Abstract
The data shown in this article are related to the subject of an article in Carbohydrate Polymers, entitled “Synthesis and characterization of chitosan alkyl urea” [1]. 1H NMR and 13C NMR spectra of chitosan n-octyl urea, chitosan n-dodecyl urea and chitosan cyclohexyl urea are displayed. The chemical shifts of proton and carbon of glucose skeleton in these chitosan derivatives are designated in detail. Besides, 1H NMR spectra of chitosan cyclopropyl urea, chitosan tert-butyl urea, chitosan phenyl urea and chitosan N,N-diethyl urea and the estimation of the degree of substitution are also presented. The corresponding explanations can be found in the above-mentioned article.
Specifications Table
| Subject area | Chemistry |
| More specific subject area | Polysaccharides modification |
| Type of data | Table, text file, graph, figure |
| How data was acquired | High-resolution liquid NMR 600 MHz spectrometer of Bruker Avance III (Sweden) with a 5 mm TCI CryoProbe equipped with Z-gradients up to 53 G/cm, and NMR 400 MHz spectrometer of Varian (USA) |
| Data format | Analyzed |
| Experimental factors | Sample solutions (20 mg/ml) were prepared with deuterated trifluoroacetic acid (TFA-D) as solvent. TFA-D was also employed as reference: δ 11.50 ppm for proton and δ 164.1 ppm for carbon |
| Experimental features | Detection temperature was set at 25 °C. Samples were scanned 64 times for 1H NMR spectra measurement, and scanned 1 h for 13C NMR measurement |
| Data source location | Qingdao, PR China; Wuhan, PR China |
| Data accessibility | Data is provided in this article |
Value of the data
-
•
Provide reference data of chemical shifts in 1H NMR and 13C NMR spectra for related chitosan derivatives.
-
•
The data showed in 1H NMR spectra suggest a facile way to estimate degree of substitution of a substituent.
-
•
The chemical shifts designation is helpful to structural analysis of polysaccharides or their derivatives.
1. Data
Data presented here is divided into two classes. In the first class, 1H NMR and 13C NMR spectra of three chitosan alkyl urea derivatives with very high degree of substitution (DS) are displayed, and the corresponding chemical shifts of proton and carbon of glucose skeleton in the chitosan derivatives are tabulated. In the second class, 1H NMR spectra of chitosan alkyl urea with relatively lower DS are showed. According to the integrals in 1H NMR spectra, the estimation of every DS is described.
2. Experimental design, materials and methods
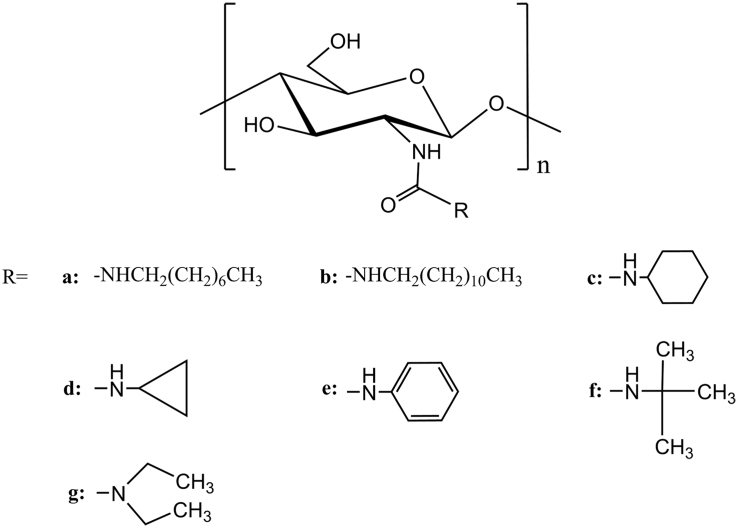
All chitosan alkyl urea derivatives (Fig. 1) were synthesized with the same method described in Ref. [1]. Specifically, chitosan firstly was reacted with methyl chloroformate yielding N-methoxyformylated chitosan, which was converted into chitosan alkyl urea via amine–ester exchange reaction with structurally different amines. After the products were washed with ethanol and dried, 1H and 13C NMR measurements of a–c were performed on a high-resolution liquid NMR 600 MHz spectrometer of Bruker Avance III (Sweden) with a 5 mm TCI CryoProbe equipped with Z-gradients up to 53 G/cm. 1H NMR measurement of d–g was conducted on a NMR 400 MHz spectrometer of Varian (USA). Sample solutions (20 mg/ml) were prepared with TFA-D as solvent. TFA-D was also used as internal standard: proton (δ 11.50 ppm) and carbon (δ 164.1 ppm). Detection temperature was set at 25 oC. Degree of substitution (DS) of alkyl group (diethylamido group for g) on chitosan alkyl urea were determined according to integrals in corresponding 1H NMR spectra.
