Abstract
A patient presented with mental status changes over several weeks. Imaging indicated several areas of hyperintensity in the white matter of the temporal and parieto-occipital lobes. Brain biopsy and CSF analysis led to the diagnosis of progressive multifocal leukoencephalopathy (PML). Though this patient had no clinical evidence of immune suppression, autopsy later indicated the presence of undiagnosed systemic sarcoidosis, which has been associated with PML.
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a severe, demyelinating disease of the central nervous system caused by reactivation of the JC virus. The course of the disease is usually subacute, characterized by altered mental status, motor deficits, ataxia, and visual symptoms. Prior to becoming a major opportunistic infection in AIDS, the disease was seen in patients with hematologic or other malignancies, those on chronic immunosuppression, or those with longstanding inflammatory disorders. The recent introduction of natalizumab for treatment of multiple sclerosis renewed interest in non-human immunodeficiency virus (HIV) related PML infections; however these still represent only a small minority of PML cases. It is often fatal and although numerous therapies have been tried, there is no clear treatment shown to be of benefit, other than institution of highly active anti-retroviral therapy (HAART) in HIV patients and cessation of immune suppressive therapies [1].
REPORT OF CASE
We describe a patient who presented to the emergency department as an urgent referral from her neuro-ophthalmologist with presumed cortical blindness. One month prior, the patient had complained to her husband of “dizziness” followed several days later by “blurry vision.” Her symptoms slowly worsened to include vague difficulties with her peripheral vision and difficulty reading. Ophthalmologic evaluation was inconclusive. The patient’s husband described other symptoms, including progressive difficulties with operating kitchen appliances.
Her medical history was significant for emphysema, hypertension, and hyperlipidemia. Her medications included aspirin, atorvastatin, verapamil, hydrochlorothiazide, tolterodine, fluticasone/salmeterol, montelukast, and albuterol as needed. On initial physical exam in the emergency department her blood pressure was 130/59, pulse 96, and temperature 96.2. She was oriented only to person, place, and day, had impaired recall and attention, and had difficulty following complex commands. Her mini-mental status exam was 24/30. Extraocular movements were normal. Visual field testing showed her to have constricted fields bilaterally, though the exam was complicated by her poor attention. Acuity was 20/70 bilaterally. Strength and sensation testing were intact.
Routine blood chemistry and blood count testing were unrevealing. Head CT scan performed in the emergency department showed multiple areas of hypodensity in the white matter bilaterally. A routine chest X-ray also performed in the emergency department showed elevated hila bilaterally, bullous changes in the middle and upper zones, also extending into the lower zones as well. Apical wall loss with fibrosis was also seen. The patient was admitted to the neurology service for further workup of her mental status change.
MRI of the head with and without gadolinium showed nonspecific scattered areas of high T2/FLAIR signal predominantly in the left parieto-occipital and right temporal lobes without abnormal contrast enhancement. These findings were thought to be most characteristic of acute disseminated encephalomyelitis (ADEM) or low-grade glioma (Figure 1).
Figure 1.
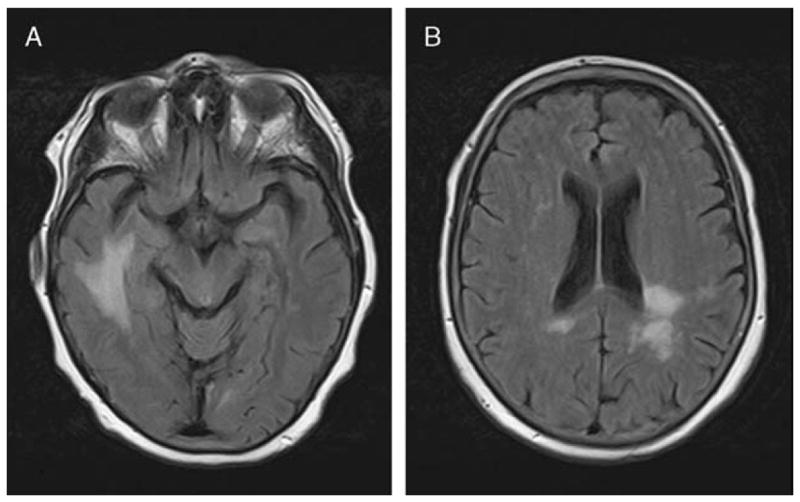
Axial MRI FLAIR sequence shows increased signal in the right temporal (a) and left parieto-occipital (b) areas.
