Abstract
Magnetoencephalography (MEG) is a noninvasive imaging method for localization of focal epileptiform activity in patients with epilepsy. This study investigates the cerebral functional abnormalities quantified by MEG coherence laterality in mesial temporal lobe epilepsy (mTLE). Resting state MEG data was analyzed using MEG coherence source imaging (MEG-CSI) method to determine the location of highly coherent anatomical sites in 12 adult mTLE patients and 12 age- and gender- matched controls. MEG coherence laterality, after Bonferroni adjustment, showed significant differences for right versus left mTLE in insular cortex and both lateral orbitofrontal and superior temporal gyri (p<0.025). None of these anatomical sites showed statistically significant differences in coherence laterality between right and left sides of controls. Coherence laterality was in agreement with the declared side of epileptogenicity in insular cortex (in 75% of patients) and both lateral orbitofrontal (83%) and superior temporal gyri (84%). Combining all significant laterality indices improved the lateralization accuracy to 92%. The proposed methodology for using MEG to investigate the abnormalities related to focal epileptogenicity and propagation can provide a further means of noninvasive lateralization.
Index Terms: Magnetoencephalography, Coherence Source Imaging, Coherence Laterality, Mesial Temporal Lobe Epilepsy
I. INTRODUCTION
Over sixty-five million people worldwide [1] and three million people in the United States are diagnosed with epilepsy, 15 to 20% of which remain medically refractory in spite of antiepileptic medical therapy [2]. Mesial temporal lobe epilepsy (mTLE) is the most common form of surgically remediable focal epilepsy, accounting for 60 – 75% of patients undergoing surgery for medically refractory epilepsy [3]. Intracranial electroencephalography (icEEG) optimizes localization of focal epileptogenicity, although it incurs great expense, and carries risks of infection, intracranial hemorrhage and elevated intracranial pressure [4–6]. This has inspired further work with noninvasive neuroimaging methods to provide better definition of focal epileptogenicity and obviate the need for invasive study in some patients and perhaps altogether [7, 8].
Magnetoencephalography (MEG) is a noninvasive method of recording the magnetic fields that principally arise from intracellular electric currents flowing in active neurons [9, 10]. MEG is used clinically to localize interictal and, sometimes, ictal activity [11–15]. Abnormal transients and oscillations can be modeled to identify underlying sources. Synchronization of neuronal activity, a characteristic of epileptogenicity, can be quantified by coherence, a measure of the strength of functional interrelation between pairs of cerebrocortical regions. Use of EEG mean phase coherence has revealed that regions of highly coherent nodes in the cerebral cortex are adjacent to seizure onset zones [16]. During the interictal period, local increases in coherence between EEG electrodes have been reported [17] as well as increased levels of synchronization in the involved hemisphere [18]. It has been shown that MEG coherence source imaging (MEG-CSI) is more sensitive than the standard single equivalent dipole (ECD) model in lateralizing the site of epileptogenicity in mTLE patients [19]. By defining the coherence laterality measure, we hypothesize that MEG-CSI method can contribute more efficient to noninvasive lateralization of mTLE patients in an asymmetric fashion.
II. Materials and Methods
A. Patients and Treatment
Twelve consecutive adult patients with refractory TLE (six females) who had undergone a MEG evaluation were selected for this study. Each had undergone inpatient video-electroencephalography, MRI, neuropsychological evaluation and intracarotid sodium amobarbital injection for evaluation of verbal memory capacity. Those lacking sufficient lateralization by this stage underwent further study by intracranially implanted electrodes for extraoperative electroencephalography. Patients were excluded if their MRI indicated cortical dysplasia, tumor, dilated ventricles or previous resection. Four patients had pathologically-proven hippocampal sclerosis. All patients underwent surgical resection and achieved an Engel class I outcome (i.e., seizure-free one year postoperatively). Twelve age- and gender-matched healthy control subjects without neurologic disorders underwent MEG study with the same parameters.
