Abstract
Purpose
To determine the prostate cancer detection rate of multi-parametric (MP) MRI at 3T. Precise one to one histopathologic correlation with MRI was possible using prostate MRI based custom-printed specimen molds following radical prostatectomy.
Materials and methods
This IRB approved prospective study included forty-five patients (mean age 60.2 years, range 49–75 years) with a mean PSA of 6.37ng/mL (range 2.3–23.7ng/mL), who had biopsy proven prostate cancer (mean Gleason score of 6.7; range 6 to 9). Prior to prostatectomy, all patients underwent prostate MRI on a 3T scanner which included tri-plane T2 weighted MRI, apparent diffusion coefficient maps of diffusion weighted MRI, dynamic contrast enhanced MRI, and spectroscopy.. The prostate specimen was whole mount sectioned in the mold allowing geometric alignment to MRI. Tumors were mapped on MRI and histopathology.. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of MRI for cancer detection were calculated. Additionally, the effects of tumor size and Gleason score on sensitivity of MP-MRI were evaluated.
Results
PPV of MP-MRI to detect prostate cancer was 98%, 98%, and 100% in overall prostate, peripheral zone, and central gland, respectively. Sensitivities of MRI sequences were higher for tumors >5mm in diameter, as well as for tumors with higher Gleason scores (>7) (p<0.05).
Conclusion
Prostate MRI at 3T allows for the detection of prostate cancer. A multi-parametric approach increases the predictive power of MRI for diagnosis. In this study, accurate correlation between MP-MRI and histopathology was obtained by the patient specific MRI-based mold technique.
Keywords: prostate cancer, prostatectomy, multi-parametric MRI, 3 Tesla, spectroscopy, custom made mold, histopathology
Introduction
Prostate cancer is the most common cancer among American men with 217,730 estimated new cases and 32,050 deaths expected in 2010 (1). Screening with prostate specific antigen (PSA) has led to an increased incidence of prostate cancer and the cancers detected are smaller, lower grade and lower stage. Validating imaging methods for prostate cancer detection has, therefore, become more challenging. Magnetic resonance imaging (MRI) including both anatomical and functional sequences has been shown to be effective in detection and local staging of prostate cancer at the pre-operative stage in various studies with different magnetic field strengths (2–15). However, there are limitations in validating MRI findings even with whole-mount histopathology as the “gold standard” because free-hand slicing can easily result in deformation of the prostatectomy specimens, thereby altering the orientation of the histologic sections compared to that of MRI (12, 16, 17). These mismatches between histopathology and MRI can make it difficult to assess the true accuracy of MRI. In this study, we describe our experience using a customized specimen mold that is based on the data extracted from the pre-surgical MRI, which allows sectioning of the prostate in the same planes as the in vivo MRI slices. The findings of multi-parametric MRI (T2 weighted [T2W] MRI, apparent diffusion coefficient [ADC] maps of diffusion weighted [DW] MRI, MR spectroscopy, dynamic contrast enhanced [DCE] MRI) were correlated with the resulting registered histopathology slices.
Materials and Methods
Study design and population
This prospectively designed, single institution study was approved by the local institutional review board and was compliant with HIPAA; informed consent was obtained from each patient. Forty-five consecutive patients were enrolled in the study between July 2008 and July 2009. The mean age of the patients was 60.2 years (median 60 years, range 49–75 years), and the mean PSA level was 6.37 ng/mL (median 5.8 ng/mL; range 2.3–23.7 ng/mL). All patients had biopsy-proven adenocarcinoma of the prostate; the mean Gleason score was 6.7 (median 7; range 6 to 9). The inclusion criteria required that robotic assisted radical prostatectomy and pelvic lymphadenectomy be performed within 180 days of imaging without any intervening treatment. Exclusion criteria included contraindications to MR imaging (cardiac pacemakers, prosthetic valves, intracranial aneurysm clips, shrapnel injury, severe claustrophobia, etc.) or inability to have an endorectal coil placed (anorectal surgery and colostomy, inflammatory bowel disease, severe hemorrhoids, etc).