Cerebrospinal fluid (CSF) analysis showed glucose 73, protein 30, WBC 1. CSF Lyme, syphilis, herpes zoster, culture, and IgG were all negative or normal. These findings were thought to be not clearly consistent with ADEM. Further serum studies, including anti-nuclear antibody, angiotensin-converting enzyme, anti-neutrophil cytoplasmic antibody, and C-reactive protein were all within normal limits. A paraneoplastic panel was negative. Electroencephalography showed generalized slowing, slightly increased over the right hemisphere, without epileptiform activity.
Clinically, the patient deteriorated somewhat rapidly over the next week as further studies were obtained. She became more agitated and confused and required a 24 hour sitter. Cerebral angiography, performed to rule out cerebral vasculitis, was normal. CT chest, abdomen, and pelvis was obtained to evaluate for an occult malignancy. There were no findings suspicious for malignancy, however a pretracheal lymph node, bi-apical scarring and pleural thinning of the lungs, and perilymphatic nodules were observed on the scan. Bronchoscopy and transbronchial biopsy were unremarkable. The pulmonary consultants therefore believed the abnormal findings on chest X-ray and CT were likely due to long-standing emphysema.
MR spectroscopy showed abnormal signal intensity within the left periatrial white matter with elevation of the choline concentration as well as a choline:creatine ratio which approached 2. This was concerning, though not specific, for neoplasm. A subsequent brain biopsy showed the presence of numerous macrophages and relative preservation of axons suggestive of a demyelinating process (Figure 2). Immunocytochemical staining of tissue sections from the biopsy specimen were positive for SV40 antigen in nuclei, which was consistent with the diagnosis of PML (Figure 2). Further testing of CSF for JC virus by polymerase chain reaction assay showed 204,497 copies of the virus, further establishing the diagnosis as PML.
Figure 2.
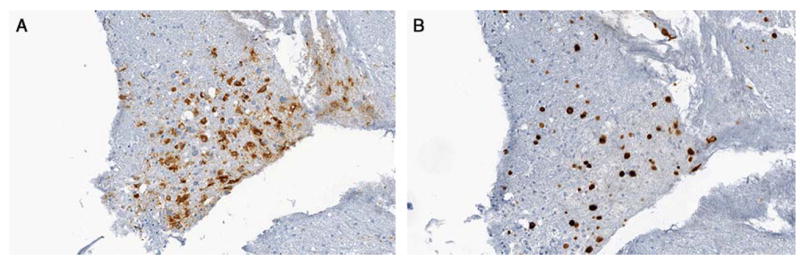
Brain biopsy - Immunostain for CD68 highlights numerous positive cells consistent with macrophages. The presence of numerous macrophages and relative preservation of axons is suggestive of a demyelinating process (original magnification X200). Brain biopsy - Immunostain for SV40 shows positivity in large nuclei. The presence of SV40 positive nuclei is suggestive of a demyelinating process such as progressive multifocal leukoencephalopathy. SV40 is a DNA virus which belongs to the genus Polyomavirus. Other Polyomaviruses including BK and JC can be associated with kidney disease and demyelinating brain diseases (original magnification X200).
Due to this unexpected diagnosis, HIV testing was repeated and was normal. CD4 and CD8 counts were 182 and 122, respectively, and on repeat testing only several days later were 627 and 269. Hematologist consultation suggested the initial finding was either spurious or related to a subclinical viral infection, unrelated to the patient’s current condition. They specifically commented that this was likely not representative of true CD4 lymphocytopenia.
The patient became progressively more confused and ultimately nonverbal. She was unable to eat and had several focal seizures of her right arm. After much discussion of the diagnosis and prognosis with the family, they opted for comfort care and the patient was discharged to hospice, where she expired about 1 week later.