Fig. 1.
Structures of chitosan alkyl urea derivatives (a–g).
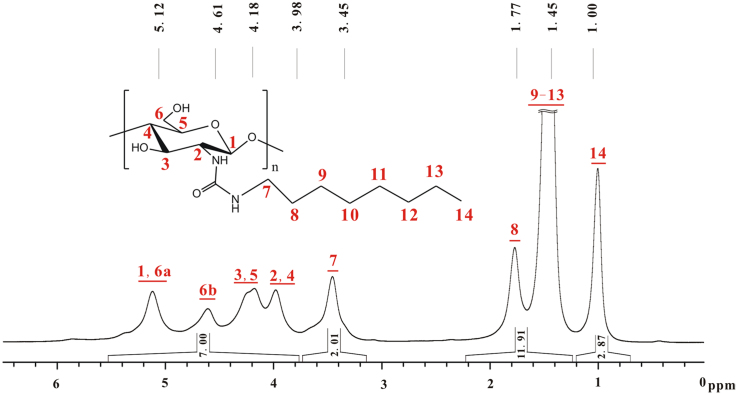
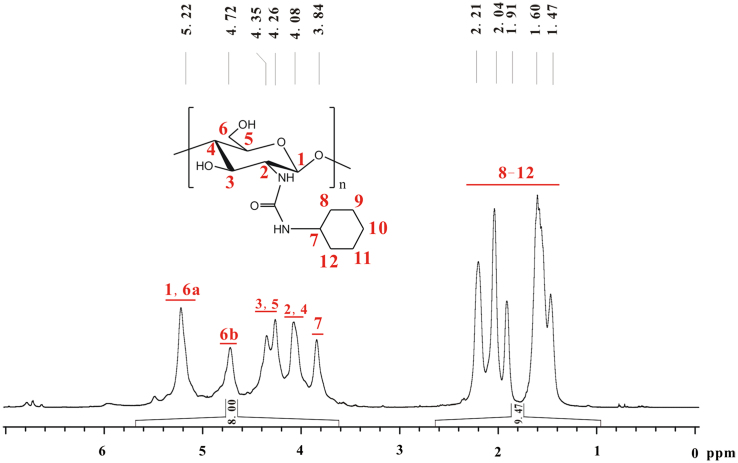
As shown in Fig. 2, the peaks from 5.12 to 3.98 ppm correspond to the seven hydrogens of glucose skeleton of chitosan, and their total integral is designated as 7.00. The peak at 3.45 and the peaks from 1.77 to 1.45 ppm theoretically correlate with two hydrogens and twelve hydrogens. The actual integrals almost match the hydrogen numbers, and the integral of the peak at 1.00 is close to 3.00. Therefore, the DS of n-octyl on a is nearly 100%.
Fig. 2.
1H NMR spectrum of a (TFA-D, 298 K, 600 MHz).