MR Imaging
All MR imaging studies were performed using a combination of an endorectal coil (BPX-30, Medrad, Pittsburgh, PA, USA) tuned to 127.8 MHz and a 16-channel cardiac coil (SENSE, Philips Medical Systems, Best, The Netherlands) on a 3T magnet (Achieva, Philips Medical Systems, Best, the Netherlands) without prior bowel preparation. The endorectal coil was inserted using a semi-anesthetic gel (Lidocaine, AstraZeneca, US) while the patient was in the left lateral decubitus position. The balloon surrounding the coil was distended with perfluorocarbon (3 mol/L-Fluorinert, 3M, St. Paul, MN, USA) to a volume of approximately 50 mL to reduce susceptibility artifacts induced by air in the coil’s balloon. The MR imaging protocol included triplanar T2W TSE, DW MRI, 3D MR Point Resolved Spectroscopy (PRESS), axial pre-contrast T1W, axial 3D fast filed echo dynamic contrast-enhanced (DCE) MRI sequences and their detailed sequence parameters were defined in a prior study (12). Mean interval between MRI and radical prostatectomy was 60 days (range 3–180 days; median 48 days). The interval between TRUS guided biopsy and MR imaging was ≥ 10 weeks to avoid post-biopsy hemorrhage related MRI signal changes.
Preparation of the Customized MRI-based Mold
Following MRI, 3D models of each prostate were generated using ANALYZE software (Mayo Clinics, AnalyzeDirect, Inc., Overland Park, KS, USA). The 3D model generation included segmentation of the prostate capsule on in vivo triplane T2W MRI, fusion of the binary objects, and the surface extraction of high resolution 3D surfaces from the binary object. Each mold was designed using commercially available 3D computer-aided design software (Solidworks, Dassault Systèmes SolidWorks Corp., Concord, MA, USA). A 3D printer (Dimension Elite 3D printer, Stratasys, Inc., Eden Prairie, MN, USA), which “prints” by depositing acrylonitrile butadiene styreneplastic plastic was used to fabricate each mold (Figure 1). Following robotic radical prostatectomy, the specimen was fixed in formalin for 2–24 hours at room temperature and then was placed in the customized 3D mold and sliced in axial 6mm sections (18). This short period of fixation makes the specimen firm and allows slicing without distortion
Figure 1.
Customized MRI-Based prostatectomy mold.
MRI and Histopathology Analysis
Tumors were mapped prospectively on multi-parametric MRI (each MRI sequence was evaluated separately, independently) prospectively by two radiologists, (BT, PLC) with an accumulated experience of prostate MRI for 4 and 12 years, respectively. MRIs were assessed in consensus. Although both reviewers were aware that patients had biopsy proven prostate cancer, they were blinded to pre-imaging serum PSA and TRUS guided biopsy results, as well as histopathology findings. Whole mount histopathology specimens sectioned in the customized mold were mapped for individual tumor foci, dimensions and Gleason scores independently by two experienced pathologists, (HM, MJM) blinded to MRI. For tumor localization on MRI and histopathology, the prostate gland was divided into axial sections (the number of axial sections varied between 4 and 7 depending on the dimension of the prostatectomy specimen). Sectioning of the gross specimen in the molds corresponded to the axial plane of the MRI sections. These whole mount sections were processed for histopathology and paraffin-embedded sections were evaluated for presence and grade of cancer. Foci of cancer were marked on each slide with two-axis measurements in millimeters. These foci were then mapped on paper. For comparative evaluation, each of the slices was divided on paper into six regions as follows, four PZ sections (right anterior, right posterior, left anterior, left posterior) and two central (CG) regions (right and left) (Figure 2). Thus, all histology sections were annotated.
Figure 2.

Illustration demonstrates region system used for MRI and whole-mount step-section histopathologic correlation. Analysis was conducted for 4 different zones of the prostate: peripheral zone (regions 1–4), central gland (regions 5, 6), anterior peripheral zone and central gland (regions 1, 4, 5, 6) and the overall prostate gland (regions 1–6).
MR Imaging and Histopathology Correlation
The customized mold provided whole mount tissue blocks that have one-to-one correspondence to the in vivo MRI. The annotated histology images of each whole mount specimen were stringently correlated with the corresponding slice of the multi-parametric MRI (Figure 3). For region-based correlation, the annotated histology region and corresponding MRI having marked tumors were simultaneously displayed on computer using MIPAV (http://mipav.cit.nih.gov/) software and no correction or approximation was performed during correlation.
