Abstract
One important modality of breast cancer therapy is surgical treatment, which has become increasingly less mutilating over the last century. Breast reconstruction has become an integrated part of breast cancer treatment due to long-term psychosexual health factors and its importance for breast cancer survivors. Both autogenous tissue-based and implant-based reconstruction provides satisfactory reconstructive options due to better surgeon awareness of “the ideal breast size”, although each has its own advantages and disadvantages. An overview of the current options in breast reconstruction is presented in this article.
Keywords: Breast cancer, Prosthetic implants, Autologous reconstruction, Plastic surgery
Highlights
-
•
Surgical treatment of breast cancer.
-
•
Breast reconstruction with both autogenous tissue-based and implant-based.
-
•
Complications.
1. Introduction
Breast cancer is the leading cause of cancer death among women worldwide, with approximately 1.7 million new diagnoses and 521,900 deaths occurring in 2012 [1]. One important modality of breast cancer therapy is surgical treatment, which has become increasingly less mutilating over the last century. Approximately 35–40% of women diagnosed with breast cancer undergo total mastectomy, a trend that is increasing [2]. Until the 1970s, breast cancer was treated with radical mastectomy involving removal of the breast, axillary lymph nodes, and pectoralis muscle. This was extremely disfiguring for patients and did not lend itself to optimal reconstructive options. In the 1970s, modified radical mastectomy was introduced, which preserved the pectoralis muscle and improved the contour of the chest wall, as well as increased the reconstructive possibilities. In the 1980s, a large randomized study conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP) demonstrated that breast conservation plus radiation had equivalent outcome to mastectomy [3].
Breast reconstruction has become an integrated part of breast cancer treatment due to long-term psychosexual health factors and its importance for breast cancer survivors [4], [5], [6]. Both autogenous tissue-based and implant-based reconstruction provides satisfactory reconstructive options due to better surgeon awareness of “the ideal breast size” [7], although each has its own advantages and disadvantages. Data from the United States indicate that between 1998 and 2008, there was an 11% increase in the use of implants per year, whereas autologous reconstruction rates remained stable [8], [9]. Indeed, the data shows that prior to 2002, autologous reconstructions were more frequently chosen compared with prostheses. However, after 2002, this relationship was reversed, and in 2008, implants outnumbered autologous reconstructions by a ratio of 2:1 (258 vs. 120 per 1000 mastectomies) [8]. Albornoz et al. [8] suggests a number of reasons behind this change: the longer time it takes to perform autologous reconstruction, a cultural shift towards acceptance of breast implants, and the way in which reconstruction is funded. In alloplastic reconstructions, patients are exposed to less surgical risk, fewer scars, less donor site morbidity, and fewer irreversible consequences. However, surgical factors like implant type, number of surgical stages, and the use of an acellular dermal matrix can influence outcomes [10], [11], [12], [13].
The authors analyze the current literature on the light of their multicentric experience in the filed of breast surgery in order to determine the latest trends in breast reconstruction.
2. Mastectomy: different techniques
The mastectomy procedure has evolved from the Halsted radical mastectomy, which involved a wide excision of all breast tissue, all overlying skin, and the pectoralis major muscle and included a full en bloc dissection of Level I, II, and III nodes [14]. The most commonly performed mastectomy is the total mastectomy, which removes all breast tissue including the nipple–areola complex (NAC) and an ellipse of skin adjacent to the nipple–areola complex.
For women who require mastectomy for the surgical management of breast cancer or as a prophylactic procedure in those with a known genetic predisposition for breast cancer, skin-sparing mastectomy with immediate reconstruction is an excellent choice that allows complete breast parenchyma resection with acceptable breast mound provision via an implant or flap. This procedure preserves as much of the patient's breast skin as possible—the breast parenchyma and nipple–areola complex are removed through a circumareolar incision (sentinel lymph node dissection may be performed through a separate incision if indicated). Skin-sparing mastectomy has been shown to have equivalent local recurrence rates to conventional mastectomy given the selection bias of reserving this approach for patients without clinical evidence of locally advanced or inflammatory breast cancer [15], [16], [17], [18].
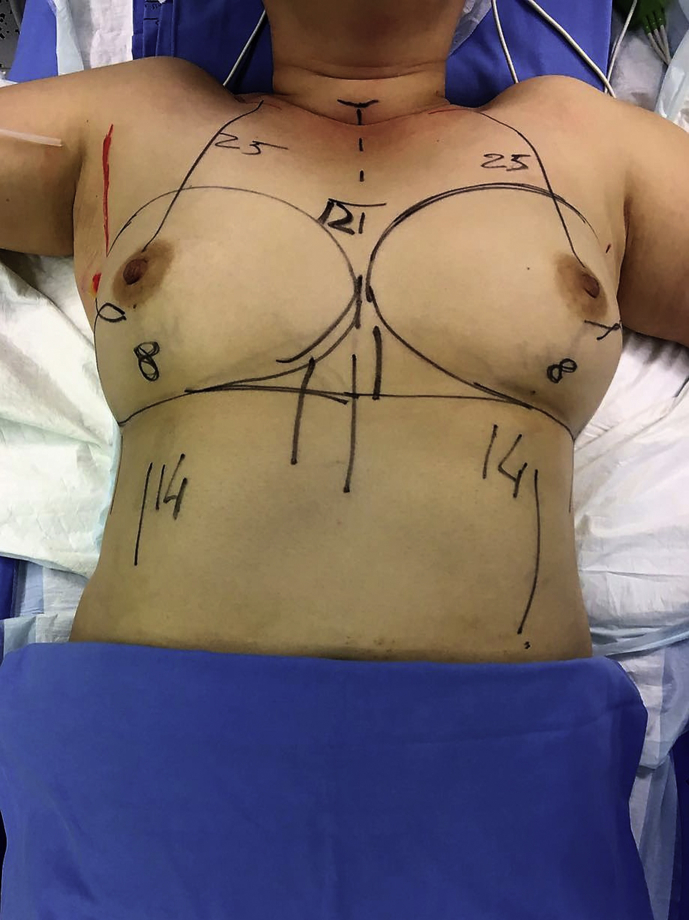
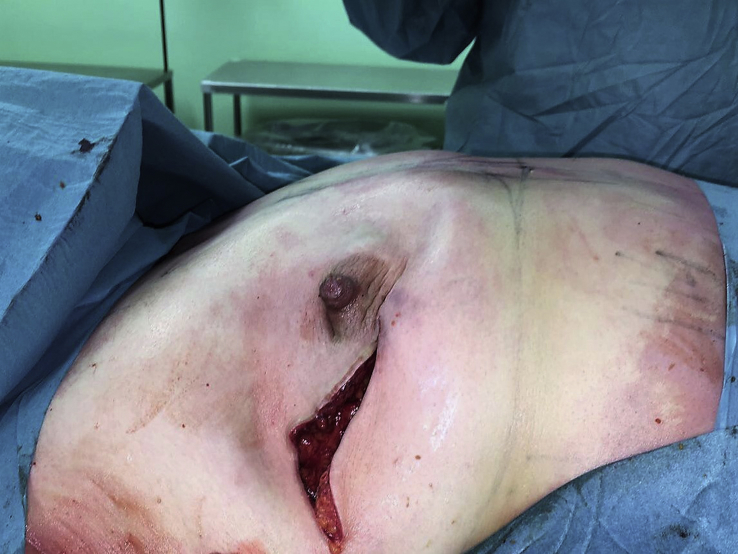
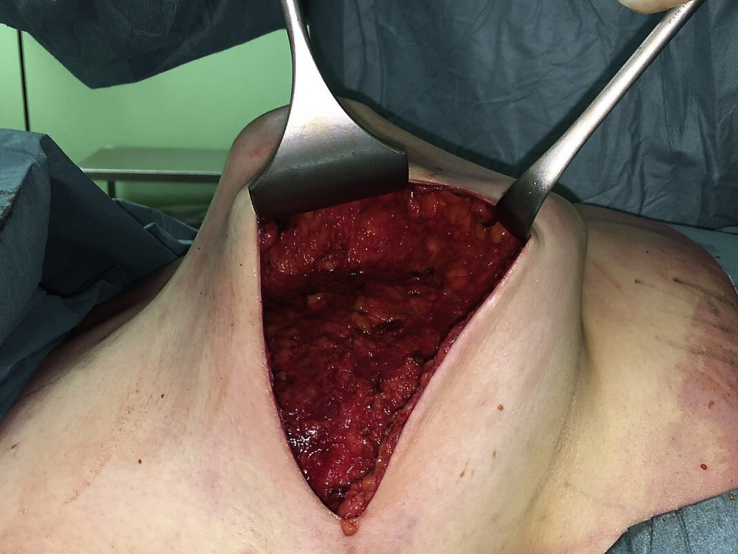
Another surgical option is nipple-sparing mastectomy, which involves a total mastectomy via a noncentral incision (such as an inframammary or axillary approach), preserving the skin, skin envelope, and cutaneous portion of the nipple–areola complex. Crowe et al. published one of the first modern series on this technique, reporting the technical feasibility of nipple-sparing mastectomy [19] (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Bilateral nipple-sparing mastectomy, preoperative design.