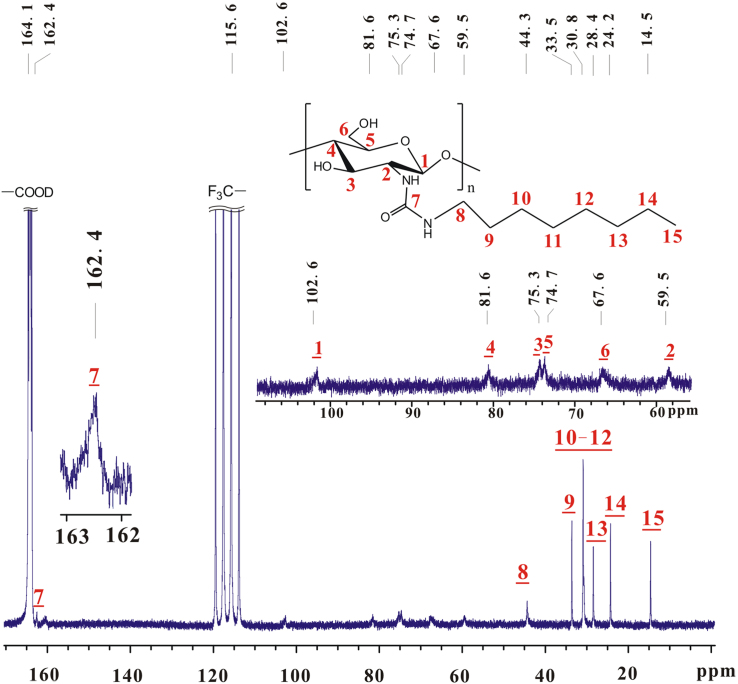
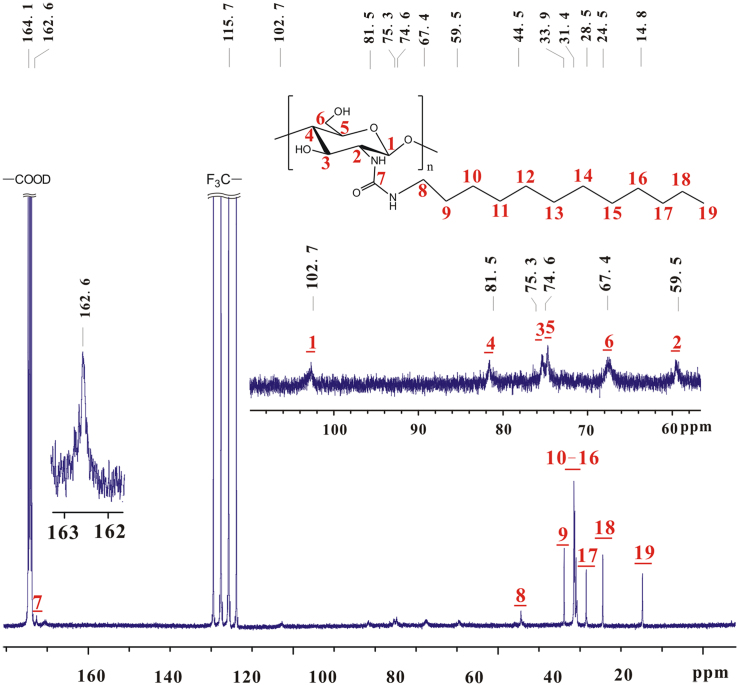
Based on Fig. 2, Fig. 3, the chemical shifts of proton and carbon in glucose skeleton of chitosan n-octyl urea (a) are listed in Table 1. The sequence of chemical shifts of C1–C6 slightly differs from that in reported [2], [3].
Fig. 3.
13C NMR spectrum of a (TFA-D, 298 K, 600 MHz).
Table 1.