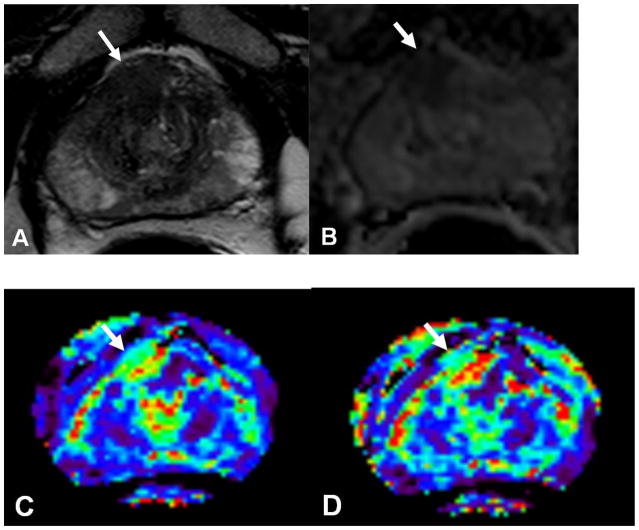
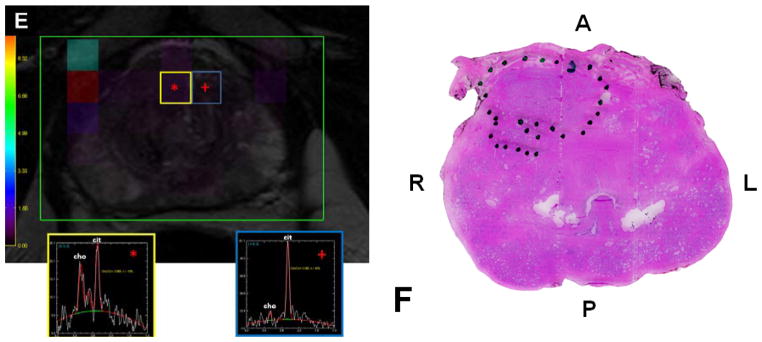
Figure 3.
Prostate cancer in 52-year-old man. (a) Axial T2-weighted and ADC map of diffusion weighted (b) MR images demonstrate a low-signal-intensity lesion (arrow) in right mid anterior central gland lesion suspicious for cancer. Ktrans (c) and kep (d) maps of dynamic contrast enhanced MRI localize tumor (arrow). MR spectroscopy (e) demonstrates increased ratio of choline (cho) to citrate (cit) in the right mid anterior central gland lesion (*) when compared with normal adjacent left side (+). Histopathologic slide (f) at mid prostate level confirms presence of tumor (Gleason score, 7) (black dotted line) detected on multi-parametric MRI (A = anterior, L = left, P = posterior, R = right).
Statistical Analysis
Statistical analysis was undertaken to assess correlation between MRI findings and histopathology. Sensitivity, specificity, positive and negative predictive values of MRI were calculated in peripheral zone (PZ), in central gland (CG), in anterior peripheral zone, which includes the anterior stroma, bilateral anterior horns of the PZ and central gland (A&CG) and in overall prostate gland. Sensitivity of an MRI sequence was defined as the probability of correctly identifying a histopathologically-proven tumor focus in a given region. Specificity was defined as the probability of correctly identifying regions negative for tumor. Estimates of sensitivity and specificity were obtained by first estimating sensitivity and specificity across all levels of zones of interest for each patient. Sensitivity and specificity were then estimated by averaging the individual-specific estimates across patients. The variance of the estimate is the sample variance divided by the number of patients. We tested for differences in sensitivity and specificity between different modalities by testing for difference in individual-specific estimates using the paired Wilcoxon rank test.