Fig. 2.
Periareolar skin incision.
Fig. 3.
All breast tissue removed.
3. Implants
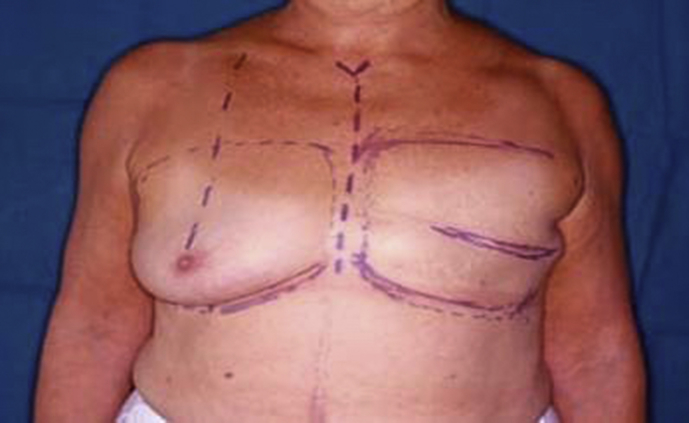
Use of prosthetic implants for breast reconstruction began in the early 1960s when Cronin and Gerow developed the silicone prosthesis and used it clinically for augmentation mammoplasty [20]. Shortly thereafter, these implants began to be used for the reconstruction of mastectomized breasts [21]. Breast reconstructions using prosthetic implants were applied in a single stage at first. Development of tissue expanders by Radovan created new possibilities in immediate or delayed reconstruction, and the popularity of single-stage reconstruction by implants was overtaken by two-stage reconstructions during the 1980s [22].
The use of implants and skin expanders are the quickest and presumably easiest methods of breast reconstruction. The prerequisite for implant-based breast reconstruction is an adequate skin envelope to cover the implant that is usually introduced into the submuscular plane by detaching the medial insertions of the pectoralis major muscle from the ribs. Saline and silicone gel implants are available as the final implant material for expander/implant-based postmastectomy reconstruction. All implant models have a bladder (or outside shell) made of solid silicone. The shell can be either textured or smooth. Modern expanders are textured to help prevent migration and early capsular contracture and to help develop a well-defined inframammary fold and ptosis [23] (Fig. 4, Fig. 5, Fig. 6, Fig. 7). Both saline and silicone implants can be either round or anatomically shaped (like a teardrop). The consensus is that there is no difference between round and shaped implants in rippling or in overall satisfaction with breast surgery outcome [24], [25]. Silicone gel implants are traditionally thought to provide a softer, more natural feeling breast compared with saline implants [26].
Fig. 4.
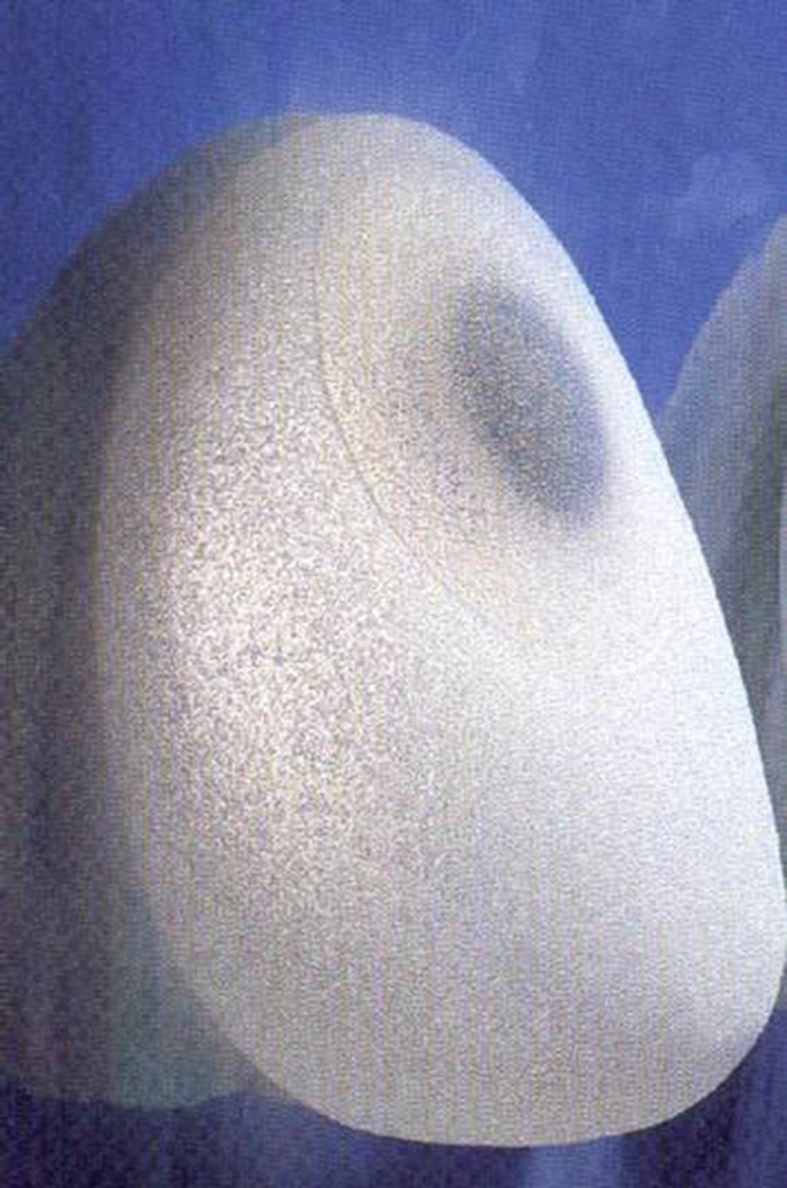
Textured implant.
Fig. 5.
