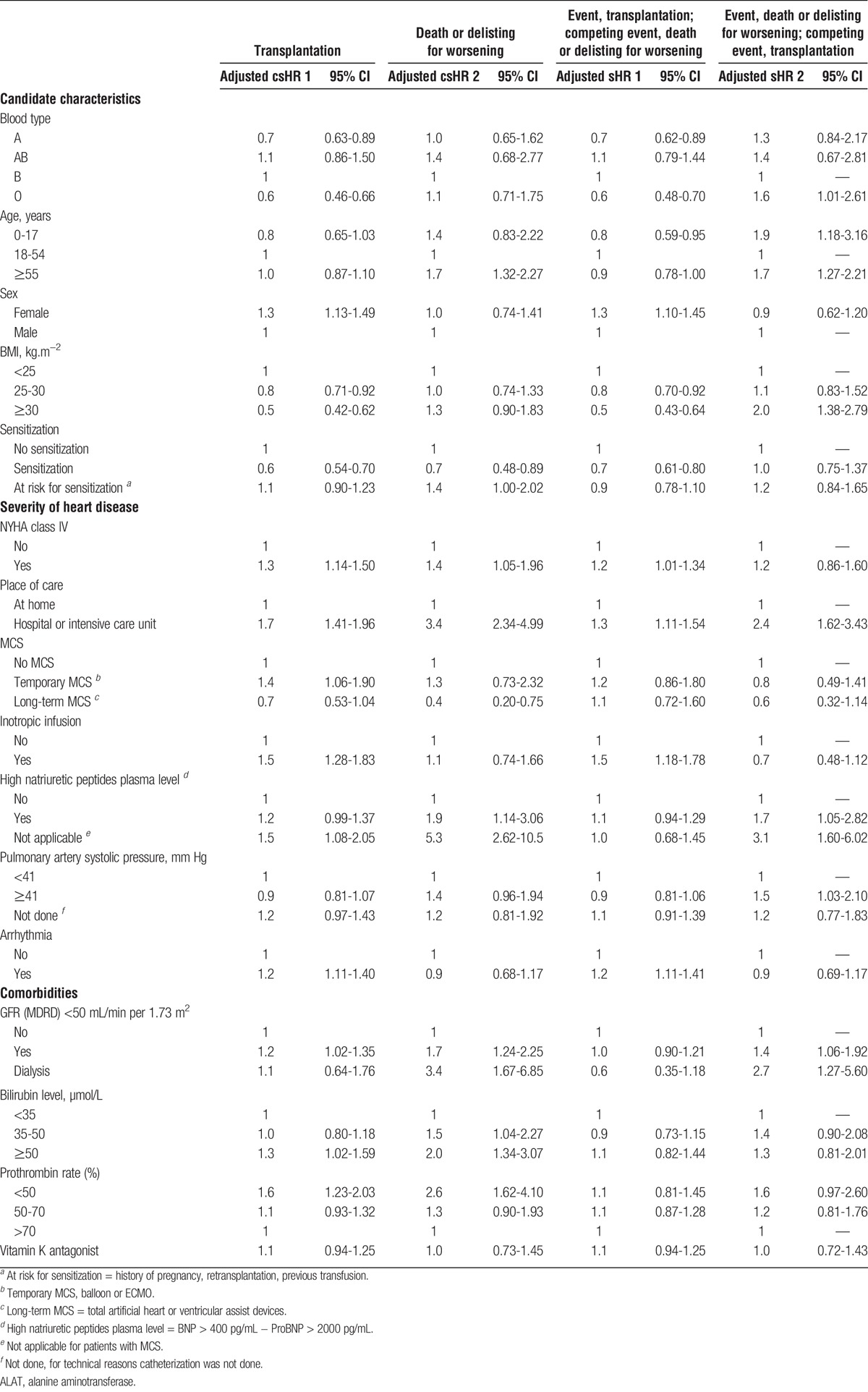
The comparison between csHR and sHR risks for transplantation and death, among factors which are associated with mortality on the waitlist, made it possible to identify those which are indicators of heart failure severity and those which are associated with poor access to transplantation.
Candidates With a Higher Risk of Death on the Waitlist Due to Lower Access to Transplantation
Blood group O candidates (vs group B) and candidates with a BMI of 25 kg/m2 or less had lower access to transplantation (csHR1). These factors were not associated with death per se (csHR2). However, an increased risk of death on the waitlist became apparent in these patients when access to transplantation was considered (sHR2).
