Abstract
Objectives
Older adults with early forms of neurodegenerative disease are at risk for functional disability, which is often defined by the loss of independence in instrumental activities of daily living (IADLs). The current study investigated the influence of mild changes in everyday functional abilities (referred to as functional limitations) on risk for development of incident functional disability.
Method
407 participants, who were considered cognitively normal or diagnosed with mild cognitive impairment (MCI) at baseline, were followed longitudinally over an average 4.1 years (range = 0.8-9.2 years). Informant-based ratings from the Everyday Cognition (ECog; Farias et al., 2008) and the Instrumental Activities of Daily Living (Lawton & Brody, 1969) scales assessed the degree of functional limitations and incident IADL disability, respectively.
Results
Cox proportional hazards models revealed that more severe functional limitations (as measured by the Total ECog score) at baseline were associated with about a four-fold increased risk of developing IADL disability a few years later. Among the ECog domains, functional limitations in Everyday Planning, Everyday Memory, and Everyday Visuospatial domains were associated with the greatest risk of incident functional disability. These results remained robust even after controlling for participants' neuropsychological functioning on tests of executive functions and episodic memory.
Conclusions
Current findings indicate that early functional limitations have prognostic value in identifying older adults at risk for developing functional disability. Findings highlight the importance of developing interventions to support everyday abilities related to memory, executive function, and visuospatial skills in an effort to delay loss of independence in IADLs.
Keywords: Alzheimer's disease, dementia, everyday function, instrumental activities of daily living, cognition, disability
Introduction
Loss of autonomy and independence are among the top concerns of older adults (Andersen, Wittrup-Jensen, Lolk, Andersen, & Kragh-Sorensen, 2004). A hallmark feature of the dementia is functional disability. Functional disability is often operationalized as the loss of the ability to independently perform instrumental activities of daily living (IADLs), such as cooking, performing household tasks, managing finances and medications, and driving (Albert et al., 2011; Barberger-Gateau et al., 2004; Peres, Helmer, Letenneur, Jacqmin-Gadda, & Barberger-Gateau, 2005; Peres, Verret, Alioum, & Barberger-Gateau, 2005). Loss of independence in IADLs, in turn, is associated with elevated risk for numerous deleterious outcomes, including greater caregiver burden (Gallagher et al., 2011; Razani et al., 2007), initiation of home-help services (Biegel, Bass, Schulz, & Morycz, 1993; Robinson, Buckwalter, & Reed, 2005), placement in residential and nursing home care (Gaugler, Kane, Kane, Clay, & Newcomer, 2003; Wattmo, Londos, & Minthon, 2014), and reduced quality of life for the affected individual and their families (Andersen et al., 2004; Conde-Sala, Garre-Olmo, Turro-Garriga, Lopez-Pousa, & Vilalta-Franch, 2009). Additionally, the long-term economic burden associated with providing care to older adults with functional disability is considerable (Gaugler et al., 2013; Zhu et al., 2008), and it is estimated that a delay in the onset of functional disability of five years would dramatically reduce the total costs for care of individuals with AD (Alzheimer's Disease) (Alzheimer's Association Expert Advisory Workgroup on NAPA, 2012). To this end, it is critical to better understand early precursors of functional disability in IADLs in order to inform when and how to intervene.
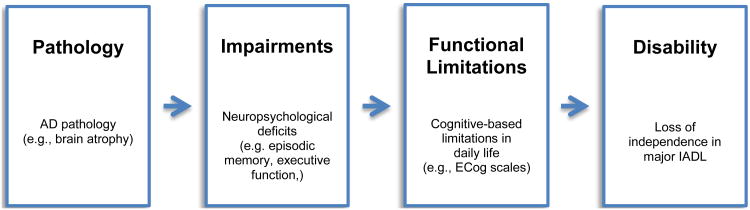
The Disablement Process Model (Verbrugge & Jette, 1994) is a useful theoretical framework from which to study the evolution of functional disability. This model has most commonly been used to conceptualize how chronic medical illnesses and physical functional limitations (e.g. difficulty with walking) lead to functional disability (A. M. Jette, 2006; D. U. Jette et al., 1997; McDonough & Jette, 2010), but it is also very applicable to understanding functional disability associated with cognitive impairment and dementia (Ali, Coulson-Thomas, Dixon, & Williams, 2013; Barberger-Gateau et al., 2004; Barberger-Gateau, Fabrigoule, Amieva, Helmer, & Dartigues, 2002; Peres, Verret, et al., 2005). According to this model (see Figure 1), disease(s) develops and disrupts basic organ systems (e.g., in the case of AD, neuropathological changes in the brain), resulting in organ-specific ‘impairments’ (e.g., impairments in cognitive processes, such as memory and executive function, among others). Cognitive/neuropsychological impairments can subsequently lead to ‘functional limitations,’ which are defined in the current study as mild restrictions in one's ability to utilize specific cognitive processes in performing everyday tasks, such as remembering a grocery list, following a map to a new location, and keeping financial records organized (Barberger-Gateau et al., 2002; Verbrugge & Jette, 1994). According to this model, functional limitations are the precusors to ‘functional disability.’ That is, as functional limitations become more severe, they can eventually lead to disability, which is defined as complete loss of independence in major domains of life (e.g., IADL domains), such as the ability to shop, drive, and manage finances independently.
Figure 1. The main disease-based disablement pathway (adapted from Verbrugge & Jette, 1994).
While the Disablement Process Model has not been extensively used in the cognitive aging and dementia literature, there is support for various aspects of this model. First, it is well documented that indicators of AD pathology such as whole brain and hippocampal atrophy are associated with neuropsychological impairment and clinical disease progression (see reviews by Buckner, 2004; Jack et al., 2010; Nelson, Braak, & Markesbery, 2009). Greater neuropsychological impairments, particularly in memory and executive functions, have also been shown in cross-sectional studies to correlate with more severe functional limitations (Farias, Park, et al., 2013) and neuropsychological performance predicts faster subsequent decline in a variety of functional outcome measures (Cahn-Weiner et al., 2007; Cahn-Weiner, Malloy, Boyle, Marran, & Salloway, 2000; Cahn-Weiner, Ready, & Malloy, 2003; Farias, Mungas, Reed, Haan, & Jagust, 2004).