Chemical shifts of proton and carbon in.glucose skeleton of chitosan n-octyl urea (a).
| 1H NMR δ (ppm) | 13C NMR δ (ppm) | ||
|---|---|---|---|
| H1 | 5.12 | C1 | 102.6 |
| H2 | 3.98 | C2 | 59.5 |
| H3 | 4.26 | C3 | 75.3 |
| H4 | 3.98 | C4 | 81.6 |
| H5 | 4.18 | C5 | 74.7 |
| H6 | 4.61, 5.12 | C6 | 67.6 |
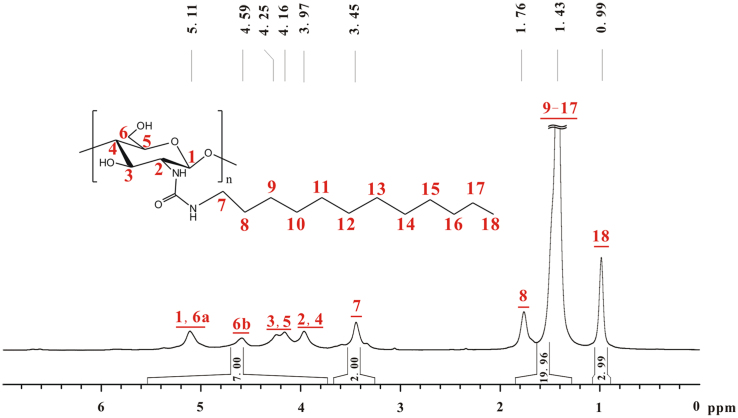
The total integral of the seven hydrogens of glucose skeleton of chitosan is designated as 7.00 (Fig. 4), other integrals match corresponding hydrogen numbers. Therefore, the DS of n-dodecyl on b is almost 100%.
Fig. 4.
1H NMR spectrum of b (TFA-D, 298 K, 600 MHz).
Based on Fig. 4, Fig. 5, the chemical shifts of proton and carbon in glucose skeleton of chitosan n-dodecyl urea (b) are listed in Table 2.
Fig. 5.
13C NMR spectrum of b (TFA-D, 298 K, 600 MHz).
Table 2.
Chemical shifts of proton and carbon in glucose skeleton of chitosan n-octyl urea (b).
| 1H NMR δ (ppm) | 13C NMR δ (ppm) | ||
|---|---|---|---|
| H1 | 5.11 | C1 | 102.7 |
| H2 | 3.97 | C2 | 59.5 |
| H3 | 4.25 | C3 | 75.3 |
| H4 | 3.97 | C4 | 81.5 |
| H5 | 4.16 | C5 | 74.6 |
| H6 | 4.59, 5.11 | C6 | 67.4 |
The peak of the hydrogen in –CH– of cyclohexyl group overlapped with that of seven hydrogens on glucose skeleton of chitosan, and the total integral of these eight hydrogens is assumed as 8.00 (Fig. 6). The DS of cyclohexyl group on c is calculated by the equation of (7+DS)/10DS=8.00/9.47. Therefore, DS=94%.
Fig. 6.
1H NMR spectrum of c (TFA-D, 298 K, 600 MHz).
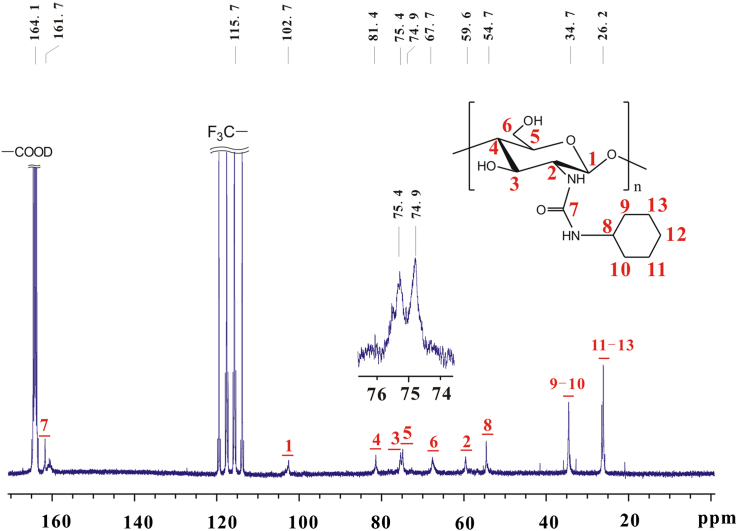
Based on Fig. 6, Fig. 7, the chemical shifts of proton and carbon in glucose skeleton of chitosan cyclohexyl urea (c) are listed in Table 3.
