Abstract
Objective
To estimate mortality in HIV-positive patients starting combination antiretroviral therapy (ART), and to discuss different approaches to calculating correction factors to account for loss to follow-up.
Methods
A total of 222,096 adult HIV-positive patients who started ART 2009–2014 in clinics participating in the International epidemiology Databases to Evaluate AIDS (IeDEA) collaboration in 43 countries in sub-Saharan Africa, Asia Pacific, Latin America, and North America were included. To allow for underascertainment of deaths due to loss to follow-up, two correction factors (one for the period 0–6 months on ART and one for later periods) or 168 correction factors (combinations of 2 genders, 3 time periods after ART initiation, 4 age groups, and 7 CD4 groups) based on tracing patients lost in Kenya and data linkages in South Africa were applied. Corrected mortality rates were compared with a worst-case scenario assuming all patients lost to follow-up had died.
Results
Loss to follow-up differed between regions; rates were lowest in Central Africa and highest in East Africa. Compared to using two correction factors (1.64 for the initial ART period and 2.19 for later), applying 168 correction factors (range 1.03–4.75) more often resulted in implausible mortality rates that exceeded the worst-case scenario. Corrected mortality rates varied widely, ranging from 0.2 per 100 person-years to 54 per 100 person-years depending on region and covariates.
Conclusions
Implausible rates were less common with the simpler approach based on two correction factors. The corrected mortality rates will be useful to international agencies, national programmes, and modelers.
Keywords: HIV infection, mortality, ART, loss to follow-up, bias, sub-Saharan Africa, Asia Pacific, Latin America, and North America, Spectrum projection software
Introduction
The survival of HIV-positive patients on antiretroviral therapy (ART) is an important indicator of ART programmes’ effectiveness and is key to informing public health policy [1,2]. Estimations of mortality in HIV-positive patients on ART rely on the complete ascertainment of deaths. However, many patients starting ART are lost to follow-up, especially in sub-Saharan Africa, and their mortality is typically higher compared to patients retained in care [3–5]. Deaths among patients lost to follow-up are not generally recorded, leading to underestimation of overall, programme-level mortality. Several correction methods to reduce this bias have been proposed [6–8], which rely on vital status information of a sample of patients lost to follow-up obtained through tracing or data linkages with civil registries [4,5,9].
The International epidemiology Databases to Evaluate AIDS (IeDEA) collaboration is a consortium of HIV cohort studies and clinical databases with regional networks in sub-Saharan Africa, North and Latin America, and Asia Pacific [10–12]. IeDEA is an important source of regional HIV/AIDS data, which has been used, for example, in the Spectrum projection package developed by Avenir Health under the auspices of the Joint United Nations Program on HIV/AIDS (UNAIDS) [13]. Spectrum is a modelling package that supports national programmes to make annual estimates of the number of people living with HIV by age and sex, the number of new infections and AIDS deaths, and the need for ART and its impact [13,14].
In 2012 the IeDEA consortium provided UNAIDS with mortality estimates for patients on ART for use in the Spectrum projection package [8]. In that study, a two-stage approach was used to adjust for biases in mortality estimation resulting from loss to follow-up. Initially, correction factors were determined based on data from programmes tracing patients lost to follow-up in Kenya [15] and from linkages with population registries in South Africa [9]. Then the correction factors were applied to adjust mortality rates from other regions [8]. The aim of this analysis is to update the previous estimates and to refine and study the mortality correction methods.
Methods
Data sources
We included longitudinal patient-level data from HIV cohorts in the seven regions of the IeDEA collaboration: Central Africa, East Africa, Southern Africa, West Africa, Asia-Pacific, Latin America (Caribbean, Central and South America) and North America [10–12]. Forty-three countries contributed data: Rwanda, Burundi, Democratic Republic of the Congo (Central Africa); Uganda, Kenya, Tanzania (East Africa); South Africa, Zambia, Zimbabwe, Malawi, Lesotho, Mozambique (Southern Africa); Côte d’Ivoire, Nigeria, Togo, Burkina Faso, Mali, Benin, Guinea-Bissau, Senegal, Guinea (West Africa); India, Singapore, Cambodia, Vietnam, Thailand, Hong Kong, Philippines, Malaysia, Taiwan, Japan, Republic of Korea, China, Indonesia (Asia Pacific); Haïti, Peru, Brazil, Chile, Argentina, Mexico, Honduras (Latin America); and USA, Canada (North America). In all of the cohorts, data were collected at enrolment, ART initiation, and at each follow-up visit. Pooling of data and their use in collaborative analyses was approved by local ethics committees and institutional review boards.