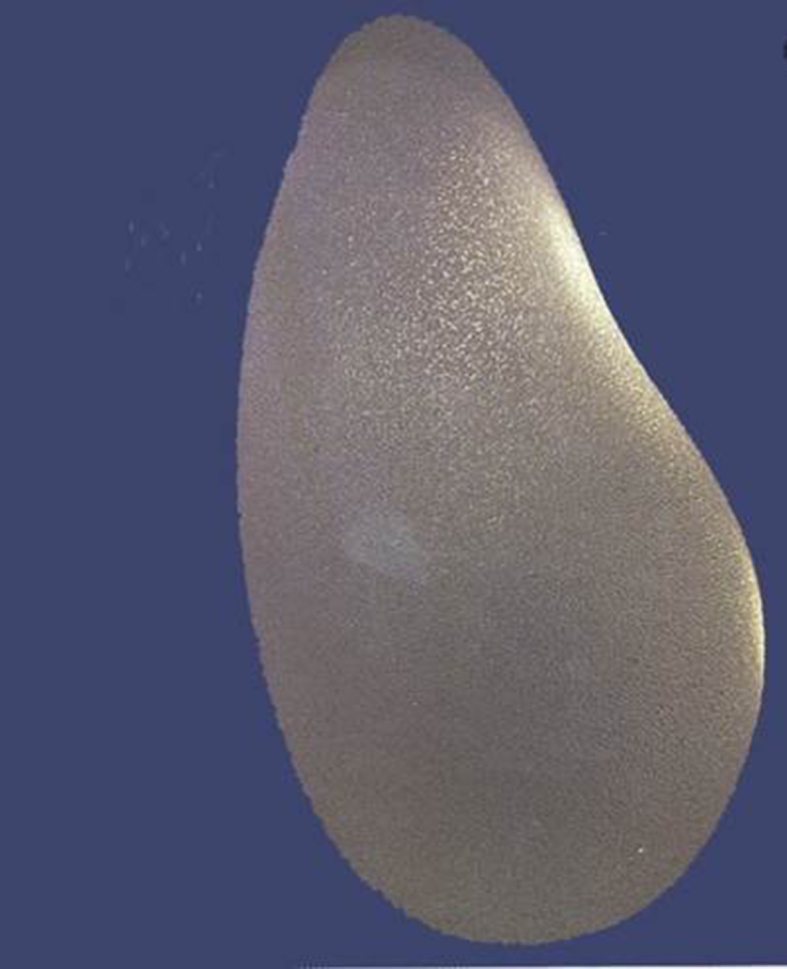
Anatomical silicone implant.
Fig. 6.
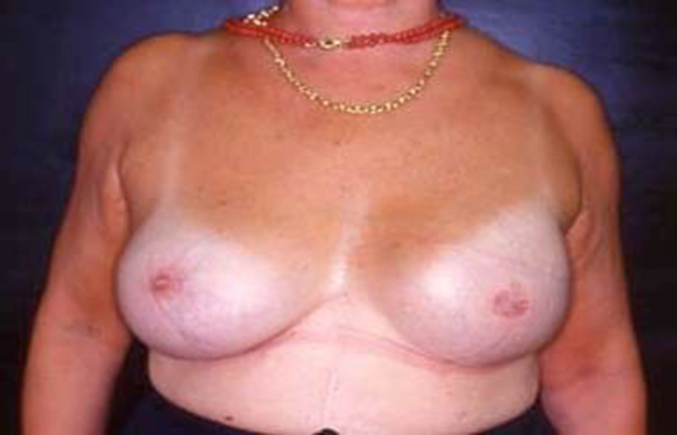
Left mastectomy before reconstruction.
Fig. 7.
Left mastectomy post two stage reconstruction.
4. Use of an acellular dermal matrix
Traditional submuscular placement of a tissue expander requires the elevation of, and coverage with, the pectoralis major and serratus anterior muscles. However, the use of an acellular dermal matrix (ADM) has been increasing [27], with the pectoralis muscle being used to cover the prosthesis anteromedially and the ADM being used to cover it laterally. Matrices are usually of human, porcine, or bovine origin. They have been shown to improve the aesthetic outcome, reduce implant-related morbidity—such as capsular contracture [28]—improve tolerance to radiotherapy, and produce a more natural anatomical reconstruction of the inframammary fold and final breast contour [29], [30].
Use of ADM allows placement of tissue expanders with greater intraoperative fill volumes; therefore, fewer expansions are required before exchange for the permanent implant. In addition, ADM may have the potential to reduce the rate of encapsulation [31]. Finally, use of ADM avoids elevation of the serratus anterior, which was once thought to decrease postoperative pain. However, a multicenter, blinded, randomized controlled trial did not demonstrate any reduction in postoperative pain with ADM use [32].
Nevertheless, several complications may occur due to the release of the pectoralis major muscle, and physiotherapy rehabilitation is frequently required [33]. The detachment of the pectoralis muscle with the aim of placing an expander/implant under the muscle can cause prolonged postoperative pain [34]. A new conservative surgical technique that preserves the pectoralis major muscle is possible with Braxon®, a non-crosslinked acellular dermal matrix [35]. This ADM is wrapped around the breast implant and sutured with absorbable sutures to the superficial surface of the pectoralis major, avoiding its detachment.
5. Breast reconstruction using flaps/autologous tissue
Autologous reconstruction uses the woman's tissue and can be performed immediately at the time of mastectomy or in a delayed fashion. Immediate reconstruction potentially exposes the patient to fewer operations, can save resource costs [35], [36], and gives the patient the best chance of a good aesthetic result [37]. In delayed reconstruction, mastectomy skin flaps are often scarred and less compliant [38], and a higher rate of free-flap thrombosis has been found (AOR, 1.42; overall free flap thrombosis rate 2.4%) [39]. However, similar rates of both major and minor complications have been reported between patients undergoing either immediate or delayed reconstruction with a transvers rectus abdomins muscle (TRAM) free flap [40].
The most favored donor site and method of choice in autologous breast reconstruction is utilization of the lower abdominal tissue [41] in the form of a TRAM, deep inferior epigastric artery perforator (DIEP), or superficial inferior epigastric artery (SIEA) flap. Autologous reconstruction can also be performed using tissue from the thigh or buttock in the form of transverse upper gracilis (TUG), superior gluteal artery perforator (SGAP), inferior gluteal artery perforator (IGAP), or profunda artery perforator (PAP) flaps. Breast reconstruction using autologous flap tissue allows a natural and durable result, although flap harvest causes “collateral damage” at the donor site, including potential surgery-related complications, scars, contour deformity, and functional impairment. Wound-related complication rates vary from 30 to 50% in the largest series [42], [43]. Ischemic complications include fat necrosis or flap loss in free-flap reconstruction. A recent meta-analysis showed that ischemic complications are higher in DIEP flaps compared with free TRAM flaps [44]. The pedicled TRAM tends to be associated with more fat necrosis than free abdominal flaps [45], [46] and an increased risk of partial and total flap loss in obese patients [47]. To decrease these types of complications, especially in “high risk” patients, a vascular delay procedure can be used in which the inferior vascular pedicle is ligated 2–3 weeks before reconstruction [48]. Criticism of the free TRAM flap has been related to morbidity from sacrificing the rectus muscle at the donor site [49], [50]. Patients reconstructed with a free TRAM flap have decreased abdominal strength and twice the risk of an abdominal bulge or hernia compared with DIEP reconstructions [51].
Technology is rapidly progressing and continues to challenge and exceed expectations, while maintaining considerations for patient safety and accessibility. Careful preoperative planning of interventions may be performed making use of new computer devices [52], [53]. The review of comprehensive imaging such as CTA and MRA can highlight other dominant perforators near potential donor sites and facilitate consideration of all feasible options for autologous breast reconstruction, but do not provide physiological information or flow characteristics. Some of these technologies serve as an aid to reduce the learning curve, potentially decrease surgery time and operative stress, and may translate into improved clinical outcomes [54], [55].
6. Autologous fat grafting
Autologous fat grafting (AFG) is an increasingly popular technique for breast reconstruction [56]. Its main use it to address any step-off and contour deformities following either implant- or autologous-based breast reconstruction [57]. Furthermore AFG can correct any asymmetry with the contralateral breast that can eventually develop in the late postoperative period following radiation therapy [58]. In literature, there's some debate regarding the safety of AFG following breast reconstruction for breast cancer; nevertheless to date there's no clear evidence of cancer recurrence following AFG [59].
The growing popularity of AFG derives from its specific characteristics. Indeed it's the ideal filler being 100% autologous thus no graft reaction can be seen [60]. Furthermore its function is more than simply filling a defect or modifying a contour but can improve the texture of the skin, scars and any late chronic degenerative side effect of radiation therapy [61]. This regenerative potential of AFG derives mainly from a population of stem cells, the adipose-derived stem cells (ASCs) localized within the so-called stromal vascular fraction of the adipose tissue [62], [63], [64], [65]. ASCs are multipotent mesenchymal stem cells that show definitive stem cell characteristics such as plastic adherence in culture, ability to maintain multipotency upon in vitro expansion, and self-renewal capacity [66], [67], [68], [69], [70], [71].