Similarly, patients younger than 18 years tended to have lower access to transplantation (csHR1), which led to a greater risk of death on the waiting list (sHR2). Of note, pediatric candidates did not have higher 1-year waiting list mortality when access to transplantation was not considered (csHR2).
Candidates With a Higher Risk of Death on the Waitlist Despite Better Access to Transplantation
Candidates hospitalized at listing had a higher risk of 1-year death due to heart failure than those who were cared for at home (csHR2). Similarly, patients with a GFR under 50 mL/min per 1.73 m2 were at a higher risk of death (csHR2). These patients remained at higher risk of death on the waiting list when transplantation was considered as a competing event (sHR2), despite better access (csHR1),
Candidates With a Higher Risk of Death on the Waiting List Despite the Same Access to Transplantation
Candidates aged 55 years or older were at a higher risk of death before transplantation than those aged between 18 and 54 years (csHR2). However, their access to transplantation did not differ from that of other patients (csHR1) and therefore did not affect their resulting risk of death (sHR2), which was significantly higher than that of younger adults.
The risk of death of candidates with high plasma natriuretic peptide levels and high systolic pulmonary artery pressure was significantly greater when transplantation was considered as a competing event (sHR2) as their access to transplantation was not significantly different from that of others (csHR1).
Other Candidates Have the Same Resulting Risk of Death on the Waiting List. Candidates With Better Access to Transplantation Which Offsets Their Increased Risk of Death Due to Heart Failure
Candidates with severe heart failure (NYHA class IV symptoms), high serum bilirubin levels (≥50 μmol/L), or low prothrombin rates (<50%) had a higher risk of 1-year death on the waitlist (csHR2). They also had better access to transplantation (csHR1), which resulted in the same risk of death on the waitlist when access to transplantation was considered (sHR2).
Candidates With Less Access to Transplantation Which Offset Their Lower Risk of Death Due to Heart Failure
Sensitized candidates or those with long-term MCS had less access to transplantation (csHR1), whereas they had a lower risk of 1-year death on the waitlist due to heart failure (csHR2). Thus, their risk of death on the waitlist within the year after registration was the same as that of other candidates when considering transplantation as a competing event (sHR2).
Candidates With Good Access to Transplantation and the Same Risk of Death Due to Heart Failure
Female candidates and candidates on temporary MCS, as well as those on inotropic support at listing, had better access to transplantation (csHR1) leading to a 1-year waiting list survival rate like that of other patients (sHR2 near 1).
The validity of the model and the proportional hazard assumption were respected.
DISCUSSION
The primary objective of current urgency-based allocation systems is to reduce waiting list mortality. In the context of graft shortage, we posit that equity for access to transplantation is ensured when all candidates on the waitlist have the same risk of death when considering access to transplantation as a competing event.
Here, we analyzed the determinants of waiting list mortality, including death and removal from the waitlist for worsening medical condition, using a competing risks analysis method that reports both Cox cause-specific hazards and Fine and Gray subdistribution hazards. The reason for using both methods is to distinguish between the effect of the candidates' medical condition and the effect of the allocation system rules.
The assessment of prognosis in heart failure is challenging. Demographics, functional status, filling pressure biomarkers, and renal and liver dysfunction have been associated with mortality in patients hospitalized for heart failure and have been incorporated into several prognostic models for in-hospital patients.18-21 Conversely, these variables have not been included in commonly used prognosis scores for ambulatory heart failure patients.22,23 The application of outpatient prognostic models to the waiting list heart failure population is likely not appropriate.
The severity of heart failure is per se associated with a high risk of death. The strength of this association is given by the csHR for death, which gives an estimation of the risk of death, independent of the access to transplantation. When patients with more severe disease are prioritized via the allocation system, they have better access to transplantation and their csHR for transplantation is high. The resulting risk of death of these patients while on the waitlist (estimated by sHR for death) is lower than their inherent risk of death because they have higher access to transplantation than others. The combination of both methods captures comprehensive information on the determinants of waitlist mortality.