We then analyzed the effect of histopathologic variables on the sensitivity of each MRI sequence accounting for the correlation among the multiple regions on the same patient. Specifically, we evaluated the effect of lesion size (greatest diameter ≤5 mm vs. >5 mm) and Gleason score (≤7 vs. >7) for each tumor focus in 4 different zones defined above. Generalized estimating equations (GEEs) with logit link and working independence correlation structure were used to estimate sensitivity and to test the effect of histopathological variables on sensitivity. GEEs were also used to evaluate the combined diagnostic accuracy of multiple MRI variables in a region on the probability of cancer within that region. Robust variance estimate and delta method were used to calculate the standard errors of positive predictive value (PPV). Eleven GEE models with different combinations MRI sequences were fitted for each zone and each modality. Wald test with robust variance estimate was used for inference. All p-values correspond to two-sided tests with a p-value <0.05 considered as statistically significant.
Results
Histopathologic findings
Whole mount histopathologic evaluation of 45 prostatectomy specimens revealed 342 tumor positive regions (281 [82%] in the PZ, 61 [18%] in the CG) among a total of 1746 regions. Of these 342 tumor positive regions, 110 (90 [82%] in PZ, 20 [18%] in CG) contained tumors ≤5 mm in diameter, whereas 232 (191 [82%] in PZ, 41 [18%] in CG) contained tumors >5 mm in diameter. Gleason scores were ≤7 in 235 (194 [82.5%] in PZ, 41 [17.5%] in CG) regions, and were >7 in 107 (87 [81%] in PZ, 20 [19%] in CG) regions. In histopathology, extracapsular extension was detected in 20 regions in 12 prostatectomy specimens. Seminal vesicle and lymph node invasions were detected in 2 and 3 patients, respectively.
MRI findings
Based on the results of the generalized estimating equations/logistic regression, all four MRI combined sequences provided significant improvement in the positive predictive value than each single MRI sequence and some doublet MRI sequences (p<0.01) (Table 1). For example, in the PZ, PPV estimated from the GEE using all four MRI sequences yielded PPV of 98%, significantly higher than using T2W MRI alone (PPV=69%) and using a combination of T2W MRI and ADC maps of DW MRI. In the CG, PPV using all four MRI sequences yield 97% PPV, compared to PPV of 87% using T2W MRI alone. Tables 2 and 3 summarize the individual sensitivity, specificity, positive predictive and negative predictive values of the 4 MRI sequences for peripheral zone (PZ), central gland (CG), anterior PZ and central gland (A&CG) and overall prostate gland. Sensitivity for T2W MRI and ADC maps of DW MRI was significantly higher than MRS and DCE MRI in the PZ and in the overall prostate gland (p<0.01 for all pair wise comparisons), whereas for CG and A&CG regions T2W MRI, ADC maps of DW MRI and DCE MRI had significantly higher sensitivity than MRS (p<0.01). PPV of T2W MRI, ADC maps of DW MRI, MRS, DCE MRI were 0.7, 0.73, 0.93, 0.86, respectively in the overall prostate gland (p<0.01); whereas negative predictive values of each MRI sequence were similar (Table 3). The results presented in Table 4 suggest that sensitivity increases with the lesion size and Gleason score. With the exception of MRS in the PZ and overall prostate gland, the sensitivities were higher for tumors that were >5mm in diameter as compared with those ≤5mm. Sensitivity improves for tumors with higher Gleason scores (>7) in each modality in all prostate zones (p<0.01) (Table 4).