Also consistent with the Disablement Process Model, there is indirect evidence to support the hypothesis that functional limitations in early disease increase risk of the development of disability or loss of IADL independence. Specifically, there is a growing body of work demonstrating that individuals with Mild Cognitive Impairment (MCI), often a prodromal state prior to a diagnosis of dementia, show mild changes in their ability to perform everyday activities (Brown, Devanand, Liu, Caccappolo, & Alzheimer's Disease Neuroimaging Initiative, 2011; Burton, Strauss, Bunce, Hunter, & Hultsch, 2009; Peres et al., 2011; Perneczky et al., 2006; Tabert et al., 2002). There is even some evidence to suggest that there are detectable functional limitations in cognitively normal elders who later go on to develop MCI (Farias, Chou, et al., 2013; Marshall et al., 2014).
Based conceptually on the Disablement Process Model, as well as on the empirical work emphasized above, the present study examined the degree to which functional limitations measured at study baseline are associated with the risk of the later development of functional disability in IADLs. Functional limitations were assessed using the Everyday Cognition scales (ECog) from which a summary ‘Total Score’ can be derived as well as six specific functional limitation domains: Everyday Memory, Everyday Language, Everyday Visuospatial abilities, and three everyday executive domains including Everyday Planning, Everyday Organization, and Everyday Divided Attention. While functional limitations as measured by the ECog are cognitively oriented, they are operationalized within the context of specific functional everyday tasks. Incident disability was operationalized as loss of the ability to independently perform IADLs. We hypothesized that more severe functional limitations at study baseline (using the ECog Total Score) would be associated with increased risk for incident disability (e.g. developing dependence in two or more IADL domains) over study follow-up. We further speculated that certain types of functional limitations on the ECog, and more specifically Everyday Memory and everyday executive functions (e.g., Everyday Planning, Everyday Organization and/or Everyday Divided Attention), would be most strongly associated with the subsequent development of disability. Because neuropsychological impairment has previously been associated with IADL disability (Boyle et al., 2003; Cahn-Weiner et al., 2007; Cahn-Weiner et al., 2000; Cahn-Weiner et al., 2003), follow-up analysis also examined whether functional limitations at study baseline predict incident disability above and beyond the degree of neuropsychological impairment present at baseline. Finally, since we anticipated that functional limitations increase risk of disability, which, in turn, should be associated with a diagnosis of dementia, secondary analysis also examined the association between functional limitations and risk of incident dementia.
Methods
Participants
Participants in this study were part of a longitudinal research cohort at the University of California, Davis Alzheimer's Disease Center (ADC) and have been described elsewhere (e.g., (Farias et al., 2008; Farias, Park, et al., 2013). Participants were selected for inclusion in the present study if they: 1) were older adults who spoke English, 2) had an informant with whom the participant had regular contact and could complete informant-based ratings, 3) considered cognitively normal or diagnosed with MCI at study baseline, and 4) had baseline data for the functional measures of interest (ECog Scale and Lawton & Brody IADL ratings) and had longitudinal data (e.g., at least one follow-up visit)for the primary disability outcome variable (IADL ratings). Exclusion criteria included an unstable major medical illness, a current severe/debilitating psychiatric disorder (milder forms of depression were acceptable), another existing neurologic condition outside of the target diseases (e.g., Alzheimer's disease (AD) and related disorders, and cerebrovascular disease), and active alcohol or drug abuse/dependence.
All participants received annual multidisciplinary clinical evaluations that included a physical and neurological exam, imaging, lab work, and neuropsychological testing from the Alzheimer's Disease Uniform Dataset Neuropsychological Battery (Weintraub et al., 2009). For participants in this study baseline diagnosis was categorized as cognitively normal or MCI. Participants with MCI were diagnosed according to standard clinical criteria according to current Alzheimer's Disease Centers Uniform Data Set guidelines (Morris et al., 2006). Consistent with the most recent diagnostic guidelines for MCI due to AD (Albert et al., 2011), it was permissible for individuals with MCI to have mild problems performing complex functional tasks, but they had to require only minimal assistance from others. Over the course of the study, some participants converted to a diagnosis of dementia (12 Normals and 77 MCI). Dementia was diagnosed using the criteria outlined in the Diagnostic and Statistical Manual for Mental Disorders–Third Edition–Revised (DSM-III-R; American Psychiatric Association, 1987), but criteria were modified so that a diagnosis of dementia was made if there were significant impairments in two or more cognitive domains. For these individuals diagnosed with dementia, performance on clinical neuropsychological tests was considered significantly impaired if the score fell below 1.5 standard deviations compared to age and education-matched norms. Neuropsychological tests used to make a clinical diagnosis were separate from the neuropsychological tests used as variables in the current study. Additionally, for clinical diagnostic purposes, everyday function was assessed using a variety of standardized tests and a clinical interview with the participant and informant. Importantly, clinical diagnoses were made completely independent of the ECog and IADL ratings; that is clinicians involved in rendering the syndromic diagnosis at each annual visit did not have access to these data. All participants signed informed consent, and all human subject involvement was approved by institutional review boards at University of California at Davis, the Department of Veterans Affairs Northern California Health Care System and San Joaquin General Hospital in Stockton, California.
Assessment of everyday functional limitations
Degree of functional limitations was operationalized as a continuous variable using the Everyday Cognition (ECog) scale. The ECog is a 39-item informant-based questionnaire. It was designed specifically to be sensitive to mild functional limitations that predate the loss of independence and has been shown to be relevant to functional changes associated with MCI (Farias et al., 2008; Farias et al., 2006). The ECog items cover six cognitively-relevant domains (Everyday Memory, Everyday Language, Everyday Spatial abilities, Everyday Planning, Everyday Organization and Everyday Divided Attention) from which domain scores can be generated in addition to a total summary score. Example items include: ‘Remembering appointments, meetings or other engagements”, “Following a map to a new location,” and “Keeping financial records organized.” Informants completing the ratings were typically spouses or adult children of the participant, and in most cases, the same informant completed the ratings throughout the study. Informant ratings were made independent of the diagnosis rendered as part of the associated annual visit. On each item of the ECog scale, informants were asked to assess the participant's current level of everyday functioning in comparison to how he/she functioned 10 years earlier. In this way, individuals served as their own control. Each item on the ECog is rated on a four-point scale: 1= better or no change compared to 10 years earlier; 2 = questionable/occasionally worse; 3 = consistently a little worse; 4= consistently much worse. Higher scores indicated more severe functional limitations. Scores were calculated by summing items and dividing by the number of items completed, which allows for some missing or non-answered items (at least half of the items need to be completed to calculate a score). The current study used the ECog Total score as well as the six domain scores to measure functional limitations. Previous confirmatory factor analysis supports use of both a global score and domain-specific scores (Farias et al., 2008). Test-retest reliability for the ECog has been shown to be good (Farias et al., 2008). Literature on the ECog has also shown evidence of content, convergent and discriminant, and external validity (Farias et al., 2008; Farias, Park, et al., 2013).