B. MEG Imaging and Analysis
For each epilepsy patient, 10 minutes of spontaneous resting state MEG data sampled at 508 Hz with band pass filters set from 0.1 to 100 Hz was acquired while the subject was asked to lie still and minimize movement. Post-acquisition data processing was performed using MEG Tools, an open-source Matlab (The Mathworks Inc., Natick, MA, USA) -based software module for cortical source imaging including single current dipole and multiresolution focal underdetermined system solution (MR-FOCUSS) methods [20–22] (http://www.megimaging.com). The data were forward and backward filtered using a 3–50Hz bandpass filter to remove 60Hz power supply signal, high frequency electronic noise, movement and synchronous breathing artifacts. In addition, Independent Component Analysis (ICA) was used to remove cardiac artifact from the MEG data and singular value decomposition (SVD) method was used to eliminate high amplitude artifacts associated with head, eye and mouth movement [23, 24]. All data were visually inspected for epileptic spikes. Raw data were reviewed by board-certified neurophysiologists.
MEG-CSI was calculated using 10 minutes of rest state MEG data. These data were prepared for source imaging by division into 80 segments, each containing 7.5 seconds of data of relatively uniform brain behavior [21, 22]. For each of these data segments, signals from neuronal sources were isolated using an ICA spatiotemporal decomposition technique designed to extract signals from distinct compact sources that exhibit burst behavior and minimal temporal overlap with other active sources. These ICA signal components have MEG spatial magnetic field patterns corresponding to one or a few spatially distinct compact sources which can be imaged accurately using MR-FOCUSS [21, 22]. In the cross-spectrum calculations, a sequence of fast Fourier transform (FFT) spectra was calculated using a 0.5 sec window with a 25% overlap for FFT amplitudes for 2 Hz width frequency bins between 3 and 50 Hz.
C. Gray Matter Model
To localize cortical source activation of epileptogenic activity, a model of gray matter was constructed for each individual’s T1-weighted high-resolution volumetric MR image. We used a probabilistic brain atlas composed of 56 structures from manually delineated MRI data constructed by Shattuck et al. as a standard volumetric head model with each location specified in MNI305 coordinates. This atlas contains all cerebral lobes and, specifically, the right and left hippocampi, limbic gyri, insular cortices, caudate, putamen, cerebellum and brainstem. Excluding the cerebellum and brainstem reduced the number of anatomical regions to 27 in each cerebral hemisphere [25] (Table 1). The realistic head model consisted of X-, Y- and Z- oriented dipoles at approximately 4000 locations to represent the same amount of gray matter identified in each individual’s MR image. These MR images were coregistered with the individual’s digitized head shape recorded at the time of MEG data collection.
Table 1.
Anatomical sites in the left and right hemisphereS
| Anatomical sites | |
|---|---|
| 1 | Angular Gyrus |
| 2 | Caudate |
| 3 | Cingulate Gyrus |
| 4 | Cuneus |
| 5 | Fusiform Gyrus |
| 6 | Gyrus Rectus |
| 7 | Hippocampus |
| 8 | Inferior Frontal Gyrus |
| 9 | Inferior Occipital Gyrus |
| 10 | Inferior Temporal Gyrus |
| 11 | Insular Cortex |
| 12 | Lateral Orbitofrontal Gyrus |
| 13 | Lingual Gyrus |
| 14 | Middle Frontal Gyrus |
| 15 | Middle Occipital Gyrus |
| 16 | Middle Orbitofrontal Gyrus |
| 17 | Middle Temporal Gyrus |
| 18 | Parahippocampal Gyrus |
| 19 | Postcentral Gyrus |
| 20 | Precentral Gyrus |
| 21 | Precuneus |
| 22 | Putamen |
| 23 | Superior Frontal Gyrus |
| 24 | Superior Occipital Gyrus |
| 25 | Superior Parietal Gyrus |
| 26 | Superior Temporal Gyrus |
| 27 | Supramarginal Gyrus |
D. MEG Coherence Laterality
The imaging results and the signal cross-spectrum were used to calculate the coherence between all pairings of each of the 54 cortical locations within each of the 24 frequency bins. Finally, for each active source, the average coherence with all other sources was calculated for each frequency and then averaged across the bandwidth of 3 to 50 Hz, ranging from 0 (no coherence) to 1 (highly coherent) [19, 20]. In these coherence imaging results, the localization of imaged brain activity is strongly dependent on the frequency bands with greatest power. When these coherence results are averaged across the full 10 minutes of data, only cortical sources that are consistently engaged in synchronous activity contribute to the final results. The MEG-CSI results were coregistered to individual volumetric MRI scans and areas of significant coherence were identified for each subject.
The coherence laterality Coh_Lat was computed to determine which hemisphere exhibited higher coherence over the entire time interval of spontaneous acquisition. It was calculated for each cortical site as:
| (1) |
where Coh(i) and Coh(i + 27) represent the coherence for the site i in the left and right hemispheres, respectively. A positive value indicates that a greater fraction of the right hemisphere was engaged in coherent activity of a cortical site compared to the left hemisphere on average for the patient across the 10 minutes of MEG data.