For two of the regions, data included information about outcomes in patients lost to follow-up: the cohorts from the Republic of South Africa had linked their patients to the national death registry, and cohorts in Kenya carried out tracing of patients lost to follow-up to ascertain their vital status. For the other countries of the East and Southern African regions and the five other regions, no information on mortality in patients lost to follow-up was available. In North America and Latin America, some cohorts linked patients to vital registries and updated the outcomes of these patients in their databases. However, they did not record whether or not a death was ascertained through linkage to a vital registry.
Inclusion criteria, collection of variables & definitions
We included patients aged ≥15 years who were ART naïve at enrolment, started ART between 2009 and 2014, and had at least one day of follow-up. ART was defined as a combination of at least three antiretroviral drugs. Data included patient gender, age, CD4 cell count at start of ART, date of starting ART, and outcome. The CD4 cell count at ART start was defined as the measurement closest to the date of starting therapy within a window of 182 days before and 14 days after ART initiation. To match the structure of the Spectrum model, age was grouped into four categories (15–24, 25–34, 35–44 and ≥45 years) and CD4 cell count at ART start into seven categories (<50, 50–99, 100–199, 200–249, 250–349, 350–499, ≥500 cells/μL). Outcomes included death and loss to follow-up. Patients were considered lost to follow-up if their last visit was more than 182 days prior to database closure and there was no record of their death or transfer [16]. The Kenyan cohorts recorded whether a patient had been traced or not and the South African linkage cohorts recorded whether a patient had a valid South African civil identification (ID) number.
Estimation of crude mortality rates
We used exponential survival models to estimate mortality rates not adjusted for loss to follow-up. We assumed a piecewise constant hazard for three time periods (0–6, 6–12 and ≥12 months after starting ART) and included the covariates gender, age, and CD4 cell count at ART start. In addition we modelled the period 0–6 months separately from the other two periods to allow for potentially different effects of covariates on mortality shortly after starting treatment. The follow-up of all patients without a death record was administratively censored at the last visit in the unadjusted analysis. Models were fit using the penalized maximum-likelihood estimation procedure proposed by Gertheiss and Tutz [17], which accounts for the ordinal nature of the CD4 covariate. This procedure smoothes coefficients across categories of the CD4 cell count at ART start so that they become more similar to those of adjacent CD4 cell count categories, which reduces overfitting and improves prediction. We determined the penalization parameter for each region separately, minimising deviances in a 10-fold cross-validation.
Correction for loss to follow-up
We multiplied the crude mortality rates by correction factors to adjust them for loss to follow-up. We calculated three different sets of correction factors, one from the Kenyan tracing data and two from the South African linkage data. For Kenya, we determined the correction factors in the same way as in 2012 [8]: first we calculated crude mortality rate estimates for all subgroups by fitting the survival model and treating patients lost to follow-up as administratively censored. Then we included the ascertained outcomes of traced patients, weighted observations as proposed by Frangakis and Rubin [18], and fitted the model to the updated data to obtain adjusted mortality rate estimates. The correction factors were derived by dividing the adjusted rate estimates by the crude estimates. This led to a Kenyan set of 168 correction factors, one for each covariate subgroup (2 genders x 3 time periods x 4 age groups x 7 CD4 groups).
For the South African data, we fitted the survival model including an additional covariate, which indicated whether or not a patient had an ID and was therefore linkable to the vital registry. The first of the two sets of correction factors was defined as the estimated effect of this linkage indicator. This entailed two correction factors, one for the initial ART period (0–6 months) and one for later ART, which were modelled separately. In a further analysis, we allowed for two-way interactions between the linkage indicator and the other covariates. We then defined the correction factors as the estimated effect of the linkage indicator and its interactions. As with Kenya, this resulted in a set with 168 correction factors. In contrast to Kenya, this approach allowed statistical testing of whether the inclusion of any of the two-way interactions improved the model.
Measuring variability
We used the bootstrap case resampling method for regression analyses to generate sampling errors both for crude mortality rate estimates and correction factors. Assuming independence of the two, we derived the total variance around the adjusted mortality estimates by adding the two variances.
Sensitivity analyses
We used sensitivity analyses to gauge the plausibility of corrected mortality rate estimates. We compared the three sets of corrected mortality rates with crude estimates and estimates from worst- and best-case scenarios. The worst-case scenario assumed that all patients lost to follow-up had died at their last visit. The best-case scenario assumed that all patients lost to follow-up were alive at the end of database closure.