Assessment of incident disability in IADLs
Disability was measured as a dichotomous variable in which the participant was coded as ‘independent’ or ‘dependent’ at each annual visit using the Lawton and Brody Instrumental Activities of Daily Living scale (Lawton & Brody, 1969). This is a widely used informant-based measure used to rate participants' abilities across eight activities, including the ability to use a telephone, shop, prepare food, complete housework, do laundry, utilize public transportation, administer medication, and handle financial responsibilities. Each item was coded dichotomously: 1 = can complete the task independently, 0 = the task must now be completed by someone else. In the current study, incident functional disability was defined as obtaining a score of zero (dependence) on two or more items of the IADL scale. To be included in this study, participants had to be coded as independent at study baseline. Inter-rater reliability is reported to be .85 for the total IADL score (Lawton & Brody, 1969). Basic activities of daily living (BADLs), while also often included in the measurement of disability, were not measured in the present study because as a whole, the sample was mildly impaired and had very few problems in BADLs.
Neuropsychological assessment
Neuropsychological functioning was assessed using the Spanish and English Neuropsychological Assessment Scales battery (SENAS). The SENAS has undergone extensive development as a battery of neuropsychological tests relevant to diseases of aging (Mungas, Reed, Crane, Haan, & Gonzalez, 2004; Mungas, Reed, Marshall, & Gonzalez, 2000; Mungas, Reed, Tomaszewski Farias, & DeCarli, 2005). Modern psychometric methods based on item response theory were used to create psychometrically matched measures across different scales. This study used two composite indices from the SENAS, episodic memory and executive function, due to previous findings that consistently reported a relationship between these abilities and everyday functioning (Bertrand & Willis, 1999; Boyle et al., 2003; Burton et al., 2009; Cahn-Weiner et al., 2007; Cahn-Weiner et al., 2000; Schmitter-Edgecombe, Woo, & Greeley, 2009). The Episodic Memory Index is a composite score derived from a multi-trial word list-learning test (Word List Learning I). The Executive Function Index was a composite measure constructed from component tasks of Category Fluency, Phonemic (letter) Fluency, and Working Memory. These measures do not have appreciable floor or ceiling effects for participants in this sample and have linear measurement properties across a broad ability range. The SENAS indices are psychometrically matched measures of domain specific neuropsychological abilities (i.e., the indices have comparable reliability and sensitivity to individual differences). SENAS development and validation are described in detail elsewhere (Gonzalez, Mungas, & Haan, 2002; Mungas et al., 2004; Mungas et al., 2000).
Statistical analyses
Two-sample t-tests, Wilcoxon rank-sum tests (for ECog scores and follow-up time), and chi-square tests (for categorical variables) were used to compare diagnostic groups on demographics, functional limitations and incident disability, and neuropsychological function. Cox proportional hazards models were used to assess associations between functional limitations and incident disability. The follow-up time from the baseline visit to either the visit at which an individual was classified as disabled or the last clinical visit (whichever came first) was used as the event time in incident disability models, with those that did not become disabled considered censored. Models were adjusted for baseline age, education, sex, and race/ethnicity. Secondary analyses assessed the association between functional limitations and incident disability independent of episodic memory and executive function. These models were then analyzed separately for those who were cognitively normal at baseline and those who were MCI at baseline to assess differences in the associations in the two diagnostic groups. Further models assessed the association between functional limitations and incident dementia; in this case, follow-up time from the baseline visit to either the visit at which an individual was classified as demented or the last clinical visit, whichever came first, was used as the event time with those that did not become demented considered censored. To further ensure that informants' reports of incident disability corroborated with objective cognitive decline, we used a mixed effects model to estimate the difference in the rates of change in episodic memory and executive functions between older adults who became disabled and those who did not become disabled. All analyses were conducted in SAS version 9.2 and a p-value < .05 was considered statistically significant.
Results
Sample characteristics at baseline
The primary sample consisted of 407 participants without dementia or disability at baseline who had ECog scores and IADL ratings collected at baseline and IADLs collected during follow-up visit (s). At baseline, participants on average were 75.3 years old (SD = 7.3), and had an average of 14.4 years (SD = 3.5, range = 0-20 years) of education. Females represented 59.2% of the sample. The racial/ethnicity breakdown was: 55.3% Caucasians, 22.7% African Americans, 15.8% Hispanics, 5.2% Asians, and 1.0% other/unknown. The majority of informants were either the spouse (51.1%) or the adult child (29.1%). Informants spent 88.7 (SD = 70.1, range=0-168) hours per week, on average with the participant. Table 1 presents baseline demographic information, ECog domain and total scores, and episodic memory and executive function composite scores on neuropsychological testing for cognitively normal older adults and MCI. Participants in this study were followed longitudinally on average for 4.1 years (range=0.8-9.2). The average time between baseline and becoming disabled or the last follow-up (whichever came first) was 3.2 years (range = 0.7-8.8 years), while the average time between baseline and becoming demented, or between baseline and the last follow-up in which the participant was seen if they never became demented over the course of follow-up, was 3.3 years (range=0.8-8.8 years).
Table 1.