E. Statistical Analysis
Two-way repeated measures analysis of variance (RMANOVA) was used to examine the relationships of the MEG coherence laterality measurements with the brain regions (i.e., a repeated factor) and the mTLE laterality type (i.e., a fixed factor) [26]. Of particular interest were tests for interaction between a region and a laterality type, since a significant interaction would imply that separate one-way ANOVAs are required to assess mTLE laterality type.
For each region, one-way ANOVA on the mTLE laterality type was performed and multiple comparisons were addressed by Bonferroni adjustments for two pairwise comparisons between laterality types (p<0.05/2=0.025). However, the one-way ANOVAs were considered statistically significant only if the overall ANOVA F-test for all mTLE laterality types was also significant after a multiple comparisons adjustment (p<0.05/27=0.0019).
F. Lateralization Response-Driven Models
The statistically significant coherence laterality measures between the left and right mTLE cohorts were considered as multivariate independent variables and incorporated into the development of response-driven models of laterality using logistic function regression [27]. In order to assess how the multinomial logistic function generalized to an independent dataset and how accurately this response model performed in practice, a cross-validation was performed using the ‘leave-one-out’ approach [28]. The probability of detection on the test data was then calculated [29, 30].
III. Results
A. MEG Coherence Laterality
The ages of the male and female subjects across any of the right and left mTLE and control cohorts were statistically comparable. MEG-CSI identified epileptic network sites for each of the 12 mTLE patients (Fig. 1).
Figure 1.
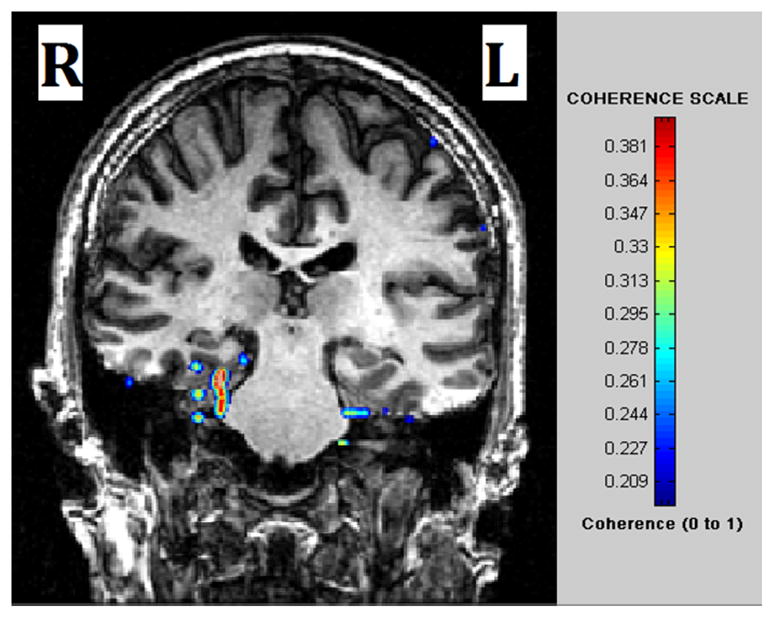
MEG -CSI map for a patient with right TLE. High coherence was detected in the right mesial temporal region.