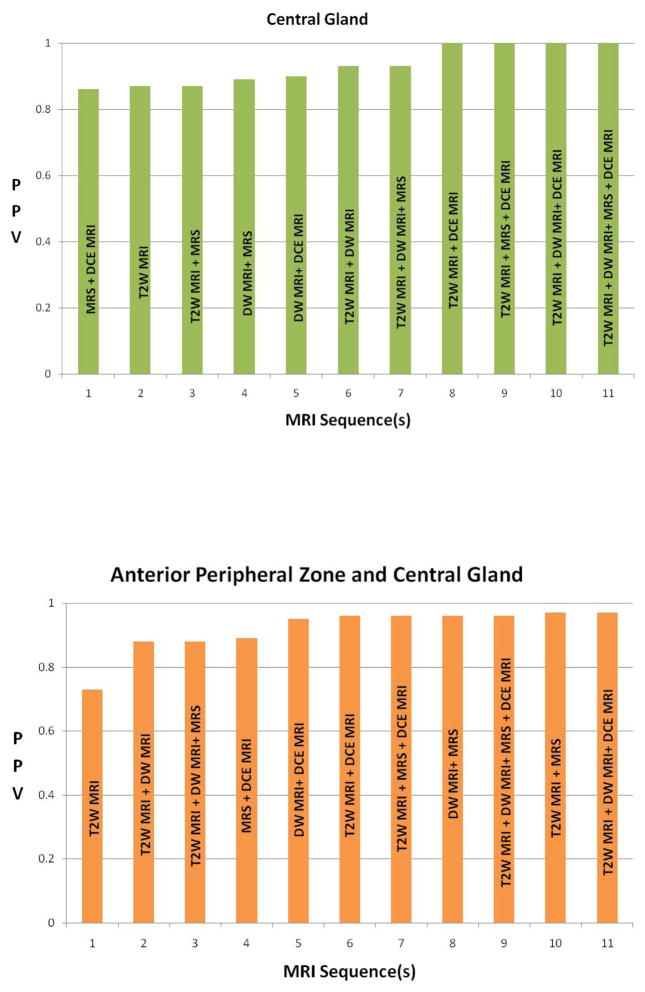
Table 1.
Bar graphs demonstrate results of the generalized estimating equations/logistic regression for tumor prediction in different prostate zones. All four MRI sequences were found to provide an additive predictive value, however this is more pronounced in the peripheral zone and in the central gland.
Table 2.
Sensitivity and specificity for each MRI sequence in different zones of prostate gland. In both peripheral zone and overall gland, except for the comparisons between T2W MRI and ADC maps of DW MRI, the four modalities had significantly different sensitivity and specificity (P<0.01), with T2W having the highest sensitivity and MRS having the highest specificity. In the central gland, most of the differences in both sensitivity and specificity were not significant. In anterior peripheral zone & central gland, T2W MRI, ADC maps of DW MRI and DCE MRI had significantly higher sensitivity than MRS (P<0.01).
| Sensitivity | Specificity | |||||||
|---|---|---|---|---|---|---|---|---|
| T2W | ADC | MRS | DCE | T2W | ADC | MRS | DCE | |
| Peripheral zone | 0.65 (0.04) | 0.57 (0.04) | 0.17 (0.04) | 0.39 (0.05) | 0.9 (0.02) | 0.93 (0.02) | 1 (0) | 0.97 (0.01) |
| Central gland | 0.15 (0.08) | 0.22 (0.09) | 0.08 (0.06) | 0.22 (0.09) | 1 (0) | 0.97 (0.01) | 1 (0) | 0.99 (0) |
| Anterior PZ& Central gland | 0.38 (0.07) | 0.44 (0.07) | 0.15 (0.05) | 0.31 (0.07) | 0.98 (0.01) | 0.97 (0.01) | 1 (0) | 0.99 (0) |
| Overall gland | 0.58 (0.04) | 0.53 (0.04) | 0.16 (0.04) | 0.38 (0.05) | 0.93 (0.01) | 0.95 (0.01) | 1 (0) | 0.98 (0.01) |
Table 3.
PPV and NPV for the each MRI sequence in different prostate zones. PPVs were significantly different among the four MRI sequences (p<0.01), whereas NPVs were similar.
| PPV | NPV | |||||||
|---|---|---|---|---|---|---|---|---|
| T2W | ADC | MRS | DCE | T2W | ADC | MRS | DCE | |
| Peripheral zone | 0.69 (0.05) | 0.74 (0.04) | 0.94 (0.04) | 0.86 (0.05) | 0.89 (0.02) | 0.87 (0.02) | 0.8 (0.02) | 0.84 (0.02) |
| Central gland | 0.87 (0.1) | 0.63 (0.13) | 0.89 (0.1) | 0.86 (0.07) | 0.92 (0.02) | 0.92 (0.02) | 0.91 (0.02) | 0.92 (0.02) |
| Anterior PZ & Central gland | 0.73 (0.09) | 0.75 (0.06) | 0.96 (0.04) | 0.89 (0.04) | 0.93 (0.01) | 0.94 (0.01) | 0.91 (0.01) | 0.92 (0.01) |
| Overall gland | 0.7 (0.05) | 0.73 (0.04) | 0.93 (0.04) | 0.86 (0.04) | 0.9 (0.01) | 0.89 (0.01) | 0.83 (0.01) | 0.87 (0.01) |
Table 4.