Demographic characteristics, ECog domain and total scores, and cognitive functioning at baseline (unless otherwise noted).
| Variables | Normals (n = 270) | MCI (n = 137) | Effect Size | p-value |
|---|---|---|---|---|
| Age | 75.4 (6.9) | 75.3 (8.1) | .01 | .9 |
| Education | 14.1 (3.6) | 15.1 (3.3) | .28 | .008 |
| Female [N (%)] | 176 (65.2) | 65 (47.4) | 17.8 | <.001 |
| Race/Ethnicity [N (%)] | <.001 | |||
| African American | 68 (25.2) | 24 (17.5) | 7.7 | |
| Caucasian | 131 (48.5) | 93 (67.9) | 19.4 | |
| Hispanic | 55 (20.4) | 9 (6.6) | 13.8 | |
| Other/Unknown | 16 (5.9) | 11 (8.0) | 2.1 | |
| MMSE | 28.2 (1.7) | 26.3 (2.9) | .90 | <.001 |
| Everyday Memory | 1.5 (0.5) | 2.5 (0.9) | 4.2 | <.001 |
| Everyday Language | 1.3 (0.4) | 1.7 (0.6) | 2.2 | <.001 |
| Everyday Visuospatial | 1.2 (0.4) | 1.5 (0.6) | 2.0 | <.001 |
| Everyday Organization | 1.3 (0.5) | 2.0 (0.9) | 2.9 | <.001 |
| Everyday Planning | 1.2 (0.4) | 1.7 (0.7) | 2.6 | <.001 |
| Everyday Divided Attention | 1.5 (0.6) | 2.1 (0.9) | 2.5 | <.001 |
| ECog Total Score | 1.4 (0.4) | 1.9 (0.6) | 3.9 | <.001 |
| SENAS Episodic Memory1 | 0.17 (0.75) | −0.81 (0.64) | 1.36 | <.001 |
| SENAS Executive Function2 | 0.08 (0.60) | −0.24 (0.55) | .54 | <.001 |
| Length of follow-up (years) | 4.3 (2.3) | 3.6 (2.1) | 1.4 | .002 |
| Became disabled during follow-up [N (%)] | 31 (11.5) | 61 (44.5) | 33 | <.001 |
| Became demented during follow-up3 [N (%)] | 12 (4.5) | 77 (56.6) | 52.1 | <.001 |
Note. Values represented are mean (standard deviations in parentheses) unless otherwise noted. Effect size refers to Cohen's d, a probability based effect size expressed as odds for the ECog scores and time to follow-up (Ruscio, 2008), or the difference in percentage, where appropriate. All p-values are based on the two-sample t-test except for the ECog scores and time to follow-up (Wilcoxon rank sum test) and the categorical variables (chi-square test).ECog = Everyday Cognition Scale; MCI = Mild Cognitive Impairment; MMSE = Mini-Mental State Exam; SENAS = Spanish English Bilingual Neuropsychological Assessment Scales.
Missing for 84 Normals and 42 MCI.
Missing for 83 Normals and 41 MCI.
Missing for 3 Normals and 1 MCI
Functional limitations at baseline and incident disability at follow-up
We examined how the risk of incident functional IADL disability (defined as being rated as dependent in two or more IADLs) was associated with functional limitations on the ECog Total Score and individual ECog domain scores at baseline, after controlling for age, education level, sex, and race/ethnicity. As hypothesized, a higher Total ECog score at baseline, reflecting more severe overall functional limitations, predicted greater risk of incident functional disability at follow up (hazard ratio (HR) = 3.9, 95% CI = 2.8 - 5.4; p < .001). When examining each individual ECog domain as a predictor of incident disability, we found that each subscale was significantly associated with increased risk of subsequent disability (p < .001). Of the six ECog domains, the greatest risk of incident functional disability was associated with more severe functional limitations in Everyday Planning (HR = 3.1, 95% CI = 2.3 - 4.3, p < .001), Everyday Memory (HR = 2.9, 95% CI = 2.3 - 3.8, p < .001), and Everyday Visuospatial (HR-2.7, 95% CI=1.9-3.8, p < .001), such that a one unit increase in baseline Everyday Planning, Everyday Memory, and Everyday Visuospatial domains was associated with approximately a 3-fold increased risk of incident functional disability after adjusting for covariates. Table 2 presents corresponding hazard ratios associated with the ECog Total and domain scores. When models were analyzed separately for the cognitively normal individuals, the ECog Total Score (HR=3.8, p < .001), Everyday Planning (HR=3.5, p < .001), and Everyday Memory (HR=3.1, p < .001) remained associated with the highest increased risk of incident disability. The other sub-domains had similar hazard ratios to those in Table 2, except for Everyday Organization (HR=2.8, p < .001). In the MCI group, however, only the ECog Total Score (HR = 1.8, p = .01), Everyday Planning (HR = 1.6, p = .02), and Everyday Memory (HR = 1.4, p = .04) were significantly associated with incident disability.
Table 2.
Associations between ECog domain and total scores at baseline, and incident functional disability and incident dementia at follow-up after controlling for baseline age, education, sex, and race/ethnicity.
| Incident disability | Incident dementia | |
|---|---|---|
|
| ||
| Independent variable | HR (95% CI) | HR (95% CI) |
| Everyday Planning | 3.1 (2.3-4.3)* | 3.0 (2.2-4.1)* |
| Everyday Memory | 2.9 (2.3-3.8)* | 3.0 (2.3-3.8)* |
| Everyday Visuospatial | 2.7 (1.9-3.8)* | 2.7 (1.9-3.6)* |
| Everyday Language | 2.3 (1.6-3.1)* | 2.5 (1.8-3.5)* |
| Everyday Organization | 2.3 (1.9-3.0)* | 2.6 (2.1-3.3)* |
| Everyday Divided Attention | 2.1 (1.6-2.7)* | 2.3 (1.8-2.9)* |
| ECog Total Score | 3.9 (2.8-5.4)* | 4.2 (3.0-5.8)* |
Note: ECog = Everyday Cognition Scales; CI = confidence interval; HR = hazard ratio.
p< .001
Functional limitations and neuropsychological functioning at baseline and incident disability at follow-up
In the next set of analyses, we examined whether ECog scores at baseline (along with age, education, sex and race/ethnicity as covariates) predicted the risk of incident disability when also considering baseline performance on neuropsychological measures of memory and executive functions. Results showed that even after accounting for neuropsychological function at baseline, a one unit increase (2 standard deviation increase) in the total ECog was associated with over a 3-fold increase in the hazard of becoming disabled (HR = 3.1, 95% CI = 2.0-4.7, p < .001); a one standard deviation increase in the total ECog was still associated with a nearly double increase in the hazard (HR=1.8). Of the six ECog domain scores, Everyday Planning, Everyday Memory, and Everyday Visuospatial were again associated with the greatest risk of becoming disabled (see Table 3). Of the neuropsychological predictors, in all models, better episodic memory performance on the SENAS was associated with a reduced risk of becoming disabled (p <.001, data not shown), while executive function was not significant (p > .3). Overall, results showed that functional limitations at baseline remained a significant predictor of risk for incident disability at follow-up independent of neuropsychological performance. When models were run separately in each diagnostic group, the general pattern remained similar in the cognitively normal group, except that Everyday Language was no longer significant (HR=1.7, p = .19). In the MCI group, the total ECog (HR = 2.4, p = .007), Everyday Planning (HR = 2.3, p = .003), Everyday Memory (HR = 2.2, p = .002), and Everyday Visuospatial (HR = 1.8, p = .04) were significantly associated with incident disability, independent of neuropsychological function.