Results
Selection of eligible patients
After excluding patients with missing CD4 cell count at the start of ART or no follow-up visit after ART was initiated, 222,096 patients were included in the analyses (Table 1): 8,043 from Central Africa, 61,315 from East Africa, 109,434 from Southern Africa, 21,713 from West Africa, 7,425 from Asia Pacific, 7,017 from Latin America and 7,149 from North America. A total of 30,292 patients were enrolled in Kenyan cohorts with tracing programmes and 35,674 South African patients in cohorts where vital registry linkage was available. Of these 35,674 patients, 13,749 (39%) had no ID and could not be linked.
Table 1.
Patient characteristics at the start of antiretroviral therapy by region.
| Regions without linkage or tracing | Regions with linkage or tracing | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Central Africa | East Africa (no tracing) | Southern Africa (no linkage) | West Africa | Asia Pacific | Latin America | North America | East Africa (tracing) | Southern Africa (linkage) | |
|
|
|
||||||||
| No. of patients | 8043 | 31023 | 73760 | 21713 | 7425 | 7017 | 7149 | 30292 | 35674 |
|
| |||||||||
| Gender | |||||||||
| Men | 2695 (34%) | 11365 (37%) | 27172 (37%) | 7193 (33%) | 5097 (69%) | 4348 (62%) | 5473 (77%) | 10849 (36%) | 12603 (35%) |
| Women | 5348 (66%) | 19658 (63%) | 46588 (63%) | 14520 (67%) | 2328 (31%) | 2669 (38%) | 1676 (23%) | 19443 (64%) | 23071 (65%) |
|
| |||||||||
| Age (years) | |||||||||
| Median (IQR) | 34 (28–42) | 33 (27–40) | 35 (29–42) | 37 (31–45) | 37 (31–44) | 36 (29–44) | 39 (30–48) | 37 (30–45) | 35 (29–42) |
| 15–24 | 1053 (13%) | 3604 (12%) | 7491 (10%) | 1324 (6%) | 516 (7%) | 816 (12%) | 840 (12%) | 2001 (7%) | 3321 (9%) |
| 25–34 | 3185 (40%) | 12537 (40%) | 28820 (39%) | 7651 (35%) | 2617 (35%) | 2505 (36%) | 2056 (29%) | 9170 (30%) | 14725 (41%) |
| 35–44 | 2409 (30 %) | 9400 (30%) | 23690 (32%) | 7517 (35%) | 2644 (36%) | 2102 (30%) | 1906 (27%) | 10302 (34%) | 11254 (32%) |
| 45+ | 1396 (17%) | 5482 (18%) | 13759 (19%) | 5221 (24%) | 1648 (22%) | 1594 (23%) | 2347 (33%) | 8819 (29%) | 6374 (18%) |
|
| |||||||||
| CD4 cell count (cells/μL) | |||||||||
| Median (IQR) | 275 (174–342) | 207 (96–324) | 198 (108–295) | 181 (82–291) | 170 (58–283) | 210 (85–205) | 336 (190–488) | 173 (74–279) | 157 (80–231) |
| < 50 | 450 (6%) | 4565 (15%) | 7586 (10%) | 3676 (17%) | 1631 (22%) | 1149 (16%) | 677 (9%) | 5489 (18%) | 5662 (16%) |
| 50–99 | 556 (7%) | 3456 (11%) | 9176 (12%) | 2707 (12%) | 973 (13%) | 772 (11%) | 406 (6%) | 4127 (14%) | 5447 (15%) |
| 100–199 | 1425 (18%) | 6997 (23%) | 20523 (28%) | 5432 (25%) | 1594 (21%) | 1422 (20%) | 787 (11%) | 7663 (25%) | 12213 (34%) |
| 200–249 | 938 (12%) | 3448 (11%) | 9938 (13%) | 2610 (12%) | 857 (12%) | 827 (12%) | 551 (8%) | 3522 (12%) | 5132 (14%) |
| 250–349 | 2923 (36%) | 6473 (21%) | 17141 (23%) | 4076 (19%) | 1471 (20%) | 1952 (28%) | 1336 (19%) | 5986 (20%) | 5367 (15%) |
| 350–499 | 961 (12%) | 3513 (11%) | 5802 (8%) | 1825 (8%) | 591 (8%) | 620 (9%) | 1702 (24%) | 1970 (7%) | 1172 (3%) |
| ≥500 | 790 (10%) | 2571 (8%) | 3594 (5%) | 1387 (6%) | 308 (4%) | 275 (4%) | 1690 (24%) | 1535 (5%) | 681 (2%) |
Baseline characteristics and loss to follow-up
For all African regions, the proportion of women among all patients initiating ART was about twice the proportion of men, whereas for non-African regions the opposite was the case (Table 1). The median age at the start of ART ranged from 33 years in the East African cohorts without tracing to 39 years in North America. The median CD4 cell count at ART initiation ranged from 157 cells/μL in Southern African cohorts with vital registry linkage to 336 cells/μL in North America.