An autopsy was obtained and showed several findings that were diagnostic for PML and, unexpectedly, systemic sarcoidosis. The heart contained dense scars in the myocardium with multi-nucleated giant cells (Figure 3). The respiratory system showed interstitial fibrosis in the bilateral apices with multi-nucleated histiocytes and giant cells present diffusely in the lung parenchyma, representing degenerated granulomas (Figure 3). There was mediastinal and hilar lymphadenopathy with hyalinized lymph nodes showing multi-nucleated histiocytes and giant cells similar to the findings in the heart and lungs (Figure 3). Neuropathologic examination showed multiple foci of rarified white matter with abundant macrophages. There were ground glass inclusions suggestive of viral infection in numerous nuclei. There was evidence of demyelination and some degree of axonal loss (Figure 3). SV40 staining was again present in multiple nuclei consistent with polyomavirus infection, as seen in the previously obtained brain biopsy. Other organ systems were essentially unremarkable.
Figure 3.
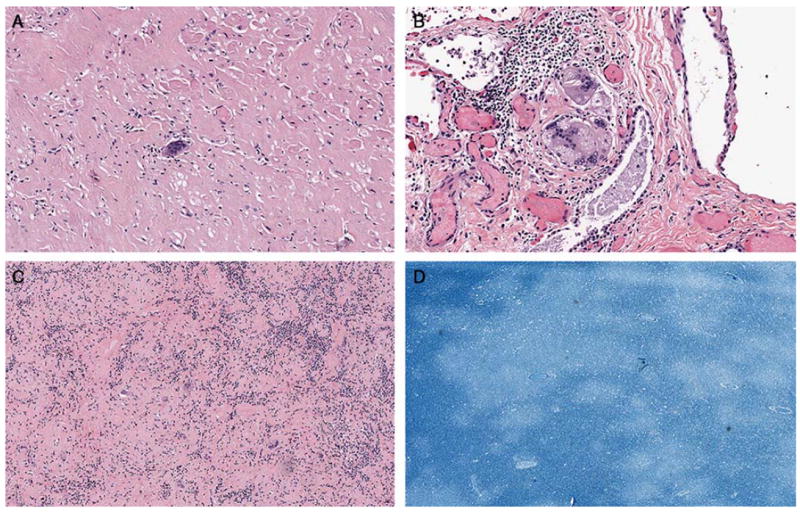
Autopsy heart - Dense scar with multinucleated giant cell in the anterior myocardium (original magnification X200). B. Autopsy right lung apex - Multinucleated giant cell with asteroid bodies which are seen in granulomatous diseases such as sarcoidosis (original magnification X200). C. Autopsy mediastinal lymph node - Hyalinized lymph node with multinucleated giant cells (original magnification X100). D. Section of brain from autopsy - Luxol fast blue stain shows areas of demyelination (original magnification X20).
DISCUSSION
This patient was notable due to the rarity of PML in patients without known immune suppression, history of malignancy, or previously diagnosed chronic inflammatory or autoimmune disease. This fact led to multiple other diagnoses being initially considered. Ultimately, brain biopsy ultimately indicated the correct diagnostic avenue to pursue. PML in association with undiagnosed and untreated sarcoidosis is exceptionally rare.
The earliest descriptions of PML noted an association with sarcoidosis. This was initially seen in 1955 by Christensen and Fog, though this was several years before PML was described as a distinct clinical entity [2]. A recent study focusing on 58 HIV-negative patients with PML, found that approximately 9% of these had underlying systemic sarcoidosis [1]. It appears that the association is unrelated to prior steroid use or other immunosuppressive therapy [2].