Table 3.
Associations between ECog domain and total scores at baseline, and incident functional disability and incident dementia at follow-up after controlling for baseline age, education level, sex, race/ethnicity, episodic memory, and executive function performance.
| Incident disability | Incident dementia | |
|---|---|---|
|
| ||
| Independent variable | HR (95% CI) | HR (95% CI) |
| Everyday Planning | 2.9 (1.9-4.2)* | 2.4 (1.6-3.7)* |
| Everyday Memory | 2.3 (1.7-3.3)* | 2.1 (1.5-2.9)* |
| Everyday Visuospatial | 2.3 (1.6-3.5)* | 2.1 (1.4-3.1)* |
| Everyday Language | 1.7 (1.1-2.7)** | 2.1 (1.3-3.3)** |
| Everyday Organization | 1.8 (1.3-2.4)* | 1.9 (1.4-2.6)* |
| Everyday Divided Attention | 1.6 (1.2-2.2)* | 1.7 (1.2-2.2)** |
| ECog Total Score | 3.1 (2.0-4.7)* | 3.2 (2.0-5.1)* |
Note: ECog = Everyday Cognition Scales; CI = confidence interval; HR = hazard ratio.
p≤ .001,
p<.05.
Incident disability and rates of cognitive change in episodic memory and executive functions
To further corroborate informants' report of incident disability, we examined the rates of objective neuropsychological change for participants who became disabled and those who did not become disabled over study follow-up. Estimates of slope differences revealed that older adults who became disabled over the course of study follow-up had a statistically significant faster rate of neuropsychological decline on measures of episodic memory (β=-.09, SE=.02, p<.001) and executive functions (β=-.07, SE=.02, p<.001) in comparison to older adults who did not become disabled.
Association of functional limitations at baseline and incident dementia at follow-up
Lastly, given that functional disability is a core feature for dementia, we examined the association of functional limitations at baseline and risk of incident dementia at follow-up. A total of 89 individuals in the sample (12 Normals and 77 MCI) progressed to dementia during follow-up; sixty-three (70.8%) of them were also disabled, while 28 of those who became disabled did not become demented. Results revealed that more severe functional limitations on the ECog Total score at baseline was associated with a 4-fold increase in incident dementia (HR = 4.2, 95% CI = 3.0-5.8, p < .001). Individual analyses of the six ECog domains were also significant (see Table 2). Again, baseline functional limitations in Everyday Planning (HR = 3.0, 95% CI = 2.2-4.1, p < .001) and Everyday Memory (HR = 3.0, 95% CI = 2.3-3.8, p < .001) predicted the greatest risk of incident dementia at follow-up. A one-unit increase at baseline in each of these ECog domains was associated with a 3-fold increased risk of incident dementia after adjusting for age, education, sex, and race/ethnicity. After accounting for episodic memory and executive function performance on the SENAS, an increase in the ECog Total score and the six ECog domain scores were each associated with an increased hazard of incident dementia, although the hazard was reduced (p < .001 for all scores except for Everyday Language and Everyday Divided Attention which had p < .05, see Table 3).
Discussion
Our main findings demonstrated that among older adults without functional disability in IADLs or dementia at baseline, more severe functional limitations at study baseline, as reported by informant ratings on the ECog, were associated with an almost four-fold increased risk of losing the ability to independently perform IADLs over the next few years. Consistent with the Disablement Process Model (Verbrugge & Jette, 1994), our results support the hypothesis that functional limitations predispose older adults with normal cognition or MCI to the development IADL disability. Such findings are consistent with prior work showing that more severe functional difficulties among older adults with MCI predict disease progression (Aretouli, Okonkwo, Samek, & Brandt, 2011; Gomar et al., 2011; Hsiung et al., 2008; Purser, Fillenbaum, Pieper, & Wallace, 2005).
Unique to this study, we also examined the relationships between different types of everyday functional limitations, as measured by the individual ECog domains, and the risk of future disability. We found that more severe functional limitations in each of the six ECog domains were associated with higher risk of becoming subsequently disabled, but there was also evidence that certain domains confer greater risk than others. Of the individual ECog domains, functional limitations in everyday activities that tax executive planning skills (e.g. anticipating events, putting together the sequence for completing a particular task, and prioritizing activities) were most strongly predictive of the loss of independence in IADLs. The functional limitation domain with the second strongest associated risk for disability was the Everyday Memory domain of the ECog, which measured functional limitations, such as difficulty remembering appointments and shopping items. Results remained similar in follow-up analysis that examined functional limitation predictors in those participants that started with a diagnosis of MCI verses those who were cognitively normal. The importance of limitations in everyday executive and memory functions in predicting greater risk for future functional decline is consistent with the body of literature that links neuropsychological measures of episodic memory and executive function to a variety of everyday functional outcomes (Cahn-Weiner et al., 2007; Cahn-Weiner et al., 2000; Cahn-Weiner et al., 2003; Farias et al., 2004; Farias, Mungas, Reed, Harvey, & DeCarli, 2009; Farias, Park, et al., 2013). In addition to everyday executive and memory functions, our findings showed that early limitations in performing everyday visuospatial tasks (e.g. following a map to a new location, finding a car in a parking lot, etc.) predicted future disability in IADLs. This is not surprising as visuospatial impairment is often an early cognitive marker of neurodegenerative disease, and common complaints, such as getting lost are frequently brought on by changes in the medial temporal lobes and parietal cortex, areas critical for navigation learning (Possin, 2010). Previous literature has also shown that neuropsychological measures of visuospatial impairments predicted IADL dependence as reported by caregivers (Glosser et al., 2002; Jefferson, Barakat, Giovannetti, Paul, & Glosser, 2006). The current findings further extend that previous work by showing that even informant ratings of early everyday executive, memory, and visuospatial functional limitations are predictive of the loss of functional independence in IADLs.