Rates of loss to follow-up differed widely between regions. Rates were lowest in Central Africa and highest in East Africa (Table 2). For all African regions, rates of loss to follow-up declined with longer ART duration. In non-African regions, the decline was less consistent: in Asia Pacific the rate of loss to follow-up was highest in the first 6 months of ART but stayed constant afterwards, in North America rates declined slightly, and in Latin America the rate of loss to follow-up stayed fairly constant.
Table 2.
Rates of loss to follow-up and duration of antiretroviral therapy by regions with and without tracing or linkage data.
| Regions without linkage or tracing | Regions with linkage or tracing | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Central Africa | East Africa (no tracing) | Southern Africa (no linkage) | West Africa | Asia Pacific | Latin America | North America | East Africa (tracing) | Southern Africa (linkage)* | |
| Loss to follow-up rate (95%-CI) (per 100 person-years) | |||||||||
| < 6 months ART | 7.3 (6.5–8.3) | 27.0 (26.1–27.9) | 14.9 (14.5–15.3) | 21.9 (21.0–22.8) | 18.0 (16.7–19.5) | 13.2 (12.0–14.5) | 16.7 (15.4–18.2) | 53.0 (51.8–54.3) | 29.5 (28.1–31.0) |
| 6–12 months ART | 6.4 (5.6–7.3) | 15.0 (14.2–15.7) | 7.9 (7.5–8.2) | 12.7 (11.9–13.5) | 10.6 (9.5–11.9) | 11.9 (10.7–13.2) | 16.4 (14.9–18.0) | 34.0 (32.9–35.2) | 20.0 (18.7–21.5) |
| ≥ 12 months ART | 5.4 (5.0–5.8) | 9.3 (8.9–9.6) | 6.3 (6.1–6.5) | 10.8 (10.4–11.1) | 10.6 (10.0–11.3) | 13.7 (12.9–14.4) | 15.8 (14.8–16.7) | 23.3 (22.7–23.9) | 17.0 (16.1–17.9) |
|
| |||||||||
| Median years on ART before lost to follow-up (IQR) | |||||||||
| 1.34 (0.55–2.39) | 0.60 (0.19–1.44) | 0.76 (0.21–1.86) | 1.11 (0.39 – 2.54) | 1.17 (0.37–2.32) | 1.46 (0.64–2.34) | 1.07 (0.46–1.95) | 0.68 (0.24–1.52) | 0.63 (0.24–1.44) | |
based on patients who could not be linked to the vital registry
Crude mortality rate estimates
Crude mortality rates were generally highest in Southern Africa, slightly lower in East and West Africa, lower in Central Africa, Latin America and Asia Pacific, and clearly lowest in North America (Supplementary Tables S1–S7). Crude mortality was highest in patients with CD4 counts <50 cells/μL at ART initiation and declined with increasing CD4 cell count, although the decline was only modest for the highest four CD4 categories. Crude mortality was highest in the first six months after starting therapy for all regions except North America, where mortality did not vary much by duration of therapy.
Correction factors and sensitivity analysis
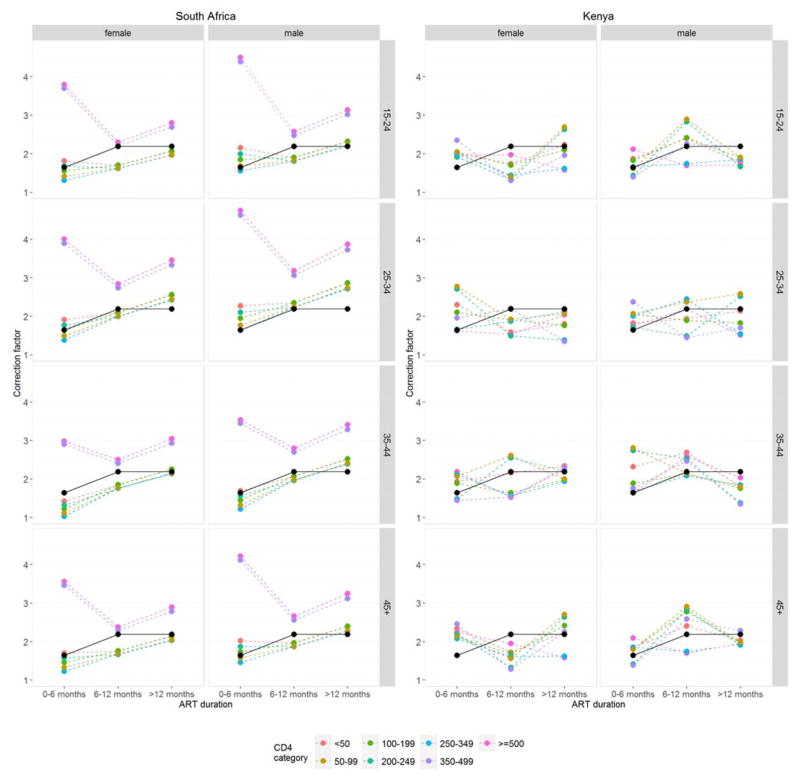
The three sets of correction factors are illustrated in Figure 1 and given in Supplementary Table S8. The South African set with two correction factors implied that underascertainment of death was less pronounced in the first six months of ART duration. The South African set with 168 correction factors contained some high values and implausible patterns, for example for CD4 categories 350–499 and ≥500 cells/μL (where sample sizes were small). None of the two-way interactions included in the model was statistically significant (P>0.05). The Kenyan set of 168 correction factors also showed some unexpected patterns, most likely due to small sample sizes in some of the combinations of ART duration, genders, age groups and CD4 groups.