Using the multivariable Cox cause-specific hazards method, we found 1-year heart failure mortality to be higher in candidates 55 years or older, hospitalized patients, patients with NYHA class IV symptoms, candidates with high natriuretic peptide levels, and candidates with a GFR less than 50 mL/min per 1.73 m2, a low prothrombin rate and, a total bilirubin concentration of 50 μmol/L or greater (adjusted csHR2 > 1). We found that long-term MCS was a predictor of lower 1-year mortality on the waiting list (adjusted csHR2, 0.4 [95% CI, 0.20-0.75]). This may be due to recent advances in the management of ventricular assist device patients.24 This finding underlines that prioritization of stable candidates on long-term MCS may not be equitable, at least within the time window of 1 year. The incidence of late complications and their impact on mortality of LVAD recipients with or without heart transplantation is beyond the scope of this study.
In addition, this study identified candidate characteristics associated with higher waiting list mortality when competing risk of transplantation was considered (Table 2 and Table S1, SDC, http://links.lww.com/TXD/A50).
Five well-recognized predictors of mortality in heart failure (age, ≥ 55 years, hospitalization, high systolic pulmonary artery pressure, elevated natriuretic peptide levels, and low GFR) were associated with waiting list mortality by the Fine and Gray method (adjusted sHR2, > 1). None of these factors was associated with improved access to transplantation except hospitalization. This finding indicates that the current allocation system does not properly prioritize specific high-risk patient subcategories. This study suggests that the prioritization of candidates based on candidate characteristics, rather than on medical management, may offer advantages.
Using the Fine and Gray method, we found 3 variables (blood type O; age, < 18 years; BMI, ≥ 30 kg/m2), which are not predictors of mortality in heart failure (adjusted csHR2 near 1), to be predictors of waiting list mortality (adjusted sHR2 > 1). These characteristics were associated with lower access to transplantation. Identical donor and recipient ABO blood type matching is the common rule in France, but ABO-compatible hearts may be allocated to HU candidates, as well as those with low access to transplantation. Consequently, the waiting time of blood type O candidates is significantly longer than for others. This study in agreement with that of Hussey et al24 suggests that blood type O hearts only be allocated to blood type O or B recipients. The current allocation system in France grants priority status to pediatric over adult candidates. Thus, 52% of pediatric candidates were transplanted with a heart from an adult donor. Nevertheless, allocation policies do not address the issue of oversized donor hearts in pediatric candidates. Efforts to improve the pediatric donation process are needed. Apart from posttransplant outcomes, obesity also raises the issue of donor-recipient size matching. The International Society for Heart and Lung Transplantation has stated that a male donor of 70 kg can be safely used for any recipient, irrespective of his weight.25 Utilization of these hearts can be increased without increasing posttransplant mortality.
With the competing risk analysis using the Fine and Gray method, we found that candidates on VA-ECMO and those on inotropic support at listing had a 1-year waiting list survival rate like that of other patients (sHR2 near 1). Their relatively low risk of death on the waiting list can be explained by better access to transplantation (adjusted csHR1 > 1), as the current allocation system in France grants HU status to candidates on VA-ECMO and inotropic support over long-term MCS patients with device-related complications and other candidates. These therapies accurately identify patients with poor outcomes. The improvement in management of these very high-risk patients without transplantation may have had a positive effect on their waiting list mortality, in addition to the positive impact of the current graft allocation algorithm. However, it has been noted that the allocation of grafts based on therapy provides a strong incentive to overuse treatments with well-known complications.26
This study has several limitations. It consisted of the analysis of registry data subject to coding errors and missing values, even if the quality of the recorded data is currently tested. Surprisingly, sensitized candidates, who have less access to grafts, had the same waiting list mortality rate as others (adjusted sHR2= 1). This point should be considered cautiously because the techniques and reporting for HLA antibody screening are heterogeneous in France. Some variables that were not incorporated in the database may have influenced waiting list mortality and affected the study results. Medical data collected at listing are likely to change over waiting time.
Mortality on waiting list is strongly associated to shortage and allocation rules aside from candidate characteristics. Local donor availability and candidate characteristics may modify the risk determinants and their relevance. Thus, a single universal set of criteria for organ prioritization cannot be defined. Here, we provide a method rather than a specific score or set of variables. This empirical approach must be appreciated in a field that shows much geographical variability and changing scenarios over time.
In conclusion, we calculated unbiased estimates of the mortality risk among heart transplant candidates on the waitlist. Prioritization based on the severity of heart failure had an impact on both transplantation and death. In the presence of these competing events, the simultaneous use of the Cox and Fine and Gray models permits comprehensive assessment of the current allocation system. The Cox proportional hazard allows estimation of the candidates' risk of death due to the severity of heart failure, and the Fine and Gray subdistribution hazards is useful for understanding how differential access to transplantation affects mortality on the waitlist.