Effects of tumor characteristics (Gleason score and tumor size at histopathology) on sensitivity of each MRI sequence in different prostate zones. With the exception of MRS in the PZ and overall prostate gland, all 4 MRI sequences had significantly higher sensitivity in detecting larger tumors and tumors with Gleason score > 7 (p<0.05 for all comparisons).
| Overall Prostate Gland | ||||
|---|---|---|---|---|
| SENSITIVITY | MRI sequence | |||
| T2W MRI | DCEMRI | MR spectroscopy | DW MRI | |
| Tumor type | ||||
| Gleason score <=7 | 0.47 (0.05) | 0.27 (0.05) | 0.14 (0.04) | 0.45 (0.05) |
| Gleason score > 7 | 0.8 (0.06) | 0.69 (0.08) | 0.32 (0.14) | 0.74 (0.07) |
| Size <= 5 mm | 0.37 (0.05) | 0.13 (0.03) | 0.08 (0.03) | 0.33 (0.06) |
| Size > 5 mm | 0.68 (0.05) | 0.53 (0.07) | 0.25 (0.08) | 0.64 (0.06) |
| Peripheral Zone | ||||
|---|---|---|---|---|
| SENSITIVITY | MRI sequence | |||
| T2W MRI | DCE MRI | MR spectroscopy | DW MRI | |
| Tumor type | ||||
| Gleason score <=7 | 0.56 (0.05) | 0.30 (0.05) | 0.17 (0.04) | 0.52 (0.06) |
| Gleason score > 7 | 0.85 (0.05) | 0.69 (0.09) | 0.30 (0.17) | 0.74 (0.08) |
| Size <= 5 mm | 0.45 (0.05) | 0.15 (0.04) | 0.10 (0.04) | 0.40 (0.07) |
| Size > 5 mm | 0.75 (0.05) | 0.55 (0.07) | 0.26 (0.09) | 0.68 (0.06) |
| Central Gland | ||||
|---|---|---|---|---|
| SENSITIVITY | MRI sequence | |||
| T2W MRI | DCE MRI | MR spectroscopy | DW MRI | |
| Tumor type | ||||
| Gleason score <=7 | 0.05 (0.05) | 0.12 (0.08) | 0 (0) | 0.10 (0.07) |
| Gleason score > 7 | 0.6 (0.21) | 0.7 (0.18) | 0.4 (0.23) | 0.75 (0.18) |
| Size <= 5 mm | 0 (0) | 0.05 (0.05) | 0 (0) | 0 (0) |
| Size > 5 mm | 0.34 (0.15) | 0.44 (0.14) | 0.2 (0.13) | 0.46 (0.16) |
| Anterior Peripheral Zone and Central Gland | ||||
|---|---|---|---|---|
| SENSITIVITY | MRI sequence | |||
| T2W MRI | DCE MRI | MR spectroscopy | DW MRI | |
| Tumor type | ||||
| Gleason score <=7 | 0.29 (0.07) | 0.18 (0.06) | 0.06 (0.03) | 0.35 (0.07) |
| Gleason score > 7 | 0.76 (0.13) | 0.79 (0.12) | 0.5 (0.18) | 0.84 (0.11) |
| Size <= 5 mm | 0.12 (0.07) | 0.07 (0.04) | 0.02 (0.02) | 0.29 (0.1) |
| Size > 5 mm | 0.57 (0.09) | 0.49 (0.1) | 0.27 (0.11) | 0.59 (0.09) |
On MRI, extracapsular extension was detected in 27 regions in 18 patients. The region basis sensitivity, specificity rates of MRI for extracapsular extension were 85%, 99%, respectively; whereas patient basis sensitivity and specificity rates were 78% and 79%, respectively.