It is important to highlight that functional limitations were independent predictors of eventual loss of independence in IADLs even after considering neuropsychological test performance in executive functioning and memory. In fact, the strength of the association between functional limitation domains and risk of disability decreased only modestly when neuropsychological performance was included in the model. This suggests that everyday functional limitation ratings and neuropsychological test performance are capturing unique aspects of functioning. Consistent with this idea, most studies report fairly moderate associations between objective neuropsychological test scores and everyday functional outcomes (Chaytor & Schmitter-Edgecombe, 2003; Spooner & Pachana, 2006), calling into question the extent to which neuropsychological test performance capture how cognitive difficulties might manifest in everyday life. It has been suggested that numerous factors, including the differing environmental demands between the neuropsychological testing setting and ‘real life’ situations, and extra-individual and non-cognitive variables (e.g. physical limitations and emotional well-being), reduces the strength of the relationship between neuropsychological test performance and function in everyday life (see review by Chaytor & Schmitter-Edgecombe, 2003; see also the literature on cognitive frailty referring to simultaneous occurrence of both physical and cognitive impairment (e.g. Panza et al., 2015). While some performance-based assessments of everyday functional tasks may be more ecologically valid (McAlister & Schmitter-Edgecombe, 2013; Tucker-Drob, 2011), the lack of current availability of many of these instruments as well as practical constraints limit their use in clinical settings. Recognizing that neuropsychological test performance and ratings of everyday functional limitations may reflect separate, yet related constructs suggests that both measurements should be included as part of many clinical evaluations. The finding that functional limitation ratings on the ECog are strongly predictive of future loss of IADLs independence even when neuropsychological test performance is considered demonstrates that these ratings are of incremental value.
To further validate our findings, we examined the association between functional limitations and risk of incident dementia. As expected, baseline total functional limitations were also associated with a higher risk of getting a diagnosis of dementia over the study follow-up period. Further, the relative strength of the relationships between specific functional limitation domains and the risk of incident dementia was very similar to the analysis using disability as the outcome. It is important to note that neither the ECog nor the Lawton and Brody IADL measure were used in making a diagnosis of dementia. For this reason, there was not 100% overlap in older adults who converted to dementia over time and those who met study criteria for developing IADL disability over time.
Current findings have important implications for clinical practice and treatment. The period of time during which an older adult exhibits mild functional limitations, but remains independent in IADLs provides a critical window of opportunity to intervene. For example, the implementation of cognitive training at that point may serve to delay the development of disability (Rebok et al., 2014; Willis et al., 2006). Potentially even more fruitful may be development of interventions that specifically target enhancement of compensatory strategies to better support everyday functional abilities in the face of declining cognition. In support of this idea, a six-week behavioral intervention program designed to teach older adults with amnestic MCI on how to use an external memory support system (e.g. calendar and note taking system), showed an improvement in memory-related everyday functional limitations (Greenaway, Duncan, & Smith, 2013; Greenaway, Hanna, Lepore, & Smith, 2008). Other interventions that have specifically focused on training techniques for everyday executive function skills, such as the Goal Management Training (Levine et al., 2011; Levine et al., 2007; Robertson, 1996) and a virtual supermarket task (Jacoby et al., 2013), have also been shown to improve performance on simulated tasks of everyday demands for older adults as well as other populations, such as traumatic brain injury. Interventions used to address everyday executive function skills for attention-deficit hyperactivity disorder (Safren et al., 2010; Solanto, Marks, Mitchell, Wasserstein, & Kofman, 2008) could also be adapted for older adult populations in order to help compensate for cognitive loss and support functional independence.
The current study has several strengths that should be noted. The sample was comprised of a well-characterized and broadly representative cohort of older adults with significant educational and ethnic diversity (roughly 40% of participants are Hispanic and African American). Additionally, participants in the study were followed for up to eight years with an average follow-up rate of 4 years. As with any study, there are also several limitations. Functional capacities, both in terms of early functional limitations and loss of independence in IADLs were measured using informant report. Informant report is subject to a number of biases (Jorm et al., 1994; Zanetti, Geroldi, Frisoni, Bianchetti, & Trabucchi, 1999), including some historical knowledge of the participant's diagnostic status. While all data, including functional ratings are collected prior to participants' annual diagnostic review, informants' knowledge of a previous diagnostic status (e.g., a diagnosis of MCI in the preceding year(s)) may contribute to increased awareness and endorsement of participants' functional limitations and IADL performance. Despite these potential biases, previous work has shown that informant-reported functional abilities can be quite accurate and are of value. For example, informant functional ratings help to discriminate diagnostic groups (Farias, Mungas, & Jagust, 2005; Rueda et al., in press; Schinka, 2010) and predict disease progression (Farias, Chou, et al., 2013). Informant-reported functional abilities have also been shown to be related to concurrently objective measures of disease, including cognition (Farias et al., 2005; Morales, Bermejo, Romero, & Del-Ser, 1997; Rueda et al., in press), brain atrophy and other indicators of brain pathology (Farias, Park, et al., 2013; Rueda et al., in press). Further, using the current study sample, those who became disabled by informant report over study follow-up also showed a statistically significant faster rate of cognitive decline on neuropsychological tests of episodic memory and executive functions; such results provide additional objective support of the validity of informant-based functional ratings.
In summary, this study demonstrates that functional limitations at early stages of the disease (e.g. when older adults are cognitively normal or have mild cognitive impairments) pose significant risk for future disability and dementia. Recognizing the early role of functional limitations on future disability and dementia will allow for early identification of older adults who are in need of intervention. To this end, further development of interventions specifically aimed at enhancing and/or supporting everyday executive and memory abilities is an important avenue of further study as delaying loss of independence would have major benefits in terms of human and economic costs.