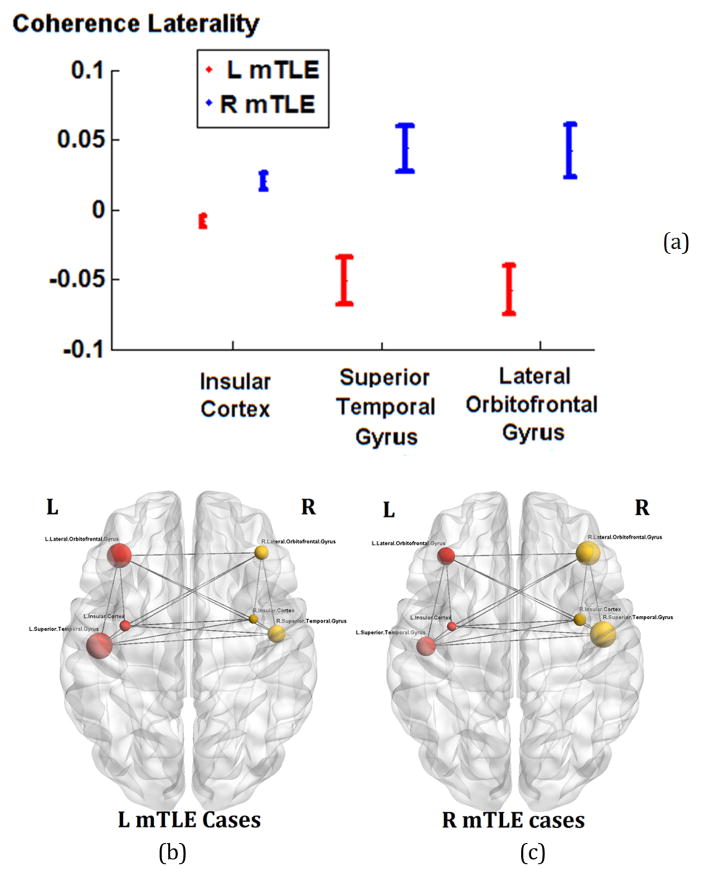
Two-way RMANOVA demonstrated significant interaction between regional and mTLE laterality type with coherence laterality measures (p<0.001). For controls, no single anatomical site showed a significant difference in coherence between the right and left sides. This finding is in concordance with the evidence that control subjects did not exhibit areas of high coherence during spontaneous MEG study [19]. For the insular cortex and the lateral orbitofrontal and superior temporal gyri, the overall ANOVA F-test in all mTLE laterality types was significant after Bonferroni adjustments (p< 0.0019). In t-tests between pairs of laterality types, the coherence laterality showed significant differences in insular cortex, and both lateral orbitofrontal and superior temporal gyri, for the right versus left mTLE cases (p<0.025; Figure 2a). Figures 2b and 2c show the MEG mean coherence in the insular cortex and the lateral orbitofrontal and superior temporal gyri overlaid upon the MNI registered brain for the left and right mTLE patient cohorts, respectively.
Figure 2.
MEG coherence laterality in insular cortex and the lateral orbitofrontal and superior temporal gyri, where significant differences between the right and left mTLE patients were seen. (a): The error bars represent the standard error of the mean coherence laterality. (b) and (c): The spheres and lines overlaid upon the MNI registered brain show the significant cortical sites and their corresponding connections, respectively. The right and left cortical sites are shown in yellow and red, respectively. The mean coherence values are represented by the size of the spheres.
B. mTLE Lateralization Models
The laterality was modeled by logistic regression of the MEG coherence laterality data in the insular cortex and the lateral orbitofrontal and superior temporal gyri. By averaging over 12 repetitions of leave-one-out cross validation, the probability of detection achieved 0.75±0.04, 0.83±0.04 and 0.84±0.02 for the laterality models of the insular cortex, the lateral orbitofrontal and superior temporal gyri, respectively. Combining the laterality measures in these three anatomical sites improved lateralization results to 0.92±0.02 of the patients, as compared to the generic MEG-CSI analysis [19] that identified the epileptogenic side for 75% of the cases under this study, which supports the findings in [19].
IV. Discussion and conclusion
In the current study, we showed that the result of the lateralization model developed based on the coherence laterality in insular cortex, lateral orbitofrontal and superior temporal gyri was in agreement with the declared side of epileptogenicity for up to 92% of the mTLE cases. It has been shown that MEG-CSI provides clinicians with valuable information regarding surgical candidacy. We used the MEG-CSI method and the coherence laterality measure to analyze the ability to detect the side of epileptogenicity. In the past 10 years, developments in the computational analysis of source localization for MEG have advanced the ability for connectivity to be imaged directly within specific regions (i.e., source space), providing a better anatomical localization as well as greater ease for co- registering to the MRI data. The high temporal resolution of MEG allows for investigations of function and effective connectivity with millisecond precision. It is possible now to study the mechanisms by which information is exchanged across brain regions, including oscillatory and synchronized neuronal activity. Only a handful of MEG coherence studies using different inverse methods (i.e., dipoles, minimum norm or beamformers) to localize source space coherence have been performed in the past decade. MEG-CSI has been used to ascertain the laterality of epileptic networks in epilepsy patients where highly coherent activity was found in epileptic neural networks while control subjects lacked similar manifestations [19]. It can provide targets for successful surgical resection with a detection rate of 77% of the patients who had high coherence in the area of the later resection. MEG-CSI uses a current distribution technique, MR-FOCUSS, to image underlying sources based on a 10 minute resting state scan. More recently, Englot et al used a beamforming technique to image underlying sources and studied regional and global functional connectivity of MEG coherence in patients with epilepsy, based on a one-minute resting state scan [31]. An Engel class I outcome was seen in 87.5% of patients where increased connectivity was found in the region of the later resection.