Discussion
The ability to accurately detect prostate cancers with MR imaging can be useful in patients for whom systematic biopsy has failed despite rising PSA values and continued suspicion of cancer. Eventually, MR imaging may be performed before the first biopsy to more precisely guide the biopsy needle into the tumor. An ancillary benefit of imaging is that it may more accurately stage tumors in the pre-treatment stage. Potentially this technology will be useful in image-guided, focal therapy as well as whole gland therapy such as surgery or radiation. Our data indicates that multi-parametric MRI of prostate at 3T enables accurate tumor detection with reasonable sensitivity and specificity values. Among MRI sequences, ADC maps of DW MRI and DCE MRI were the two most helpful for tumor detection in the central gland, where a significant overlap between tumors and benign prostatic hyperplastic changes usually occurs. A combination of DW MRI and DCE MRI demonstrated the most promising sensitivity for anterior PZ and CG tumors. Traditionally, systemic TRUS guided biopsy undersamples the CG and the anterior PZ.
MRI has better sensitivity for detecting larger (> 5 mm in diameter) and more aggressive (Gleason score of > 7) tumors, indicating that it may preferentially detect clinically relevant tumors (19). Additionally, for overall prostate cancer detection, multi-parametric MRI performed better than any individual MRI sequence did. The improved value of performing sequential MRI at 3T which includes T2W MRI, ADC maps of DW MRI, choline/citrate ratio of MR spectroscopy and permeability parameters derived from DCE MRI can be demonstrated in this data.
The customized mold provided tissue blocks that permitted a direct one-to-one correlation with the in vivo MRI. The use of the customized mold enabled more exact correlation between each MRI parameter and the histopathologic specimen, without a requirement for a correction or an approximation approach. In prior studies, to correct the mismatches between MRI and histopathology several methods have been proposed. Scheidler J et al used a methodology which considered tumor sites detected on MRI and histopathology if they were in the same sextant within a range of one section (+/− 3–4 mm craniocaudally), provided that they were in the same anterior or posterior prostatic hemisphere (16). Villers et al matched MRI with histopathology based on anatomical landmarks such as gland contours (17). Other groups accept a distance of 8–10mm (~2 sections) as evidence of a match between MR images and histopathology (9, 20, 21). Prior to the current specimen mold technique, a nearest neighbor approach had been used by our group to validate MRI with a more standardized and unbiased method (12). Moreover, the majority of the studies correlating MRI with histopathology do not directly acknowledge the difficulties in matching imaging to histopathology. The use of a customized specimen mold in this study allowed us to better validate MRI in prostate cancer detection and localization, and has helped us to improve our results in terms of tumor detection and local staging at MRI when compared to our prior correlation results (12).
Our study has several limitations. First, the radiologists reviewing the MRI knew that all patients included in the study had biopsy proven cancer and this could lead to bias during interpretation of MR images. Second, the customized MRI-based specimen mold is a relatively expensive method to apply; therefore we do not advocate it for routine clinical use. However, such a systematic method can be useful;in multi-center clinical trials. It was used for research purposes only in this study. Finally, we sliced the prostate in 6mm sections, whereas the MRI was obtained in 3mm slice thicknesses; in future studies we intend to slice the sections at 3mm intervals.