Acknowledgments
This study was supported by the following grants from the National Institute on Aging (grant numbers: AG031252, AG010220, AG031563, AG10129, and AG030514). The authors have no conflicts of interest to report. No competing financial interests exist.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Coulson-Thomas YM, Dixon RA, Williams DR. Genetic variation comparison of 15 autosomal STR loci in an immigrant population living in the UK (British Pakistanis) with an ancestral origin population from Pakistan. Forensic Sci Int Genet. 2013 doi: 10.1016/j.fsigen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association Expert Advisory Workgroup on NAPA. Workgroup on NAPA's scientific agenda for a national initiative on Alzheimer's disease. Alzheimers Dement. 2012;8(4):357–371. doi: 10.1016/j.jalz.2012.04.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-III-R. 3rd. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sorensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2:52. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretouli E, Okonkwo OC, Samek J, Brandt J. The fate of the 0.5s: predictors of 2-year outcome in mild cognitive impairment. J Int Neuropsychol Soc. 2011;17(2):277–288. doi: 10.1017/S1355617710001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberger-Gateau P, Alioum A, Peres K, Regnault A, Fabrigoule C, Nikulin M, Dartigues JF. The contribution of dementia to the disablement process and modifying factors. Dement Geriatr Cogn Disord. 2004;18(3-4):330–337. doi: 10.1159/000080127. [DOI] [PubMed] [Google Scholar]
- Barberger-Gateau P, Fabrigoule C, Amieva H, Helmer C, Dartigues JF. The disablement process: a conceptual framework for dementia-associated disability. Dement Geriatr Cogn Disord. 2002;13(2):60–66. doi: 10.1159/000048635. doi:48635. [DOI] [PubMed] [Google Scholar]
- Bertrand RM, Willis SL. Everyday problem solving in Alzheimer's patients: a comparison of subjective and objective assessments. Aging Ment Health. 1999;3(4):281–293. [Google Scholar]
- Biegel DE, Bass DM, Schulz R, Morycz R. Predictors of in-home and out-of-home service use by family caregivers of Alzheimer's disease patients. J Aging Health. 1993;5(4):419–438. doi: 10.1177/089826439300500401. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- Brown PJ, Devanand DP, Liu X, Caccappolo E Alzheimer's Disease Neuroimaging Initiative. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55(5):570–581. doi: 10.1159/000228918. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Farias ST, Julian L, Harvey DJ, Kramer JH, Reed BR, et al. Chui H. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007;13(5):747–757. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clin Neuropsychol. 2000;14(2):187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Ready RE, Malloy PF. Neuropsychological predictors of everyday memory and everyday functioning in patients with mild Alzheimer's disease. J Geriatr Psychiatry Neurol. 2003;16(2):84–89. doi: 10.1177/0891988703016002004. [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: a review of the literature on everyday cognitive skills. Neuropsychol Rev. 2003;13(4):181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- Conde-Sala JL, Garre-Olmo J, Turro-Garriga O, Lopez-Pousa S, Vilalta-Franch J. Factors related to perceived quality of life in patients with Alzheimer's disease: the patient's perception compared with that of caregivers. Int J Geriatr Psychiatry. 2009;24(6):585–594. doi: 10.1002/gps.2161. [DOI] [PubMed] [Google Scholar]
- Farias ST, Chou E, Harvey DJ, Mungas D, Reed B, DeCarli C, et al. Beckett L. Longitudinal trajectories of everyday function by diagnostic status. Psychol Aging. 2013;28(4):1070–1075. doi: 10.1037/a0034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20(9):827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed B, Haan MN, Jagust WJ. Everyday functioning in relation to cognitive functioning and neuroimaging in community-dwelling Hispanic and non-Hispanic older adults. J Int Neuropsychol Soc. 2004;10(3):342–354. doi: 10.1017/S1355617704103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, Decarli C. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord. 2006;20(4):217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Park LQ, Harvey DJ, Simon C, Reed BR, Carmichael O, Mungas D. Everyday cognition in older adults: associations with neuropsychological performance and structural brain imaging. J Int Neuropsychol Soc. 2013;19(4):430–441. doi: 10.1017/S1355617712001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Ni Mhaolain A, Crosby L, Ryan D, Lacey L, Coen RF, et al. Lawlor BA. Dependence and caregiver burden in Alzheimer's disease and mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2011;26(2):110–114. doi: 10.1177/1533317510394649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE, Hovater M, Roth DL, Johnston JA, Kane RL, Sarsour K. Analysis of cognitive, functional, health service use, and cost trajectories prior to and following memory loss. J Gerontol B Psychol Sci Soc Sci. 2013;68(4):562–567. doi: 10.1093/geronb/gbs078. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Kane RL, Kane RA, Clay T, Newcomer R. Caregiving and institutionalization of cognitively impaired older people: utilizing dynamic predictors of change. Gerontologist. 2003;43(2):219–229. doi: 10.1093/geront/43.2.219. [DOI] [PubMed] [Google Scholar]
- Glosser G, Gallo J, Duda N, de Vries JJ, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(3):198–206. [PubMed] [Google Scholar]
- Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE, Alzheimer's Disease Neuroimaging I. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer's disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68(9):961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- Gonzalez HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol. 2002;16(4):439–451. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- Greenaway MC, Duncan NL, Smith GE. The memory support system for mild cognitive impairment: randomized trial of a cognitive rehabilitation intervention. Int J Geriatr Psychiatry. 2013;28(4):402–409. doi: 10.1002/gps.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway MC, Hanna SM, Lepore SW, Smith GE. A behavioral rehabilitation intervention for amnestic mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2008;23(5):451–461. doi: 10.1177/1533317508320352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung GY, Alipour S, Jacova C, Grand J, Gauthier S, Black SE, et al. Feldman HH. Transition from cognitively impaired not demented to Alzheimer's disease: an analysis of changes in functional abilities in a dementia clinic cohort. Dement Geriatr Cogn Disord. 2008;25(6):483–490. doi: 10.1159/000126499. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Averbuch S, Sacher Y, Katz N, Weiss PL, Kizony R. Effectiveness of executive functions training within a virtual supermarket for adults with traumatic brain injury: a pilot study. IEEE Trans Neural Syst Rehabil Eng. 2013;21(2):182–190. doi: 10.1109/TNSRE.2012.2235184. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Barakat LP, Giovannetti T, Paul RH, Glosser G. Object perception impairments predict instrumental activities of daily living dependence in Alzheimer's disease. J Clin Exp Neuropsychol. 2006;28(6):884–897. doi: 10.1080/13803390591001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86(5):726–734. [PubMed] [Google Scholar]