This more appropriate method helps to understand which factors have a true impact on the death of heart transplant candidates on the waitlist in the current system and will be used to assess the future French allocation system.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the technicians involved in the clinical studies and those who entered the data into the database, Ms. Mirela Duman (Agence de la Biomédecine) and Ms. Pascale Weber (La Pitié Salpétrière Paris Hospital) for their participation in the working group on new items for CRISTAL, and Dr. Franck Assogba for his practical knowledge on competing risk.
Footnotes
Published online 18 July, 2017.
The authors declare no conflicts of interest.
C.C. participated in the study concept and design, statistical analysis, interpretation of results, and writing of the article. C.L. participated in the study concept and design, interpretation of results, writing of the article, and critical revision of the article. A.L. participated in the study concept, interpretation of results, and critical revision of the article. P.T. participated in the study concept, interpretation of results, and critical revision of the article. C.J. participated in the critical revision of the article. L.S. participated in the critical revision of the article. O.B. participated in the interpretation of results and critical revision of the article. R.D. participated in the study concept, interpretation of results, writing of the article, and study supervision.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant. 2006;25:1024–1042. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69:1167. [DOI] [PubMed] [Google Scholar]
- 5.Banner NR, Rogers CA, Bonser RS. Effect of heart transplantation on survival in ambulatory and decompensated heart failure. Transplantation. 2008;86:1515–1522. [DOI] [PubMed] [Google Scholar]
- 6.Stehlik J, Mehra MR, Sweet SC, et al. The International Society for Heart and Lung Transplantation Registries in the era of big data with global reach. J Heart Lung Transplant. 2015;34:1225–1232. [DOI] [PubMed] [Google Scholar]
- 7.Meyer DM, Rogers JG, Edwards LB, et al. The future direction of the adult heart allocation system in the United States. Am J Transplant. 2015;15:44–54. [DOI] [PubMed] [Google Scholar]
- 8.Smits JM, de Vries E, De Pauw M, et al. Is it time for a cardiac allocation score? First results from the Eurotransplant pilot study on a survival benefit-based heart allocation. J Heart Lung Transplant. 2013;32:873–880. [DOI] [PubMed] [Google Scholar]
- 9.Dorent R, Cantrelle C, Jasseron C, et al. Heart transplantation in France: current status. Presse Med. 2014;43:813–822. [DOI] [PubMed] [Google Scholar]
- 10.Agence de la biomédecine. 2015 Annual Report. https://www.agence-biomedecine.fr/Tous-nos-rapports-et-etudes. Published 2015.
- 11.Dorent R, Epailly E, Sebbag L. The effect of graft allocation system on outcomes in heart transplantation in France: has the time come to take calculated survival benefit into account? J Heart Lung Transplant. 2011;30:1299–1300. [DOI] [PubMed] [Google Scholar]
- 12.Jasseron C, Legeai C, Jacquelinet C, et al. Prediction of waitlist mortality in adult heart transplant candidates: the candidate risk score. Transplantation. 2017. doi: 10.1097/TP.0000000000001724. [DOI] [PubMed] [Google Scholar]
- 13.Yuan YC. Multiple imputation for missing data: concepts and new development. http://hbanaszak.mjr.uw.edu.pl/TempTxt/Yang_200X_Multiple%20Imputation%20for%20MIssing%20Data%20Concepts%20and%20New%20Developments.pdf. Published 2016.
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 15.Wolbers M, Koller MT, Stel VS, et al. Competing risks analyses: objectives and approaches. Eur Heart J. 2014;35:2936–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latouche A, Allignol A, Beyersmann J, et al. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66:648–653. [DOI] [PubMed] [Google Scholar]
- 17.Wolbers M, Blanche P, Koller MT, et al. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonarow GC, Adams KF, Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. [DOI] [PubMed] [Google Scholar]
- 19.Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. [DOI] [PubMed] [Google Scholar]
- 20.Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaronson KD, Schwartz JS, Chen TM, et al. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. [DOI] [PubMed] [Google Scholar]
- 23.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 24.Hussey JC, Parameshwar J, Banner NR. Influence of blood group on mortality and waiting time before heart transplantation in the United Kingdom: implications for equity of access. J Heart Lung Transplant. 2007;26:30–33. [DOI] [PubMed] [Google Scholar]
- 25.Costanzo MR. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson LW. The urgent priority for transplantation is to trim the waiting list. J Heart Lung Transplant. 2013;32:861–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