Conclusions
Prostate MRI at 3T allows for the detection of prostate cancer. In particular, a multi-parametric approach increases the predictive power of MRI for diagnosis. The patient-specific mold provides evenly spaced tissue blocks of uniform thickness which correspond directly to the MRI slice planes, leading to improved co-registration with histology as compared with prior free-hand methods. MRI may provide the urologist an imaging modality to better manage their patients for prostate cancer. This imaging platform may also allow a more accurate method for cancer detection when compared to traditional systemic non-guided biopsies. Compared to the traditional biopsy method, multiparametric imaging may allow earlier diagnosis of anterior prostate lesions and can guide needle biopsies more accurately than systematic methods. This work may also provide the basis for image guided minimally invasive, focal treatments of prostate cancer.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 2.Kim CK, Park BK, Kim B. Localization of prostate cancer using 3T MRI: comparison of T2-weighted and dynamic contrast-enhanced imaging. J Comput Assist Tomogr. 2006;30:7–11. doi: 10.1097/01.rct.0000185384.27765.09. [DOI] [PubMed] [Google Scholar]
- 3.Torricelli P, Cinquantini F, Ligabue G, Bianchi G, Sighinolfi P, Romagnoli R. Comparative evaluation between external phased array coil at 3 T and endorectal coil at 1.5 T: preliminary results. J Comput Assist Tomogr. 2006;30:355–61. doi: 10.1097/00004728-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Park BK, Kim B, Kim CK, Lee HM, Kwon GY. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr. 2007;31:534–8. doi: 10.1097/01.rct.0000250108.85799.e1. [DOI] [PubMed] [Google Scholar]
- 5.Fütterer JJ, Heijmink SW, Scheenen TW, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449–58. doi: 10.1148/radiol.2412051866. [DOI] [PubMed] [Google Scholar]
- 6.Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest Radiol. 2007;42:842–7. doi: 10.1097/RLI.0b013e3181461d21. [DOI] [PubMed] [Google Scholar]
- 7.Ocak I, Bernardo M, Metzger G, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol. 2007;189:849. doi: 10.2214/AJR.06.1329. [DOI] [PubMed] [Google Scholar]
- 8.Scheenen TW, Heijmink SW, Roell SA, et al. Three-dimensional proton MR spectroscopy of human prostate at 3 T without endorectal coil: feasibility. Radiology. 2007;245:507–16. doi: 10.1148/radiol.2451061444. [DOI] [PubMed] [Google Scholar]
- 9.Heijmink SW, Fütterer JJ, Hambrock T, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–95. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 10.Miao H, Fukatsu H, Ishigaki T. Prostate cancer detection with 3-T MRI: comparison of diffusion-weighted and T2-weighted imaging. Eur J Radiol. 2007;61:297–302. doi: 10.1016/j.ejrad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Hricak H, Shukla-Dave A, Akin O, Ishill NM, Carlino LJ, Reuter VE, Eastham JA. Clinical stage T1c prostate cancer: evaluation with endorectal MR imaging and MR spectroscopic imaging. Radiology. 2009;253:425–34. doi: 10.1148/radiol.2532081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turkbey B, Pinto P, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenktrantz AB, Neil J, Kong X, et al. Prostate cancer: Comparison of 3D T2-weighted with conventional 2D T2-weighted imaging for image quality and tumor detection. AJR. 2010;194:446–52. doi: 10.2214/AJR.09.3217. [DOI] [PubMed] [Google Scholar]
- 14.Riches SF, Payne GS, Morgan VA, et al. MRI in the detection of prostate cancer: combined apparent diffusion coefficient, metabolite ratio, and vascular parameters. AJR. 2009;193:1583–91. doi: 10.2214/AJR.09.2540. [DOI] [PubMed] [Google Scholar]
- 15.Kitajima K, Kaji Y, Fukabori Y, et al. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging. 2010;31:625–31. doi: 10.1002/jmri.22075. [DOI] [PubMed] [Google Scholar]
- 16.Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, Zaloudek CJ, Nelson SJ, Carroll PR, Kurhanewicz J. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging--clinicopathologic study. Radiology. 1999;213:473–80. doi: 10.1148/radiology.213.2.r99nv23473. [DOI] [PubMed] [Google Scholar]
- 17.Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176:2432–7. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Shah V, Pohida T, Turkbey B, et al. A method for correlating in vivo prostate magnetic resonance imaging and histopathology using individualized magnetic resonance-based molds. Rev Sci Instrum. 2009;80:104301. doi: 10.1063/1.3242697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Albert PS, Kurdziel K, Choyke PL. Imaging localized prostate cancer: current approaches and new developments. AJR Am J Roentgenol. 2009;192:1471–80. doi: 10.2214/AJR.09.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fütterer JJ, Engelbrecht MR, Huisman HJ, Jager GJ, Hulsbergen-van De Kaa CA, Witjes JA, Barentsz JO. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005;237:541–9. doi: 10.1148/radiol.2372041724. [DOI] [PubMed] [Google Scholar]
- 21.Fütterer JJ, Heijmink SW, Scheenen TW, Jager GJ, Hulsbergen-Van de Kaa CA, Witjes JA, Barentsz JO. Prostate cancer: local staging at 3-T endorectal MR imaging--early experience. Radiology. 2006;238:184–91. doi: 10.1148/radiol.2381041832. [DOI] [PubMed] [Google Scholar]