Figure 1.
Correction factors. The South African set of two correction factors is shown in black, the two sets of 168 correction factors derived from the South African and Kenyan data are shown in colour.
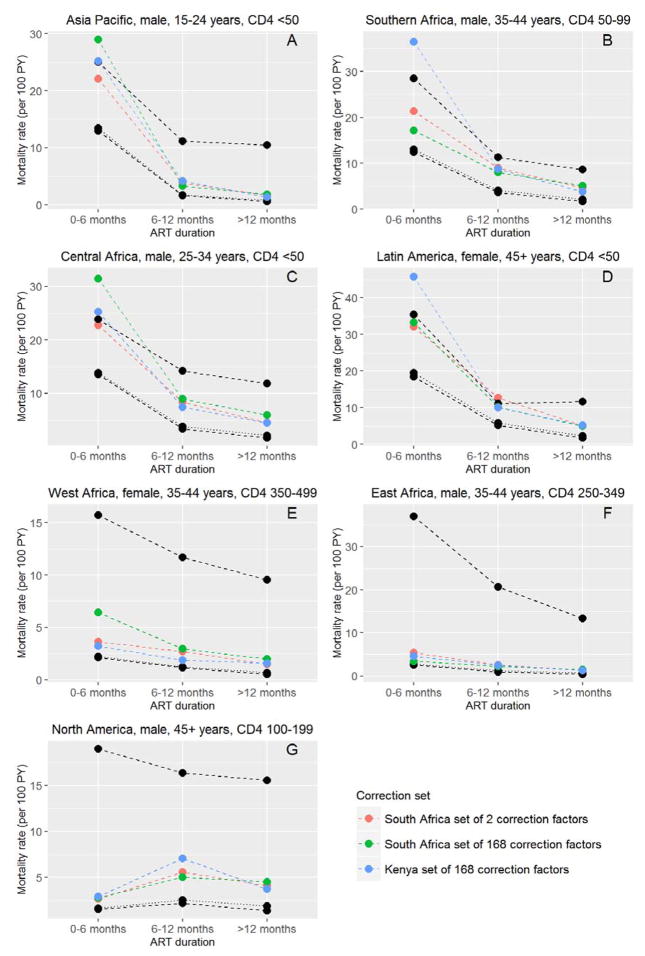
Implausible corrected mortality rates that exceeded the worst-case scenario were observed for Southern Africa, Central Africa, Latin America, and Asia Pacific. There were 16 such instances for mortality corrections with the South African set of two correction factors, 18 instances with the South African set of 168 factors, and 29 with the Kenyan set of 168 factors (Table 3). Some of these implausible rates are shown in Figure 2, Panels A–D. In West Africa, North America, and East Africa, worst-case mortality estimates were high and none of the corrected rates exceeded them (Figure 2, Panels E–G). There were no correction factors below 1; therefore no corrected mortality rate estimates fell below the best case scenario.
Table 3.