With increasingly sophisticated signal processing methods and the use of multimodal neuroimaging and neurophysiological biomarkers, noninvasive investigational techniques may ultimately supplant invasive monitoring as a means of localizing focal epileptogenicity and establishing surgical candidacy.
Acknowledgments
Research supported in part by NIH grant R01-EB013227.
Contributor Information
Mohammad R. Nazem-Zadeh, Departments of Research Administration and Radiology, Henry Ford Health System, Detroit, MI, USA
Susan M. Bowyer, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA
John E. Moran, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA
Esmaeil Davoodi-Bojd, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA.
Andrew Zillgitt, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA.
Hassan Bagher-Ebadian, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA.
Fariborz Mahmoudi, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA.
Kost V. Elisevich, Department of Clinical Neurosciences, Spectrum Health Medical Group, Grand Rapids, MI, USA
Hamid Soltanian-Zadeh, Departments of Neurology, Radiology, Radiation Oncology, and Research Administration, Henry Ford Health System, Detroit, MI, USA. Control and Intelligent Processing Center of Excellence (CIPCE), School of Electrical and Computer, University of Tehran, Tehran, Iran.
References
- 1.England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum. 2012 [Google Scholar]
- 2.Kohrman MH. What is Epilepsy? Clinical Perspectives in the Diagnosis and Treatment. Journal of Clinical Neurophysiology. 2007;24(2):87–95. doi: 10.1097/WNP.0b013e3180415b51. [DOI] [PubMed] [Google Scholar]
- 3.Engel J., Jr Surgery for seizures. New England Journal of Medicine. 1996;334(10):647–653. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 4.Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D, Najm I, Bingaman W. Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia. 2012;53(10):1722–1730. doi: 10.1111/j.1528-1167.2012.03633.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuzniecky R, Bilir E, Gilliam F, Faught E, Palmer C, Morawetz R, Jackson G. Multimodality MRI in mesial temporal sclerosis: relative sensitivity and specificity. Neurology. 1997;49(3):774–778. doi: 10.1212/wnl.49.3.774. [DOI] [PubMed] [Google Scholar]
- 6.Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: A systematic review and meta-analysis. Epilepsia. 2013;54(5):828–839. doi: 10.1111/epi.12073. [DOI] [PubMed] [Google Scholar]
- 7.Aghakhani Y, Liu X, Jette N, Wiebe S. Epilepsy surgery in patients with bilateral temporal lobe seizures: A systematic review. Epilepsia. 2014 doi: 10.1111/epi.12856. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Liu Q, Mei S, Zhang X, Liu W, Chen H, Xia H, Zhou Z, Wang X, Li Y. Identifying the affected hemisphere with a multimodal approach in MRI-positive or negative, unilateral or bilateral temporal lobe epilepsy. Neuropsychiatric disease and treatment. 2014;10:71. doi: 10.2147/NDT.S56404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen DJ, Hosaka JJ. Magnetic field produced by a current dipole. Journal of Electrocardiol. 1976;9:409–417. doi: 10.1016/s0022-0736(76)80041-6. [DOI] [PubMed] [Google Scholar]
- 10.Hamalainen M, Hari R, Ilmoniemi J, Knuutila J, Lounamaa O. Magnetoencephalography-theory, instrumentation and applications to noninvasive studies of the working human brain. Review of Modern Physics. 1993;65(2):413–497. [Google Scholar]
- 11.Barkley GL, Baumgartner C. MEG and EEG in Epilepsy. Journal of Clinical Neurophysiology. 2003;20:163–178. doi: 10.1097/00004691-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ebersole JS, Hawes-Ebersole SH. Clinical Application of Dipole Models in the Localization of Epileptiform Activity. Journal of Clinical Neurophysiology. 2007;24(2):120– 129. doi: 10.1097/WNP.0b013e31803ece13. [DOI] [PubMed] [Google Scholar]