Fig. 7.
13C NMR spectrum of c (TFA-D, 298 K, 600 MHz).
Table 3.
Chemical shifts of proton and carbon in glucose skeleton of chitosan cyclohexyl urea (c).
| 1H NMR δ (ppm) | 13C NMR δ (ppm) | ||
|---|---|---|---|
| H1 | 5.22 | C1 | 102.7 |
| H2 | 4.08 | C2 | 59.6 |
| H3 | 4.35 | C3 | 75.4 |
| H4 | 4.08 | C4 | 81.4 |
| H5 | 4.26 | C5 | 74.9 |
| H6 | 4.72, 5.22 | C6 | 67.7 |
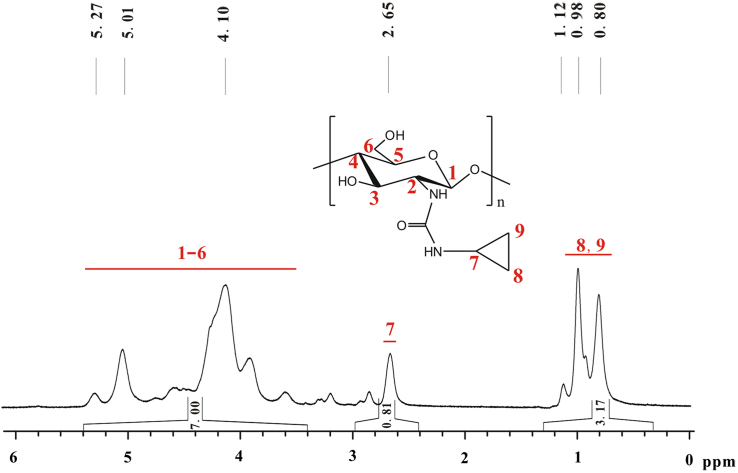
The total integral of seven hydrogens on glucose skeleton of chitosan is designated as 7.00 (Fig. 8). The DS of cyclopropyl group on d equals (A/4)×100%. A refers to total integral of the peaks from 1.12 to 0.80, in which there are four hydrogens. DS=(3.17/4)×100%≈79%.
Fig. 8.
1H NMR spectrum of d (TFA-D, 298 K, 400 MHz).
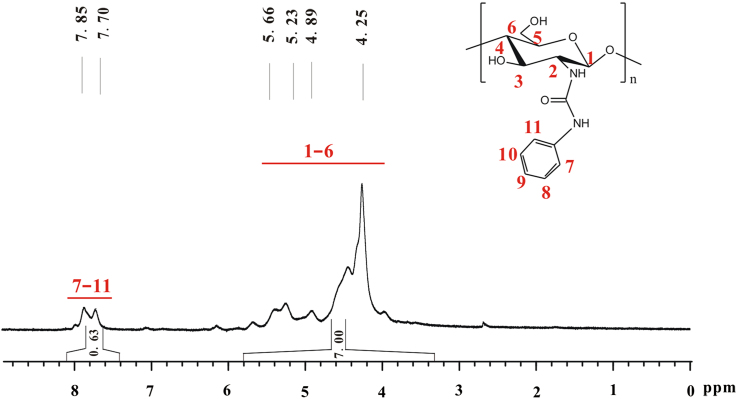
The total integral of seven hydrogens on glucose skeleton of chitosan is designated as 7.00 (Fig. 9). The DS of phenyl group on e equals (A/5)×100%. A refers to total integral of the peaks at 7.85 and 7.70, in which there are five hydrogens. DS=(0.63/5)×100%≈13%.
Fig. 9.