A literature search results in only a handful of cases where sarcoidosis, systemic or pulmonary, is thought to be directly related to coincident PML. Völker et al in 2007 listed only 8 published reports (a few of which listed several cases) of sarcoidosis and PML occurring together [4]. There have been few additional reports identified by literature search in more recent years. Furthermore, PML as the first manifestation of sarcoidosis has been infrequently reported, and in these cases it has been related inflammatory involvement of the bone marrow [5]. Our patient did not show clear evidence of hematologic involvement – the bone marrow specimen showed no evidence of sarcoidosis, though the sample was technically inadequate for diagnostic purposes, and her initially low CD4/CD8 count essentially normalized on subsequent testing. Though perplexing, CD4/CD8 lymphocytopenia can be seen in systemic sarcoidosis, independent of granulomatous involvement of the bone marrow [6]. Ultimately, there have been few, if any, reported cases of PML presenting as the first clinical manifestation of sarcoidosis without clear immune suppression, making this case particularly noteworthy.
The effectiveness of treatment for PML is uncertain in non-HIV patients. There have been numerous small trials with various experimental treatments, including cytarabine, cidofivir, topotecan, and mefloquine, all with varying results. There is no clear evidence, however, that any of these treatments are commonly effective in reversing the course of disease [1,3,5,7-11]. These treatment options were not considered for our patient. Within about 3 weeks, the disease left her completely dependent. When the patient was unable to feed on her own, the family opted for comfort care.
Though the vast majority of PML cases occur in HIV affected individuals, our case suggests that PML should be a diagnostic consideration in patients presenting with classic clinical and radiographic findings, despite lack of evidence of immune suppression or a chronic inflammatory condition. The association of PML and sarcoidosis is rare, but appears increasingly valid, even without prior use of immune suppressive medications. In our case, delay in diagnosis likely did not affect this patient’s clinical course due to the rapidity of her demise. However, in cases where experimental treatments are being considered, it is important to recognize the spectrum of patients who can be affected by this devastating disease.
Footnotes
The authors disclose no financial disclosures or conflicts of interest.
References
- 1.Koralnik I. Progressive Multifocal Leukoencephalopathy Revisited: Has the Disease Outgrown Its Name? Ann Neurol. 2006;60:162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbloom M, Uphoff D. The Association of Progressive Multifocal Leukoencephalopathy and Sarcoidosis. Chest. 1983;83:572–575. doi: 10.1378/chest.83.3.572. [DOI] [PubMed] [Google Scholar]
- 3.Owczarczyk K, et al. Progressive multifocal leucoencephalopathy in a patient with sarcoidosis – successful treatment with cidofivir and mirtazipine. Rheumatology. 2007;46:890. doi: 10.1093/rheumatology/kem049. [DOI] [PubMed] [Google Scholar]
- 4.Volker HU, Kraft K. Progressive Multifocal Leukoencephalopathy Developing In Advanced Pulmonal Sarcoidosis. Clin Neurol Neurosurg. 2007;109(7):624–30. doi: 10.1016/j.clineuro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.DeRaedt S, Lacor P. Progressive Multifocal Leukoencephalopathy As First Manifestation of Sarcoidosis. Clin Neurol Neurosurg. 2008;110(2):186–9. doi: 10.1016/j.clineuro.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Sweiss NJ, et al. Significant CD4, CD8, and CD19 Lymphopenia in Peripheral Blood of Sarcoidosis Patients Correlates with Severe Disease Manifestations. PLoS One. 2010 Feb 5;5(2):e9088. doi: 10.1371/journal.pone.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yagi T, et al. Progressive multifocal leukoencephalopathy developed in incomplete Heerfordt syndrome, a rare manifestation of sarcoidosis, without steroid therapy responding to cidofivir. Clin Neurol Neurosurg. 2010 Feb;112(2):153–6. doi: 10.1016/j.clineuro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Houston S. Failure of cidofivir therapy in progressive multifocal leukoencephalopathy unrelated to human immunodeficiency virus. Clin Infect Dis. 2001 Jan;32(1):150–2. doi: 10.1086/317539. [DOI] [PubMed] [Google Scholar]
- 9.Royal WR, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003 Jun;9(3):411–9. doi: 10.1080/13550280390201740. [DOI] [PubMed] [Google Scholar]
- 10.Brickelmaier M, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009 May;53(5):1840–9. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman Roberta. Malaria drug fails to fulfill promise in PML. Neurology Today. 2011 Apr 21;:8. [Google Scholar]