ASCs can be isolated from the harvested adipose tissue by either mechanical and/or mechanical means [72], [73], [74], [75], [76]. Once the pellet of stem cells is obtained it can be added to the processed lipoaspirates prior to fat grafting, creating a cell-assisted lipoarpirates [77]. Indeed one of the main drawbacks of AFG is its unpredictable reabsorption rate of the grafted adipose tissue that seems to be improved by the adjunction of ASCs [60].
The Coleman technique for AFG is by far the most commonly used [78], [79]. The adipose tissue is infiltrated with a tumescent solution (e.g., Klein solution) [80] and then manually harvested with 3-mm, blunt-edged, 2- hole cannula connected to a 10-mL syringe. The lipoaspirate is subsequently centrifuged for 3 min at 3000 rpm in order to isolate the adipose tissue for the oil and watery fraction and finally injected. The entire procedure can be performed under local assisted anesthesia.
7. Timing of reconstruction
Alloplastic reconstruction can be performed concomitantly with mastectomy (immediate or single-stage reconstruction) or weeks, months, or years later (delayed or two-stage reconstruction) [81].
Single-stage reconstruction may have positive psychological implications compared with delayed reconstruction: decreased distress, improved freedom of dress, better body image and self-esteem, decreased anxiety and depression, and improved feelings of sexual attractiveness and satisfaction. The greatest benefit of immediate reconstruction may be potentially fewer operations. Reconstruction of small breasts using skin-sparing mastectomy can be done with this method. The most suitable patients for this reconstruction method have less ptotic, round-shaped breasts, with healthy mastectomy flaps and less than 300 g of tissue to be resected [82], [83], [84]. It is difficult to obtain symmetry in single-stage reconstruction. In addition, the rate of complications such as infection, skin necrosis and implant exposure are higher in comparison with two-stage methods. The final breast form from single-stage reconstruction methods is typically smaller and less ptotic than that from two-stage methods [85].
Delayed reconstruction allows the patient additional time to consider her restorative options and represents the most commonly practiced form of breast reconstruction with implants [86]. In this method, a temporary device called a tissue expander is placed in a submuscular pocket during the first operation. At a later time, the tissue expander is removed, and breast reconstruction is completed with a permanent implant. Two-stage reconstruction has several advantages over the single-stage method. Indeed the tissue quality overlying the implant improves after the tissue-expander placement, while re-adjustment of the position of the permanent implant and manipulation of the inframammary fold are possible during the second stage, providing a better final breast shape. The period between the first and second stage also gives time for the patient and surgeon to deal with treatment matters such as chemotherapy and radiotherapy.
Tissue expansion/implant-based reconstruction requires enough of a healthy skin envelope for a tension-free closure. The native skin and/or muscle envelope may not be adequate to undergo expansion if there are multiple scars or a history of previous radiation injury, or if there was a large amount of skin resected during mastectomy. In these cases, the use of an autologous flap (most commonly, the latissimus dorsi myocutaneous flap) can provide coverage of the expander and, eventually, the implant. Patients requiring a salvage mastectomy after failed lumpectomy/irradiation can benefit from a latissimus dorsi/implant reconstruction [87]. Furthermore, use of an autologous flap in previously irradiated breasts appears to reduce the incidence of implant-related complications [88].
8. Drain management
Drains are typically placed after immediate reconstruction, especially after axillary dissection, but are rarely placed after delayed or second-stage reconstruction. In these cases, prior to skin closure, a closed bulb suction drain is placed over the pectoralis muscle so that it is not in contact with the implant. Drains are typically removed when drainage is less than 30 ml in a 24-h period, with many surgeons removing drains at 7–14 days postoperation irrespective of output.
9. Radiotherapy and prosthetic breast reconstruction
Postmastectomy radiation therapy (PMRT) is used for tumors with a size of 5 cm or less and one to three positive nodes [89]. In general, the timing of radiotherapy is dictated by whether patients require neoadjuvant chemotherapy [90]. For those requiring neoadjuvant chemotherapy, radiation therapy is delivered with the tissue expander in place, before it is exchanged for the permanent device. If adjuvant chemotherapy is delivered, radiation can be delivered either before or after the exchange procedure to the permanent implant.
Radiation injury usually occurs in two phases, acute and chronic. The acute phase is characterized by an inflammatory reaction that may consist of swelling, edema, erythema, desquamation, and ulceration [91], [92]. Overall, this acute radiation dermatitis occurs in 95% of patients treated with radiation [93].
The chronic phase consist of fibrosis as a reaction to radiation injury, and it primarily effects the skin and subcutaneous breast tissue. The signs and symptoms include varying degrees of permanent skin retraction and induration, chest and shoulder pain, and restricted arm and neck movement [94]. Radiation-induced fibrosis typically presents several months after radiotherapy and may progress for several years. Rates of capsular contracture have varied from 29% [95] to 68% [96] in patients with radiotherapy, compared with 10% [97] to 40% [98] of those without radiotherapy. The risk of significant capsular contracture (Baker Grade III or IV) has also been found to be higher in irradiated breasts [99], [100].
10. Complications
10.1. Risk factors
Patient selection begins with a physical examination and includes consideration of co-morbidities (diabetes, hypertension, immunodeficiency), tobacco use history, body habitus, and breast characteristics (fat versus glandular type). The occurrence of adverse events can be directly attributed to a variety of factors including elevated BMI, tobacco use, and poorly controlled diabetes mellitus [101]. Tobacco use is associated with poor perfusion, delayed healing, reconstructive failure, and increased rates of reoperation [102], [103]. Nicotine is a powerful vasoconstrictor and will impact circulation at the level of the capillaries and small vessels. By avoiding tobacco, wound-related complications could be reduced as much as threefold, from 23.5% to 7.7% [104].
Poorly controlled diabetes mellitus (types I and II) is a known factor associated with compromised healing as well [105]. Hyperglycemic states can interfere with normal wound healing and contribute to increased rates of incisional dehiscence and soft tissue infection, which can have significant consequences, especially in obese patients having mastectomy. Ideally, serum glucose should be less than 200 mg/dl, and urine glucose should be absent.
When considering breast reconstruction with prosthetic devices, obese and morbidly obese women are at risk for adverse events. Women with a BMI <30 are usually considered good candidates, whereas women with a BMI >40 are not because of increased incidences of seroma, compromised healing, infection, and poor cosmetic outcome [106].
Mammary hypertrophy in and of itself is not a contraindication to prosthetic breast reconstruction; however, there are several strategies that can increase the likelihood of a successful outcome. Most women with mammary hypertrophy will have large mastectomy skin excision patterns because traditional skin or nipple sparing is usually not an option. In these cases, we can use oncoplastic breast techniques such as superior/inferior pedicle mammoplasty with an inverted T/J scar.
10.2. Infections
Prosthesis-based breast surgery, both reconstructive and aesthetic, is associated with an even higher infection risk, and postoperative prophylactic antibiotics are routinely prescribed [107]. Breast implant infections involve 2–2.5% of patients and represent the leading cause of morbidity after reconstructive and aesthetic surgery [108]. Although antibiotic prophylaxis has been shown by several studies to prevent surgical site infection, extended antibiotic prophylaxis may lead to systemic side effects, super-infection, and the development of resistant organisms. We feel that extended systemic antibiotic prophylaxis (24 h postoperatively) can significantly reduce infection risk, especially in implant breast reconstruction. Topical antibiotic irrigation may decrease capsular contraction risk, but it might not reduce the infection rate. A cephalosporin is generally recommended for antibiotic prophylaxis to cover for the most commonly identified implant-associated bacteria. Antibiotic prophylaxis may be extended postoperatively in cases with certain individual risk factors.