1H NMR spectrum of e (TFA-D, 298 K, 400 MHz).
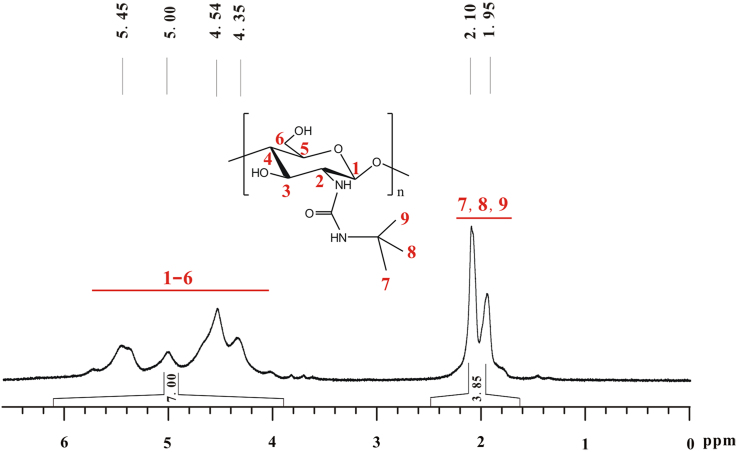
The total integral of seven hydrogens on glucose skeleton of chitosan is designated as 7.00 (Fig. 10). The DS of tert-butyl group on f equals (A/9)×100%. A refers to total integral of the peaks at 2.10 and 1.95, in which there are nine hydrogens. DS=(3.85/9)×100%≈43%.
Fig. 10.
1H NMR spectrum of f (TFA-D, 298 K, 400 MHz).
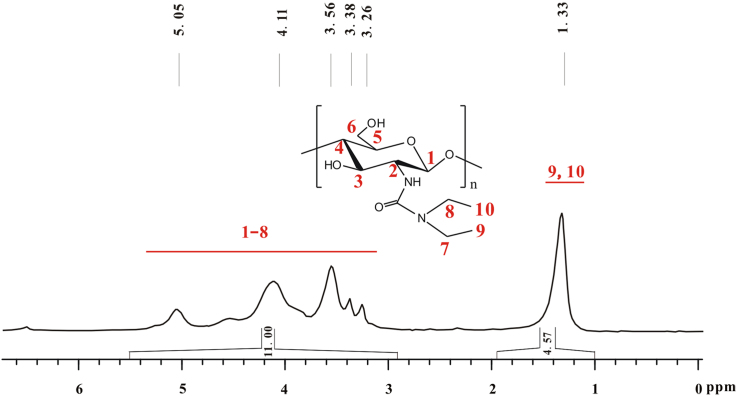
The peak of four hydrogens in two ethyl groups overlapped with that of seven hydrogens on glucose skeleton of chitosan, and the total integral of these eleven hydrogens is assumed as 11.00 (Fig. 11). The DS of N,N-diethylamido group on g is calculated by the equation of (7+4DS)/6DS=11.00/4.57. Therefore, DS=67%.
Fig. 11.
1H NMR spectrum of g (TFA-D, 298 K, 400 MHz).
Acknowledgments
Financial support from the National Natural Science Foundation of China (51373127) and the Scientific Research Foundation of Wuhan Institute of Technology (K201518) is gratefully acknowledged.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.03.099.
Appendix A. Supplementary material
Supplementary material
References
- 1.Wang J., Jiang J.-Z., Chen W., Bai Z.-W. Synthesis and characterization of chitosan alkyl urea. Carbohydr. Polym. 2016;145:78–85. doi: 10.1016/j.carbpol.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Heux L., Brugnerotto J., Desbrieres J., Versali M.-F., Rinaudo M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules. 2000;1:746–751. doi: 10.1021/bm000070y. [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas G., Paredes J.C., Cabrera G., Casals P. Synthesis and characterization of chitosan alkyl carbamates. J. Appl. Polym. Sci. 2002;86:2742–2747. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material