Implausible corrected mortality rates exceeding the worst-case scenario estimates, by set of correction factors used.
| South African correction factors | Kenyan correction factors | ||
|---|---|---|---|
| Set of 2 | Set of 168 | Set of 168 | |
| Southern Africa* | 6/168 (3.6%) | 7/168 (4.2%) | 10/168 (6.0%) |
| Central Africa | 4/168 (2.4%) | 5/168 (3.0%) | 10/168 (6.0%) |
| Latin America | 5/168 (3.0%) | 5/168 (3.0%) | 7/168 (4.2%) |
| Asia Pacific | 1/168 (0.6%) | 1/168 (0.6%) | 2/168 (1.2%) |
| East Africa | 0/168 (0%) | 0/168 (0%) | 0/168 (0%) |
| West Africa | 0/168 (0%) | 0/168 (0%) | 0/168 (0%) |
| North America | 0/168 (0%) | 0/168 (0%) | 0/168 (0%) |
non-linkage cohorts only
Figure 2.
Examples of sensitivity analyses for Asia Pacific (A), Southern Africa (B), Central Africa (C), Latin America (D), West Africa (E), East Africa (F) and North America (G). The different corrected mortality estimates are compared with worst-case scenario estimates (upper black dashed line) and best-case scenario estimates (lower black dashed line) and crude mortality (black dotted line). Crude mortality and mortality from best-case scenario are closely similar.
Final corrected mortality rates
In the final analysis, we used the South African set of two correction factors to adjust mortality rates in all regions except in East Africa, where we used the Kenyan set of 168 correction factors. The corrected mortality rate estimates with bootstrapped 95% confidence intervals, which are used in the newest update of the Spectrum projection package, are reported in Supplementary Tables S9 to S15. Similar patterns as described above for the crude mortality estimates were evident in the corrected estimates.
Discussion
Estimates of mortality and life expectancy in HIV-positive populations rely on the complete ascertainment of deaths. However, the proportion of patients lost to follow-up in HIV care programmes is high, especially in sub-Saharan Africa [3]. Patients who are lost to follow-up typically experience higher rates of mortality than those remaining in care [4,5], so failing to account for deaths among all patients who started ART leads to an underestimation of overall, programme-level mortality. Use of information from tracing patients lost to follow-up and linkages with vital registries has repeatedly resulted in upward revision of mortality estimates [7,19,20].
In this study, we multiplied crude mortality rates by correction factors to adjust for biases resulting from loss to follow-up. We used three different sets of correction factors that were calculated based on the outcomes of tracing patients lost to follow-up in Kenya, and linkage of HIV programme data with the national death registry in South Africa. Corrected mortality estimates differed depending on the set of correction factors, and implausible corrected rates were observed with all three sets. Implausible rates were more common with the two sets of 168 correction factors than with the simpler approach based on two factors only. None of the two-way interactions included in the model for the South African set of 168 correction factors reached conventional levels of statistical significance. The poorer performance of the two large sets of correction factors might therefore be due to overfitting, which occurred despite using a penalized maximum-likelihood estimation procedure [17] that will have reduced overfitting to some extent. In the final analysis, we decided to use the more conservative approach based on two correction factors only for all regions except for East Africa. For East Africa we used the Kenyan correction factors, as they might better represent the regional pattern of mortality among patients lost to follow-up in East Africa.
Compared to the earlier analysis published in 2012 [8], the mortality estimates calculated in this study were somewhat higher. This is probably explained by the different composition of the study population, with a shift to countries with higher mortality. For example, in the previous analysis the Republic of South Africa was the only country in the Southern African region. In the present study we also included data from Zambia, Zimbabwe, Malawi, Mozambique and Lesotho. Determinants of mortality were however similar for the 2012 and the current analysis, with high mortality rates in the first 6 months of ART and with low CD4 cell counts, which decreased with longer ART duration and higher CD4 cell counts. It would be worthwhile to examine trends over calendar years as recent studies have shown that in African programmes mortality among patients lost to follow-up appears to have declined in recent years [5,21]. This may be due to higher CD4 cell counts at the start of ART and to an increase in undocumented transfers to other clinics [5,21]. For example, a study in Lilongwe, Malawi found that among patients lost to follow-up and found to be alive on tracing, a majority (56%) were still on ART sourced from another clinic [22]. Similarly, a study of adults starting ART in Uganda, Tanzania, and Kenya found that 59% of patients interviewed had reconnected to care at a different clinic [23].
Our study has several limitations. The comparisons with worst- and best-case scenarios were useful to detect implausible mortality rates, but the range between estimates from the two scenarios was wide. It is impossible to know with any precision how appropriate mortality corrections were. In general, by applying correction factors originating from one region to correct mortality estimates in another region, we assumed that both mortality in patients lost to follow-up and rates of loss to follow-up were similar in the two regions. As determinants and rates of loss to follow-up and mortality differ between treatment programmes within and across countries and regions, it seems unlikely that the situation in South Africa and Kenya and the correction factors from these countries accurately capture the underestimation of mortality rates in other African countries, the Asia Pacific region, and countries in North or Latin America [8]. This in turn might be the reason why none of the three sets achieved corrected mortality estimates that stayed within best- and worst case boundaries in all regions. Also, since in Latin America and North America linkage to vital registries was performed occasionally in some cohorts, the crude mortality estimates might already capture some of the mortality of patients lost to follow-up. Applying the South African correction factors to these regions might thus result in an overestimation of mortality.