10.3. Seroma
The incidence of seroma after prosthetic breast reconstruction varies from 0.2 to 20% [109], [110], [111], [112], [113], [114], [115]. Obesity and acellular dermal matrix use have been implicated as specific risk factors [116]. A number of variables purport to contribute to seroma formation, including the creation of a large, irregular dead space by mastectomy; insertion of a foreign body; movement of the chest wall; lymphatic disruption; and the post-operative inflammatory reaction [117], [118]. Given these pathophysiologic insults—all in the setting of a relatively hypovascular milieu—seroma is not unexpected. Whether seroma serves as a nidus for infection leading to implant loss, or is simply a marker of poor wound healing and occult infection; indeed seroma seems to correlate with higher subsequent complications [119].
10.4. Implant exposure
In cases of immediate reconstruction, tension and mastectomy flap overdissection are associated with skin flap necrosis at the incision site. One of the benefits of complete submuscular placement is that local wound care is all that is necessary provided skin necrosis is minor and the underlying muscle is healthy. When radiation over an expanded implant is poorly titrated, full thickness skin loss remains a possibility. In severe cases, the expander may need to be removed. When the underlying device is a tissue expander, continued expansion to the desired volume or to match a contralateral implant may not be possible as volume must be removed to allow healing subsequent to any necessary debridement. In the event of permanent implant exposure, debridement and immediate wound closure is necessary. If enough skin is lost so that primary closure is not possible, the implant should be removed and additional skin transferred by autologous reconstruction.
10.5. Anaplastic large cell lymphoma
Recently, there have been concerns raised about anaplastic large cell lymphoma (ALCL) associated with breast implants. The reported cases involved various lymphoma subtypes with the majority being ALCL, of which 66 were confirmed as ALK negative. However, there was no association with any particular implant type. The more aggressive cases and reported low death rate appeared to be related to the presence of breast masses at the time of presentation rather than effusion. The remaining reports were occasional case reports of T-cell lymphoma, follicular lymphoma, marginal zone B-cell lymphoma, primary effusion lymphoma, and lymphoplasmacytic lymphoma [120].
11. Conclusion
Breast reconstruction has evolved in time as new surgical techniques developed along with neo-/adjuvant treatments. Breast reconstruction itself can basically be classified into four categories: implant- and expander-based breast reconstruction, flap-based breast reconstruction (using vascularized autologous tissue), a combination of both (flap and implant), and breast reconstruction using fat grafting. Nevertheless, fat grafting is predominantly used to refine post-reconstructive asymmetries. Breast reconstruction can be either performed at the time of surgery in immediate setting or delayed. Finally alloplastic breast reconstruction can be further performed in immediate settings as one- or two-stage approach when there's the need for tissue expansion.
Alloplastic (either as direct-to-implant or implant/tissue expander-based) breast reconstruction recently surpassed autologous one as the most employed reconstructive approach. Indeed advocates of alloplastic reconstruction highlight the main drawback of autologous one as: longer time operative time, longer hospital stay, higher donor site morbidity and further scar. Conversely alloplastic reconstructions, expose patients to less surgical risk, fewer scars, less donor site morbidity, and fewer irreversible consequences. Nevertheless autologous reconstruction can achieve is a one-stage reconstruction strategy that can achieve a more natural cosmetic outcome and is the only one able to reconstruct large breast when patients do not want contralateral matching surgery.
This vary of reconstructive strategy should be all part of the armamentarium of breast surgeons, which should be able to discuss with the patients the best approach for her. Indeed breast reconstruction should be individualized for each patient by taking into consideration not only tumor oncology, neo-/adjuvant treatments, and genetic predisposition, but also the patient's condition and wishes regarding its timing. Thus offering patients an opportunity for breast reconstruction is an important component of breast cancer treatment. Despite the complex decision-making process that includes many different aspects, the overall number of breast reconstructions has recently increased considerably.
Ethical approval
Nothing to declare.
Sources of funding
Nothing to declare.
Author contribution
Marco Gardani: writing the paper.
Nicolo Bertozzi: revised the paper.
Edoardo Raposio, Michele Pio Greco: study concept.
Francesco Simonacci, Mariangela Pesce, Pierluigi Santi: photos.
Conflicts of interest
Nothing to declare.
Guarantor
Marco Gardani.
Consent
Nothing to declare.
Registration of research studies
Nothing to declare.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.DellaCroce F.J., Wolfe E.T. Breast reconstruction. Surg. Clin. North Am. 2013;93:445–454. doi: 10.1016/j.suc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B., Montague E., Redmond C. Findings from NSABP protocol no. B-04-comparison of radical mastectomy with alterative treatments for primary breast cancer. I. Radiation comoplicance and its relation to treatment outcome. Cancer. 1980;46:1–13. doi: 10.1002/1097-0142(19800701)46:1<1::aid-cncr2820460102>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Cano S.J., Klassen A., Pusic A.L. The science behind quality-of-life measurement: a primer for plastic surgeons. Plast. Reconstr. Surg. 2009;123:98e–106e. doi: 10.1097/PRS.0b013e31819565c1. [DOI] [PubMed] [Google Scholar]
- 5.Cano S.J., Klassen A.F., Scott A.M., Cordeiro P.G., Pusic A.L. The BREAST-Q: further validation in independent clinical samples. Plast. Reconstr. Surg. 2012;129:293–302. doi: 10.1097/PRS.0b013e31823aec6b. [DOI] [PubMed] [Google Scholar]
- 6.Cohen W.S., Mundy L.R., Ballard T.N.S. The breast-Q in surgical research: a review of the literature 2009–2015. J. Plast. Reconstr. Surg. 2016;69:149–162. doi: 10.1016/j.bjps.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raposio E., Belgrano V., Santi P., Chiorri C. Which is the ideal breast size? Some social clues for plastic surgeons. Ann. Plast. Surg. 2016;76:340–345. doi: 10.1097/SAP.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 8.Albornoz C.R., Bach P.B., Mehrara B.J. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast. Reconstr. Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 9.Albornoz C.R., Cordeiro P.G., Pusic A.L. Diminishing relative contraindications for immediate breast reconstruction: a multicenter study. J. Am. Coll. Surg. 2014;219:788–795. doi: 10.1016/j.jamcollsurg.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy C.M., Klassen A.F., Cano S.J. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010;116:5584–5591. doi: 10.1002/cncr.25552. [DOI] [PubMed] [Google Scholar]
- 11.Macadam S.A., Ho A.L., Cook E.F. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast. Reconstr. Surg. 2010;125:761–771. doi: 10.1097/PRS.0b013e3181cb5cf8. [DOI] [PubMed] [Google Scholar]
- 12.Antony A.K., McCarthy C.M., Cordeiro P.G. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast. Reconstr. Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- 13.Salzberg C.A., Ashikari A.Y., Koch R.M. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm) Plast. Reconstr. Surg. 2011;127:514–524. doi: 10.1097/PRS.0b013e318200a961. [DOI] [PubMed] [Google Scholar]