- Jette DU, Manago D, Medved E, Nickerson A, Warzycha T, Bourgeois MC. The disablement process in patients with pulmonary disease. Phys Ther. 1997;77(4):385–394. doi: 10.1093/ptj/77.4.385. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Korten AE, Mackinnon AJ, Scott R. Complaints of cognitive decline in the elderly: a comparison of reports by subjects and informants in a community survey. Psychol Med. 1994;24(2):365–374. doi: 10.1017/s0033291700027343. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Levine B, Schweizer TA, O'Connor C, Turner G, Gillingham S, Stuss DT, et al. Robertson IH. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Stuss DT, Winocur G, Binns MA, Fahy L, Mandic M, et al. Robertson IH. Cognitive rehabilitation in the elderly: effects on strategic behavior in relation to goal management. J Int Neuropsychol Soc. 2007;13(1):143–152. doi: 10.1017/S1355617707070178. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Zoller AS, Kelly KE, Amariglio RE, Locascio JJ, Johnson KA, et al. For The Alzheimer's Disease Neuroimaging. Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2014;11(9):853–861. doi: 10.2174/1567205011666141001120903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister C, Schmitter-Edgecombe M. Naturalistic assessment of executive function and everyday multitasking in healthy older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20(6):735–756. doi: 10.1080/13825585.2013.781990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. 2010;26(3):387–399. doi: 10.1016/j.cger.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales JM, Bermejo F, Romero M, Del-Ser T. Screening of dementia in community-dwelling elderly through informant report. Int J Geriatr Psychiatry. 1997;12(8):808–816. [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14(2):209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc. 2005;11(5):620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Barulli MR, Santamato A, Seripa D, Pilotto A, Logroscino G. Cognitive Frailty - Epidemiological and Neurobiological Evidence of an Age-related Clinical Condition: A Systematic Review. Rejuvenation Res. 2015 doi: 10.1089/rej.2014.1637. [DOI] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Giannini M, Seripa D, Pilotto A, Logroscino G. Nutrition, frailty, and Alzheimer's disease. Front Aging Neurosci. 2014;6:221. doi: 10.3389/fnagi.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres K, Helmer C, Amieva H, Matharan F, Carcaillon L, Jacqmin-Gadda H, et al. Dartigues JF. Gender differences in the prodromal signs of dementia: memory complaint and IADL-restriction. a prospective population-based cohort. J Alzheimers Dis. 2011;27(1):39–47. doi: 10.3233/JAD-2011-110428. [DOI] [PubMed] [Google Scholar]
- Peres K, Helmer C, Letenneur L, Jacqmin-Gadda H, Barberger-Gateau P. Ten-year change in disability prevalence and related factors in two generations of French elderly community dwellers: data from the PAQUID study. Aging Clin Exp Res. 2005;17(3):229–235. doi: 10.1007/BF03324602. [DOI] [PubMed] [Google Scholar]
- Peres K, Verret C, Alioum A, Barberger-Gateau P. The disablement process: factors associated with progression of disability and recovery in French elderly people. Disabil Rehabil. 2005;27(5):263–276. doi: 10.1080/09638280400006515. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Sorg C, Hartmann J, Komossa K, Alexopoulos P, et al. Kurz A. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing. 2006;35(3):240–245. doi: 10.1093/ageing/afj054. [DOI] [PubMed] [Google Scholar]
- Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. 2010;16(6):466–487. doi: 10.1080/13554791003730600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. J Am Geriatr Soc. 2005;53(11):1966–1972. doi: 10.1111/j.1532-5415.2005.53566.x. [DOI] [PubMed] [Google Scholar]
- Razani J, Kakos B, Orieta-Barbalace C, Wong JT, Casas R, Lu P, et al. Josephson K. Predicting caregiver burden from daily functional abilities of patients with mild dementia. J Am Geriatr Soc. 2007;55(9):1415–1420. doi: 10.1111/j.1532-5415.2007.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Group, A. S. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH. Goal Management Training: A Clinical Manual. PsyConsult; Cambridge: 1996. [Google Scholar]
- Robinson KM, Buckwalter KC, Reed D. Predictors of use of services among dementia caregivers. West J Nurs Res. 2005;27(2):126–140. doi: 10.1177/0193945904272453. discussion 141-127. [DOI] [PubMed] [Google Scholar]
- Rueda AD, Lau KL, Saito N, Harvey D, Risacher SL, Aisen PS, et al. Alzheimer's Disease Neuroimaging Initiative. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer's disease. Alzheimers Dement. doi: 10.1016/j.jalz.2014.09.002. in press. doi: http://dx.doi.org/10.1016/j.jalz.2014.09.002. [DOI] [PMC free article] [PubMed]
- Ruscio J. A probability-based measure of effect size: robustness to base rates and other factors. Psychol Methods. 2008;13(1):19–30. doi: 10.1037/1082-989X.13.1.19. [DOI] [PubMed] [Google Scholar]
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, Otto MW. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304(8):875–880. doi: 10.1001/jama.2010.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA. Use of informants to identify mild cognitive impairment in older adults. Curr Psychiatry Rep. 2010;12(1):4–12. doi: 10.1007/s11920-009-0079-9. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23(2):168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Marks DJ, Mitchell KJ, Wasserstein J, Kofman MD. Development of a new psychosocial treatment for adult ADHD. J Atten Disord. 2008;11(6):728–736. doi: 10.1177/1087054707305100. [DOI] [PubMed] [Google Scholar]
- Spooner DM, Pachana NA. Ecological validity in neuropsychological assessment: a case for greater consideration in research with neurologically intact populations. Arch Clin Neuropsychol. 2006;21(4):327–337. doi: 10.1016/j.acn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. Devanand DP. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM. Neurocognitive functions and everyday functions change together in old age. Neuropsychology. 2011;25(3):368–377. doi: 10.1037/a0022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Wattmo C, Londos E, Minthon L. Solitary living in Alzheimer's disease over 3 years: association between cognitive and functional impairment and community-based services. Clin Interv Aging. 2014;9:1951–1962. doi: 10.2147/CIA.S71709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. Morris JC. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Group, A. S. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti O, Geroldi C, Frisoni GB, Bianchetti A, Trabucchi M. Contrasting results between caregiver's report and direct assessment of activities of daily living in patients affected by mild and very mild dementia: the contribution of the caregiver's personal characteristics. J Am Geriatr Soc. 1999;47(2):196–202. doi: 10.1111/j.1532-5415.1999.tb04578.x. [DOI] [PubMed] [Google Scholar]
- Zhu CW, Leibman C, McLaughlin T, Zbrozek AS, Scarmeas N, Albert M, et al. Stern Y. Patient dependence and longitudinal changes in costs of care in Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;26(5):416–423. doi: 10.1159/000164797. [DOI] [PMC free article] [PubMed] [Google Scholar]