The correction factors themselves might also be subject to bias: in Kenya and elsewhere tracing programmes do not trace a random sample of the patients who were lost to follow-up but are imbedded into routine patient outreach efforts [20,24,25]. Moreover, not all patients lost are successfully located. In a recent systematic review of tracing studies, we found that about 80% of patients could be located but this percentage varied widely across studies [21]. With the linkage data from South Africa this problem is less important: bias will only arise if patients with an ID differ systematically from those without ID and the effect of this bias cannot be minimised by the covariates included in the model. One study found that patients with ID are similar to the patients without ID in terms of their demographic and baseline clinical characteristics [9].
These limitations notwithstanding, the large number of patients included from many different countries and settings is an important strength of this analysis. In the absence of empirical data from tracing patients lost to follow-up [4,5] or from linkages with vital registries [9], the application of the correction factors calculated in this study are likely to result in more appropriate estimates of mortality at the treatment programme and population level than naïve, uncorrected estimates. The mortality estimates can be used by treatment programme managers and policy makers, and to inform mathematical modelling and projections such as those produced by the Spectrum modelling package [13,14].
To overcome the limitations of using correction factors derived from empirical data from only two African countries, we are in the process of compiling a large database of outcomes from efforts by ART treatment programmes to trace and ascertain the vital status of patients lost to follow-up. We identified 32 eligible studies from 12 countries in sub-Saharan Africa [21] and are in the process of obtaining the individual patient data from these studies. In a future update of this analysis, we will be able to include setting-specific data for many more countries than in the present analysis, thus improving the accuracy of corrected mortality estimates for HIV-positive patients who started ART in sub-Saharan Africa, the region where loss to follow-up is common [3]. Similar studies in other regions are warranted.
In conclusion, the tracing of patients lost to follow-up should be an integral part of ART programmes and ideally be done on a continuous basis, with dedicated data collection and analysis, so that patients who interrupted ART can be reconnected to care, records can be updated for patients who self-transferred to another clinic, and programme and country-level estimates of mortality in people on ART can be corrected appropriately for loss to follow-up.
Supplementary Material
Acknowledgments
We thank all patients, care providers and data managers in the different IeDEA regions. We are grateful to John Stover, Peter Ghys, Timothy Hallett, Mary Mahy, and Jeff Eaton for helpful discussions and suggestions, and to Christopher Ritter for revision of the manuscript. The African regions of the International epidemiology Databases to Evaluate AIDS (IeDEA) are supported by the National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases (NIAID) as part of the IeDEA (Grants 5U01AI069919-04, 5U01-AI069924-05, 1U01 AI069927, U01AI069911-01). The Caribbean, Central, and South America network of IeDEA is supported by NIAID Grant 5U01- AI69923-06. The North American AIDS Cohort Collaboration on Research and Design of IeDEA is supported by Grants U01-AI069918, U10-AA13566, U01-AI31834, U01- AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-HD32632, U10-EY08057, U10-EY08052, U10-EY08067, UL1-RR024131, UL1-RR024131, M01-RR-00052, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, M01-RR025747, P30-AI27757, P30-AI27767, P30-AI27763, P30-AI50410, P30-AI54999, R01-DA04334, R01-DA12568, R01-DA11602, R01-AA16893, R24-AI067039, Z01-CP010176, AHQ290-01-0012, N02-CP55504, AI-69432, AI-69434, K01-AI071725, K23-AI610320, K23-EY013707, K24-DA00432, K01-AI093197, U10-AA13566, R01-AG029154, and K23 AG024896 from the National Institutes of Health; contract CDC200-2006-18797 from the Centers for Disease Control and Prevention; Grants TGF-96118, HCP-97105, CBR-86906, CBR-94036, KRS-86251, and 169621 from the Canadian Institutes of Health Research; the Canadian HIV Trials Network, project 24; and the Government of British Columbia. The TREAT Asia HIV Observational Database, TREAT Asia Studies to Evaluate Resistance, and the Australian HIV Observational Database are initiatives of TREAT Asia, a programme of amfAR, The Foundation for AIDS Research, with support from the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds, and the US National Institutes of Health’s NIAID, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and NCI, as part of the IeDEA (U01AI069907). Queen Elizabeth Hospital and the Integrated Treatment Center received additional support from the Hong Kong Council for AIDS Trust Fund. The Kirby Institute is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, The University of New South Wales. The Measurement and Surveillance of HIV Epidemics (MeSH) consortium is funded by the Bill & Melinda Gates Foundation (Grant Nr. OPP 1120138). The content of this publication is solely the responsibility of the authors and does not represent the official views of any of the institutions and funders mentioned.