- 14.Riddle V. Radical mastectomy; the technique and the complications. Br. J. Surg. 1948;36:113–129. doi: 10.1002/bjs.18003614202. [DOI] [PubMed] [Google Scholar]
- 15.Slavin S.A., Schnitt S.J., Duda R.B. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast. Reconstr. Surg. 1998;102:49–62. doi: 10.1097/00006534-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Yi M., Kronowitz S.J., Meric-Bernstam F. Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer. 2011;117:916–924. doi: 10.1002/cncr.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroll S.S., Khoo A., Singletary S.E. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast. Reconstr. Surg. 1999;104:421–425. doi: 10.1097/00006534-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Carlson G.W. Local recurrence after skin-sparing mastectomy: a manifestation of tumor biology or surgical conservatism? Ann. Surg. Oncol. 1998;5:571–572. doi: 10.1007/BF02303823. [DOI] [PubMed] [Google Scholar]
- 19.Crowe J.P., Patrick R.J., Yetman R.J. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch. Surg. 2008;143:1106–1110. doi: 10.1001/archsurg.143.11.1106. [DOI] [PubMed] [Google Scholar]
- 20.Cronin T.D., Gerow F.J. Augmentation mammaplasty: a new “natural feel” prostheses. Excerpta Medica Int. Congr. Ser. 1963;66:41–46. [Google Scholar]
- 21.Cronin T.D. Subcutaneous mastectomy and gel implants. AORN J. 1969;10:81–85. doi: 10.1016/s0001-2092(08)70677-x. [DOI] [PubMed] [Google Scholar]
- 22.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast. Reconstr. Surg. 1982;69:195–208. doi: 10.1097/00006534-198202000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Fan J., Raposio E., Wang J., Nordström R.E. Development of the inframammary fold and ptosis in breast reconstruction with textured tissue expanders. Aesthet. Plast. Surg. 2002;26:219–222. doi: 10.1007/s00266-002-1477-0. [DOI] [PubMed] [Google Scholar]
- 24.Macadam S.A., Ho A.L., Lennox P.A. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plast. Reconstr. Surg. 2013;131:431–441. doi: 10.1097/PRS.0b013e31827c6d55. [DOI] [PubMed] [Google Scholar]
- 25.Gahm J., Edsander-Nord A., Jurell G. No differences in aesthetic outcome or patient satisfaction between anatomically shaped and round expandable implants in bilateral breast reconstructions: a randomized study. Plast. Reconstr. Surg. 2010;126:1419–1427. doi: 10.1097/PRS.0b013e3181ef8b01. [DOI] [PubMed] [Google Scholar]
- 26.Cordeiro P.G. Breast reconstruction after surgery for breast cancer. N. Engl. J. Med. 2008;359:1590–1601. doi: 10.1056/NEJMct0802899. [DOI] [PubMed] [Google Scholar]
- 27.Sbitany H., Serletti J.M. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast. Reconstr. Surg. 2011;128:1162–1169. doi: 10.1097/PRS.0b013e318230c29e. [DOI] [PubMed] [Google Scholar]
- 28.Spear S.L., Howard M.A., Boehmler J.H., Ducic I., Low M., Abbruzzesse M.R. The infected or exposed breast implant: management and treatment strategies. Plast. Reconstr. Surg. 2004;113:1634–1644. doi: 10.1097/01.prs.0000117194.21748.02. [DOI] [PubMed] [Google Scholar]
- 29.Stump A., Holton L.H., III, Connor J., Harper J.R., Slezak S., Silverman R.P. The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plast. Reconstr. Surg. 2009;124:82–91. doi: 10.1097/PRS.0b013e3181ab112d. [DOI] [PubMed] [Google Scholar]
- 30.Komorowska-Timek E., Oberg K.C., Timek T.A., Gridley D.S., Miles D.A. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast. Reconstr. Surg. 2009;123:807–816. doi: 10.1097/PRS.0b013e318199eef3. [DOI] [PubMed] [Google Scholar]
- 31.Basu C.B., Leong M., Hicks M.J. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast. Reconstr. Surg. 2010;126:1842–1847. doi: 10.1097/PRS.0b013e3181f44674. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy C.M., Lee C.N., Halvorson E.G. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast. Reconstr. Surg. 2012;130:57S–66S. doi: 10.1097/PRS.0b013e31825f05b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiernan T., Martin L. Use of acellular dermal matrix is comparable to expander based breast reconstructions for post operative physiotherapy requirements. Surg. Curr. Res. 2013;3:136–137. [Google Scholar]
- 34.Khoo A., Kroll S.S., Reece G.P. A comparison of resource costs of immediate and delayed breast reconstruction. Plast. Reconstr. Surg. 1998;101:964–968. doi: 10.1097/00006534-199804040-00011. [DOI] [PubMed] [Google Scholar]
- 35.Berna G., Cawthorn S.J., Papaccio G., Balestrieri N. Evaluation of a novel breast reconstruction tech- nique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J. Surg. 2014 doi: 10.1111/ans.12849. [DOI] [PubMed] [Google Scholar]
- 36.Neyt M.J., Blondeel P.N., Morrison C.M. Comparing the cost of delayed and immediate autologous breast reconstruction in Belgium. Br. J. Plast. Surg. 2005;58:493–497. doi: 10.1016/j.bjps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Chevray P.M. Timing of breast reconstruction: immediate versus delayed. Cancer J. 2008;14:223–229. doi: 10.1097/PPO.0b013e3181824e37. [DOI] [PubMed] [Google Scholar]
- 38.Serletti J.M., Fosnot J., Nelson J.A. Breast reconstruction after breast cancer. Plast. Reconstr. Surg. 2011;127:124e–135e. doi: 10.1097/PRS.0b013e318213a2e6. [DOI] [PubMed] [Google Scholar]
- 39.Masoomi H., Clark E.G., Paydar K.Z. Predictive risk factors of free flap thrombosis in breast reconstruction surgery. Microsurgery. 2014;34:589–594. doi: 10.1002/micr.22250. [DOI] [PubMed] [Google Scholar]
- 40.DeBono R., Thompson A., Stevenson J.H. Immediate versus delayed free TRAM breast reconstruction: an analysis of perioperative factors and complications. Br. J. Plast. Surg. 2002;55:111–116. doi: 10.1054/bjps.2002.3747. [DOI] [PubMed] [Google Scholar]
- 41.Sigurdson L., Lalonde D.H. MOC-PSSM CME article: breast reconstruction. Plast. Reconstr. Surg. 2008;121:1–12. doi: 10.1097/01.prs.0000294668.32874.18. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan S.R., Fletcher D.R., Isom C.D. True incidence of all complications following immediate and delayed breast reconstruction. Plast. Reconstr. Surg. 2008;122:19–28. doi: 10.1097/PRS.0b013e3181774267. [DOI] [PubMed] [Google Scholar]
- 43.Selber J.C., Kurichi J.E., Vega S.J. Risk factors and complications in free TRAM flap breast reconstruction. Ann. Plast. Surg. 2006;56:492–497. doi: 10.1097/01.sap.0000210180.72721.4a. [DOI] [PubMed] [Google Scholar]
- 44.Man L.X., Selber J.C., Serletti J.M. Abdominal wall following free TRAM or DIEP flap reconstruction: a metaanalysis and critical review. Plast. Reconstr. Surg. 2009;124:752–764. doi: 10.1097/PRS.0b013e31818b7533. [DOI] [PubMed] [Google Scholar]
- 45.Andrades P., Fix R.J., Danilla S. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann. Plast. Surg. 2008;60:562–567. doi: 10.1097/SAP.0b013e31816fc372. [DOI] [PubMed] [Google Scholar]
- 46.Garvey P.B., Buchel E.W., Pockaj B.A. DIEP and pedicled TRAM flaps: a comparison of outcomes. Plast. Reconstr. Surg. 2006;117:1711–1719. doi: 10.1097/01.prs.0000210679.77449.7d. [DOI] [PubMed] [Google Scholar]
- 47.Moran S.L., Serletti J.M. Outcome comparison between free and pedicled TRAM flap breast reconstruction in the obese patient. Plast. Reconstr. Surg. 2001;108:1954–1960. doi: 10.1097/00006534-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Kanchwala S.K., Bucky L.P. Optimizing pedicled transverse rectus abdominis muscle flap breast reconstruction. Cancer J. 2008;14:236–240. doi: 10.1097/PPO.0b013e318180bce5. [DOI] [PubMed] [Google Scholar]
- 49.Blondeel N., Vanderstraeten G.G., Monstrey S.J. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br. J. Plast. Surg. 1997;50:322–330. doi: 10.1016/s0007-1226(97)90540-3. [DOI] [PubMed] [Google Scholar]
- 50.Chen C.M., Halvorson E.G., Disa J.J. Immediate postoperative complications in DIEP versus free/muscle-sparing TRAM flaps. Plast. Reconstr. Surg. 2007;120:1477–1482. doi: 10.1097/01.prs.0000288014.76151.f7. [DOI] [PubMed] [Google Scholar]