- 13.Knowlton RC. Can magnetoencephalography aid epilepsy surgery? Epilepsy Curr. 2008;8(1):1–5. doi: 10.1111/j.1535-7511.2007.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, Lopez N. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008;71(13):990–6. doi: 10.1212/01.wnl.0000326591.29858.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englot DJ, Nagarajan SS, Imber BS, Raygor KP, Honma SM, Mizuiri D, Mantle M, Knowlton RC, Kirsch HE, Chang EF. Epilepsia. 2015. Epileptogenic zone localization using magnetoencephalography predicts seizure freedom in epilepsy surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, McKhann JG, Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage. 2007;35(1):140–148. doi: 10.1016/j.neuroimage.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towle VL, Carder RK, Khorasani L, Lindberg D. Electrocorticographic coherence patterns. J Clin Neurophysiol. 1999;16(6):528–547. doi: 10.1097/00004691-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kraskov A. Applications of synchronization and interdependence measures in particular to EEG of epilepsy patients. John Von Neumann Institute for Computing, NIC; 2004. [Google Scholar]
- 19.Elisevich K, Shukla N, Moran JE, Smith B, Schultz L, Mason K, Barkley GL, Tepley N, Gumenyuk V, Bowyer SM. An assessment of MEG coherence imaging in the study of temporal lobe epilepsy. Epilepsia. 2011:1110–1119. doi: 10.1111/j.1528-1167.2011.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran J, Manoharan A, Bowyer SM, Mason KM, Tepley N, Morrell M, Greene D, Smith BJ, Barkley GL. MEG Coherence Imaging Compared to Electrocortical Recordings from NeuroPace Implants to Determine the Location of Ictal Onset in Epilepsy Patients. 15th International Conference on Biomagnetism; 2006; Vancouver, BC Canada: Elsiver; pp. 673–676. [Google Scholar]
- 21.Moran JE, Bowyer S, Tepley N. Multi-Resolution FOCUSS: A source imaging technique applied to MEG data. Brain Topography. 2005;18(1) doi: 10.1007/s10548-005-7896-x. [DOI] [PubMed] [Google Scholar]
- 22.Moran JE, Drake CL, Tepley N. ICA methods for MEG imaging. Neurol Clin Neurophysiol. 2004;2004:72. [PubMed] [Google Scholar]
- 23.Duda R, Hart P. Pattern Classification and Scene Analysis. Wiley-Interscience; New York: 1973. Linear Discriminant Functions; pp. 130–185. [Google Scholar]
- 24.Tufts DW, Kumaresan R, KI Data Adaptive Signal Estimation by Singular Value Decomposition of a Data Matrix. Proceedings of the IEEE. 1982;70(6):684– 685. [Google Scholar]
- 25.Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39(3):1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazem-Zadeh M-R, Elisevich K, Air EL, Schwalb JM, Divine G, Kaur M, Wasade VS, Mahmoudi F, Shokri S, Bagher-Ebadian H, Soltanian-Zadeh H. DTI-based Response-Driven Modeling of mTLE Laterality. NeuroImage Clinical. 2015 doi: 10.1016/j.nicl.2015.10.015. [DOI] [PMC free article] [PubMed]
- 27.Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied logistic regression. Wiley. com; 2013. [Google Scholar]
- 28.Picard RR, Cook RD. Cross-validation of regression models. Journal of the American Statistical Association. 1984;79(387):575–583. [Google Scholar]
- 29.Nazem-Zadeh M-R, Schwalb JM, Bagher-Ebadian H, Jafari-Khouzani K, Elisevich KV, Soltanian-Zadeh H. A Bayesian averaged response-driven multinomial model for lateralization of temporal lobe epilepsy. Biomedical Imaging (ISBI), 2014 IEEE 11th International Symposium on; 2014; pp. 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazem-Zadeh MR, Elisevich KV, Schwalb JM, Bagher-Ebadian H, Mahmoudi F, Soltanian-Zadeh H. Lateralization of temporal lobe epilepsy by multimodal multinomial hippocampal response-driven models. Journal of the neurological sciences. 2014;347(1):107–118. doi: 10.1016/j.jns.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Englot DJ, Hinkley LB, Kort NS, Imber BS, Mizuiri D, Honma SM, Findlay AM, Garrett C, Cheung PL, Mantle M, Tarapore PE, Knowlton RC, Chang EF, Kirsch HE, Nagarajan SS. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain Research. 2015;138(8):2249–62. doi: 10.1093/brain/awv130. [DOI] [PMC free article] [PubMed] [Google Scholar]