Footnotes
Contributions of authors
NA, CTY, LFJ and ME contributed to the concept of the study. EZ, LFJ, CTY, KA, EB, DN, ML, and BES contributed to data collection. EZ was responsible for data management. NA performed all statistical analyses with the help of LFJ and CTY. NA drafted the manuscript with the help of ME. All authors commented on earlier drafts of the manuscript and have read and approved the final manuscript.
Conflict of interest
ML received unrestricted grants from Boehringer Ingelhiem, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, ViiV HealthCare, consultancy and presentation fees from Gilead Sciences, DSMB sitting fees from Sirtex Pty Ltd. All other authors have no conflict of interest to disclose.
References
- 1.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11:e1001718. doi: 10.1371/journal.pmed.1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69:98–108. doi: 10.1097/QAI.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N, LSW, JS-W, et al. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: Systematic review and meta-analysis. Trop Med Int Heal. 2015;20:365–379. doi: 10.1111/tmi.12434. [DOI] [PubMed] [Google Scholar]
- 6.Kiragga AN, Castelnuovo B, Musomba R, Levin J, Kambugu A, Manabe YC, et al. Comparison of methods for correction of mortality estimates for loss to follow-up after ART initiation: a case of the Infectious Diseases Institute, Uganda. PLoS One. 2013;8:e83524. doi: 10.1371/journal.pone.0083524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egger M, Spycher BD, Sidle J, Weigel R, Geng EH, Fox MP, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yiannoutsos CT, Johnson LF, Boulle A, Musick BS, Gsponer T, Balestre E, et al. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect. 2012;88(Suppl 2):i33–43. doi: 10.1136/sextrans-2012-050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LF, Dorrington RE, Laubscher R, Hoffmann CJ, Wood R, Fox MP, et al. A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. J Int AIDS Soc. 2015;18:20628. doi: 10.7448/IAS.18.1.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. doi: 10.1093/ije/dyr080. Published Online First: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan CC, Cahn P, Gotuzzo E, Padgett D, Pape JW, Wolff M, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007;36:969–976. doi: 10.1093/ije/dym073. [DOI] [PubMed] [Google Scholar]
- 12.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stover J, Andreev K, Slaymaker E, Gopalappa C, Sabin K, Velasquez C, et al. Updates to the spectrum model to estimate key HIV indicators for adults and children. AIDS. 2014;28(Suppl 4):S427–34. doi: 10.1097/QAD.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82(Suppl 3):iii45–50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yiannoutsos CT, An MW, Frangakis CE, Musick BS, Braitstein P, Wools-Kaloustian K, et al. Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in Western Kenya. PLoS One. 2008;3:e3843. doi: 10.1371/journal.pone.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;8:e1001111. doi: 10.1371/journal.pmed.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gertheiss J, Tutz G. Penalized Regression with Ordinal Predictors. Munich: 2008. [Google Scholar]
- 18.Frangakis CE, Rubin DB. Addressing an idiosyncrasy in estimating survival curves using double sampling in the presence of self-selected right censoring. Biometrics. 2001;57:333–342. doi: 10.1111/j.0006-341x.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 19.Braitstein PKA, Brinkhof MWG, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 20.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zürcher K, Anne M, Anderegg N, Olga T, Couvillon MJ, Nash D, et al. Outcomes of HIV-positive Patients Lost to Follow-up in African Treatment Programs: Systematic Review and Meta-Regression Analysis. 2016 Submitted. [Google Scholar]
- 22.Tweya H, Feldacker C, Estill J, Jahn A, Ng’ambi W, Ben-Smith A, et al. Are they really lost? “true” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PLoS One. 2013;8:e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. 2016;62:935–44. doi: 10.1093/cid/civ1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tweya H, Gareta D, Chagwera F, Ben-Smith A, Mwenyemasi J, Chiputula F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the “Back-to-Care” project in Lilongwe, Malawi. Trop Med Int Heal. 2010;15(Suppl 1):82–89. doi: 10.1111/j.1365-3156.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 25.Anglaret X, Toure S, Gourvellec G, Tchehy A, Zio L, Zaho M, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir DeficSyndr. 2004;35:320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.