- 51.Man L.X., Selber J.C., Serletti J.M. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast. Reconstr. Surg. 2009;124:752–764. doi: 10.1097/PRS.0b013e31818b7533. [DOI] [PubMed] [Google Scholar]
- 52.Raposio E., Cicchetti S., Adami M., Ciliberti R.G., Santi P.L. Computer planning for breast reconstruction by tissue expansion: an update. Plast. Reconstr. Surg. 2004;113:2095–2097. doi: 10.1097/01.prs.0000121189.51406.12. [DOI] [PubMed] [Google Scholar]
- 53.Raposio E., Caregnato P., Barabino P., Gualdi A., Orefice A., Spagnolo A., Capello C., Santi P.L. Computer-based preoperative planning for breast reconstruction in the woman with unilateral breast hypoplasia. Minerva Chir. 2002;57:711–714. [PubMed] [Google Scholar]
- 54.Porro I., Schenone A., Fato M., Raposio E., Molinari E., Beltrame F. An integrated environment for plastic surgery support: building virtual patients, simulating interventions, and supporting intraoperative decisions. Comput. Med. Imaging Graph. 2005;29:385–394. doi: 10.1016/j.compmedimag.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Mohan A.T., Cyr M.S. Advances in imaging technologies for planning breast reconstruction. Gland. Surg. 2016;5:242–254. doi: 10.3978/j.issn.2227-684X.2016.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groen J.W., Negenborn V.L., Twisk D.J., Rizopoulos D., Ket J.C., Smit J.M., Mullender M.G. Autologous fat grafting in onco-plastic breast reconstruction: a systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J. Plast. Reconstr. Aesthet. Surg. 2016;69:742–764. doi: 10.1016/j.bjps.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 57.De Blacam C., Momoh A.O., Colakoglu S. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast. Reconstr. Surg. 2011;128:411e–418e. doi: 10.1097/PRS.0b013e31822b669f. [DOI] [PubMed] [Google Scholar]
- 58.Cigna E., Ribuffo D., Sorvillo V. Secondary lipofilling after breast reconstruction with implants. Eur. Rev. Med. Pharmacol. Sci. 2012;16:1729–1734. [PubMed] [Google Scholar]
- 59.Largo R.D., Tchang L.A., Mele V. Efficacy, safety and complications of autologous fat grafting to healthy breast tissue: a systematic review. J. Plast. Reconstr. Aesthet. Surg. 2014;67:437–448. doi: 10.1016/j.bjps.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Simonacci F., Bertozzi N., Grieco M.P., Grignaffini E., Raposio E. Autologous fat transplantation for breast reconstruction: a literature review. Ann. Med. Surg. (Lond) 2016;12:94–100. doi: 10.1016/j.amsu.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rigotti G., Marchi A., Galiè M. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose- derived adult stem cells. Plast. Reconstr. Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 62.Caruana G., Bertozzi N., Boschi E., Pio Grieco M., Grignaffini E., Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Ann. Ital. Chir. 2015;86:1–4. [PubMed] [Google Scholar]
- 63.Raposio E., Bertozzi N., Bonomini S., Bernuzzi G., Formentini A., Grignaffini E., Pio Grieco M. Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy. Wounds. 2016;28:126–131. [PubMed] [Google Scholar]
- 64.Simonacci F., Bertozzi N., Grieco M.P., Grignaffini E., Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017;20:49–60. doi: 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raposio E., Calderazzi F. Fat grafting for chronic heel pain following surgery for adult flatfoot deformity: pilot study. Foot (Edinb) 2017;31:56–60. doi: 10.1016/j.foot.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Scanarotti C., Bassi A.M., Catalano M., Guida C., Coradeghini R., Falugi C., Aluigi M., Santi P., Raposio E. Neurogenic-committed human pre-adipocytes express CYP1A isoforms. Chem. Biol. Interact. 2010;184:474–483. doi: 10.1016/j.cbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Raposio E., Guida C., Baldelli I., Benvenuto F., Curto M., Paleari L., Filippi F., Fiocca R., Robello G., Santi P.L. Characterization and induction of human pre-adipocytes. Toxicol In Vitro. 2007;21:330–334. doi: 10.1016/j.tiv.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 68.Coradeghini R., Guida C., Scanarotti C., Sanguineti R., Bassi A.M., Parodi A., Santi P.L., Raposio E. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010;191:466–477. doi: 10.1159/000273266. [DOI] [PubMed] [Google Scholar]
- 69.Aluigi M.G., Coradeghini R., Guida C., Scanarotti C., Bassi A.M., Falugi C., Santi P., Raposio E. Pre-adipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic molecules. Cell Biol. Int. 2009;33:594–601. doi: 10.1016/j.cellbi.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Raposio E., Guida C., Coradeghini R., Scanarotti C., Parodi A., Baldelli I., Fiocca R., Santi P.L. In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008;41:739–754. doi: 10.1111/j.1365-2184.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertozzi N., Simonacci F., Grieco M.P., Grignaffini E., Raposio E. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann. Med. Surg. 2017;20:41–48. doi: 10.1016/j.amsu.2017.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raposio E., Bertozzi N. Isolation of ready-to-use Adipose-derived Stem Cell (ASC) pellet for clinical applications and a comparative overview of alternate methods for ASC isolation. Curr. Protoc. Stem Cell Biol. 2017;16 doi: 10.1002/cpsc.29. 41:1F.17.1-1F.17. [DOI] [PubMed] [Google Scholar]
- 73.Raposio E., Bonomini S., Calderazzi F. Isolation of autologous adipose tissue-derived mesenchymal stem cells for bone repair. Orthop. Traumatol. Surg. Res. 2016;102:909–912. doi: 10.1016/j.otsr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Raposio E., Caruana G., Bonomini S., Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2014;133:1406–1409. doi: 10.1097/PRS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 75.Raposio E., Caruana G., Petrella M., Bonomini S., Grieco M.P. A standardized method of isolating adipose-derived stem cells for clinical applications. Ann. Plast. Surg. 2016;76:124–126. doi: 10.1097/SAP.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 76.Fisher C., Grahovac T.L., Schafer M.E. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast. Recconstr Surg. 2013;132:351–361. doi: 10.1097/PRS.0b013e3182958796. [DOI] [PubMed] [Google Scholar]
- 77.Yoshimura K., Sato K., Aoi N., Kurita M., Hirohi T., Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthet. Plast. Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coleman S.R. Structural fat grafting: more than a permanent filler. Plast. Reconstr. Surg. 2006;118:108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 79.Coleman S.R. Facial augmentation with structural fat grafting. Clin. Plast. Surg. 2006;33:567–577. doi: 10.1016/j.cps.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Klein J.A. Tumescent technique for local anesthesia improves safety in large- volume liposuction. Plast. Reconstr. Surg. 1993;92:1085–1098. [PubMed] [Google Scholar]
- 81.Rosson G.D., Shridharani S.M., Magarakis M. Quality of life before reconstructive breast surgery: a preoperative compar- ison of patients with immediate, delayed, and major revision reconstruction. Microsurgery. 2013;33:253–258. doi: 10.1002/micr.22081. [DOI] [PubMed] [Google Scholar]
- 82.Pinsolle V., Grinfeder C., Mathoulin-Pelissier S. Complications analysis of 266 immediate breast reconstructions. J. Plast. Reconstr. Aesthet. Surg. 2006;59:1017–1024. doi: 10.1016/j.bjps.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 83.Roostaeian J., Sanchez I., Vardanian A. Comparison of immediate implant placement versus the staged tissue expander technique in breast reconstruction. Plast. Reconstr. Surg. 2012;129:909e–918e. doi: 10.1097/PRS.0b013e31824ec411. [DOI] [PubMed] [Google Scholar]
- 84.Malata C.M., McIntosh S.A., Purushotham A.D. Immediate breast reconstruction after mastectomy for cancer. Br. J. Surg. 2000;87:1455–1472. doi: 10.1046/j.1365-2168.2000.01593.x. [DOI] [PubMed] [Google Scholar]
- 85.Mesbahi A.N., McCarthy C.M., Disa J.J. Breast reconstruction with prosthetic implants. Cancer J. 2008;14:230–235. doi: 10.1097/PPO.0b013e31817fb7c3. [DOI] [PubMed] [Google Scholar]
- 86.Spear S.L., Mesbahi A.N. Implant-based reconstruction. Clin. Plast. Surg. 2007;34:63–73. doi: 10.1016/j.cps.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Disa J.J., McCarthy C.M., Mehrara B.J. Immediate latissimus dorsi/prosthetic breast reconstruction following salvage mastectomy after failed lumpectomy/irradiation. Plast. Reconstr. Surg. 2008;121:159e–164e. doi: 10.1097/01.prs.0000304235.75016.02. [DOI] [PubMed] [Google Scholar]
- 88.Chang D.W., Barnea Y., Robb G.L. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast. Reconstr. Surg. 2008;122:356–362. doi: 10.1097/PRS.0b013e31817d6303. [DOI] [PubMed] [Google Scholar]
- 89.Frasier L.L., Holden S., Holden T. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncol. 2015:1–7. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cordeiro P.G., Albornoz C.R., McCormick B. What is the optimum timing of postmastectomy radiotherapy in two-stage prosthetic reconstruction: radiation to the tissue expander or permanent implant? Plast. Reconstr. Surg. 2015;135:1509–1517. doi: 10.1097/PRS.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mehta V.K., Goffinet D. Postmastectomy radiation therapy after TRAM flap breast reconstruction. Breast J. 2004;10:118–122. doi: 10.1111/j.1075-122x.2004.21286.x. [DOI] [PubMed] [Google Scholar]
- 92.Ryan J.L. Ionizing radiation: the good, the bad, and the ugly. J. Investig. Dermatol. 2012;132:985–993. doi: 10.1038/jid.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salvo N., Barnes E., Van draanen J. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr. Oncol. 2010;17:94–112. doi: 10.3747/co.v17i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coles C.E., Moody A.M., Wilson C.B. Reduction of radiotherapy-induced late complications in early breast cancer: the role of intensity-modulated radiation therapy and partial breast irradiation. Part I—normal tissue complications. Clin. Oncol. R. Coll. Radiol. 2005;17:16–24. doi: 10.1016/j.clon.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Fodor J., Gulyás G., Polgár C. Radiotherapy and delayed breast reconstruction with implant: ex-amination of compatibility. Magy. Onkol. 2002;46:323–326. [PubMed] [Google Scholar]
- 96.Cordeiro P.G., Pusic A.L., Disa J.J. Irradiation after immediate tissue expander/implant breast re-construction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast. Reconstr. Surg. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 97.Rosato R.M., Dowden R.V. Radiation therapy as a cause of capsular contracture. Ann. Plast. Surg. 1994;32:342–345. doi: 10.1097/00000637-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 98.Krueger E.A., Wilkins E.G., Strawderman M. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001;49:713–721. doi: 10.1016/s0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- 99.Cowen D., Gross E., Rouannet P. Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast CancerRes Treat. 2010;121:627–634. doi: 10.1007/s10549-010-0791-5. [DOI] [PubMed] [Google Scholar]
- 100.Spear S.L., Baker J.L., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast. Reconstr. Surg. 1995;96:1119–1124. [PubMed] [Google Scholar]
- 101.Avashia Y.J., Mohan R., Berhane C., Oeltjen J.C. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast. Reconstr. Surg. 2013;131:453–461. doi: 10.1097/PRS.0b013e31827c6d90. [DOI] [PubMed] [Google Scholar]
- 102.Phillips B.T., Bishawi M., Dagum A.B., Khan S.U., Bui D.T. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast. Reconstr. Surg. 2013;131:1–13. doi: 10.1097/PRS.0b013e3182729c39. [DOI] [PubMed] [Google Scholar]
- 103.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 104.Goh S.C., Thorne T.H., Williams G., Laws S.A., Rainsbury R.M. Breast reconstruction using permanent Becker expander implants: an 18 year experience. Breast. 2012;21:764–768. doi: 10.1016/j.breast.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 105.McGrath M.H., Burkhardt B.R. The safety and efficacy of breast implants for augmentation mammaplasty. Plast. Reconstr. Surg. 1984;74:550–560. doi: 10.1097/00006534-198410000-00019. [DOI] [PubMed] [Google Scholar]
- 106.Phillips B.T., Fourman M.S., Dagum A.B. Results of a prospective randomized clinical trial assessing postoperative antibiotic use in immediate breast reconstruction. J. Am. Coll. Surg. 2013;217 doi: 10.1016/j.jamcollsurg.2016.02.018. S88–S88. [DOI] [PubMed] [Google Scholar]
- 107.Pittet B., Montandon D., Pittet D. Infection in breast implants. Lancet Infect. Dis. 2005;5:94–106. doi: 10.1016/S1473-3099(05)01281-8. [DOI] [PubMed] [Google Scholar]
- 108.Kim J.Y., Davila A.A., Persing S. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast. Reconstr. Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 109.Ho G., Nguyen T.J., Shahabi A., Hwang B.H., Chan L.S., Wong A.K. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann. Plast. Surg. 2012;68:346–356. doi: 10.1097/SAP.0b013e31823f3cd9. [DOI] [PubMed] [Google Scholar]
- 110.Sbitany H., Serletti J.M. A cellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast. Reconstr. Surg. 2011;128:1162–1169. doi: 10.1097/PRS.0b013e318230c29e. [DOI] [PubMed] [Google Scholar]
- 111.Chun Y.S., Verma K., Rosen H. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast. Reconstr. Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 112.Yuen J.C., Yue C.J., Erickson S.W. Comparison between freeze-dried and ready-to-use AlloDerm in alloplastic breast reconstruction. Plast. Reconstr. Surg. Glob. Open. 2014;2:e119. doi: 10.1097/GOX.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cordeiro P.G., McCarthy C.M. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: Part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast. Reconstr. Surg. 2006;118:832–839. doi: 10.1097/01.prs.0000232397.14818.0e. [DOI] [PubMed] [Google Scholar]
- 114.Newman M.I., Swartz K.A., Samson M.C., Mahoney C.B., Diab K. The true incidence of near-term postoperative complications in prosthetic breast reconstruction utilizing human acellular dermal matrices: a meta-analysis. Aesthet. Plast. Surg. 2011;35:100–106. doi: 10.1007/s00266-010-9631-6. [DOI] [PubMed] [Google Scholar]
- 115.Kuroi K., Shimozuma K., Taguchi T. Pathophysiology of seroma in breast cancer. Breast Cancer. 2005;12:288–293. doi: 10.2325/jbcs.12.288. [DOI] [PubMed] [Google Scholar]
- 116.Woodworth P.A., McBoyle M.F., Helmer S.D., Beamer R.L. Seroma formation after breast cancer surgery: incidence and predicting factors. Am. Surg. 2000;66:444–450. [PubMed] [Google Scholar]
- 117.Srivastava V., Basu S., Shukla V.K. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J. Breast Cancer. 2012;15:373–380. doi: 10.4048/jbc.2012.15.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yilmaz K.B., Dogan L., Nalbant H. Comparing scalpel, electrocautery and ultrasonic dissector effects: the impact on wound complications and pro-inflammatory cytokine levels in wound fluid from mastectomy patients. J. Breast Cancer. 2011;14:58–63. doi: 10.4048/jbc.2011.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adams W.P., Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin. Plast. Surg. 2009;36:119–126. doi: 10.1016/j.cps.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 120.Rupani A., Frame J.D., Kamel D. Lymphomas associated with breast implants: a review of the literature. Aesthet. Surg. J. 2015;35:533–544. doi: 10.1093/asj/sjv016. [DOI] [PubMed] [Google Scholar]