Abstract
BACKGROUND: Since invasive bladder cancer (BC) is one of the most lethal urological malignant tumors worldwide, understanding the molecular mechanisms that trigger the migration, invasion, and metastasis of BC has great significance in reducing the mortality of this disease. Although RelA/p65, a member of the NF-kappa B transcription factor family, has been reported to be upregulated in human BCs, its regulation of BC motility and mechanisms have not been explored yet. METHODS: NF-κBp65 expression was evaluated in N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN)–induced high invasive BCs by immunohistochemistry staining and in human BC cell lines demonstrated by Western Blot. The effects of NF-κBp65 knockdown on BC cell migration and invasion, as well as its regulated RhoGDIα and FBW7, were also evaluated in T24T cells by using loss- and gain-function approaches. Moreover, the interaction of FBW7 with RhoGDIα was determined with immunoprecipitation assay, while critical role of ubiquitination of RhoGDIα by FBW7 was also demonstrated in the studies. RESULTS: p65 protein was remarkably upregulated in the BBN-induced high invasive BCs and in human BC cell lines. We also observed that p65 overexpression promoted BC cell migration by inhibiting RhoGDIα expression. The regulatory effect of p65 on RhoGDIα expression is mediated by its upregulation of FBW7, which specifically interacted with RhoGDIα and promoted RhoGDIα ubiquitination and degradation. Mechanistic studies revealed that p65 stabilizing the E3 ligase FBW7 protein was mediated by its attenuating pten mRNA transcription. CONCLUSIONS: We demonstrate that p65 overexpression inhibits pten mRNA transcription, which stabilizes the protein expression of ubiquitin E3 ligase FBW7, in turn increasing the ubiquitination and degradation of RhoGDIα protein and finally promoting human BC migration. The novel identification of p65/PTEN/FBW7/RhoGDIα axis provides a significant insight into understanding the nature of BC migration, further offering a new theoretical support for cancer therapy.
Abbreviations: BC, bladder cancer; BBN, N-butyl-N-(4-hydroxybutyl)-nitrosamine; CHX, cycloheximide; RT-PCR, reverse transcription-polymerase chain reaction; NF-κB, transcription factors of the nuclear factor kappa B; RhoGDI, RHO guanosine diphosphate dissociation inhibitors; FBW7, F-box and WD repeat domain-containing 7; PTEN, phosphatase and tensin homolog; GFP, green fluorescent protein; MEF, murine embryonic fibroblasts
Background
The nuclear factor kappa B (NF-κB) family consists of five proteins—p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1), and p100/52 (NF-κB2)—that form homo- and heterodimeric complexes by associating with each other to transcriptionally regulate target genes [1].Transactivation of the NF-κB can be initiated by a vast array of stimuli relating to many biological processes such as inflammation, immunity, differentiation, cell growth, tumorigenesis, and apoptosis [2], [3], [4]. The NF-κB family may play the role of a double-edged sword in cancer development due to the emergence of the tumor suppressor role of some NF-κB subunits in cancer [5], [6], [7]. Examination of The Cancer Genome Atlas database shows that the loss of NF-κB expression is associated with bladder cancer (BC) development [8]. Increased nuclear expression of p65/RelA, seen in BC patients, can negatively affect survival of patients with the muscular invasive disease [7]. This suggests that p65 may have an oncogenic role in progression and metastasis of human BC. In current studies, we found that p65 protein expression was remarkably increased in N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN)–induced high invasive BC tissues and human high-grade invasive BC cell lines.
The RHO guanosine diphosphate dissociation inhibitor (RhoGDI) is a cellular regulatory protein that acts primarily by controlling the cellular distribution and activity of RhoGTPases [9], [10]. The alternated expression of RhoGDIs has been reported in a variety of cancers, which could mediate several processes during tumorigenesis and cancer progression [11], [12], [13]. RhoGDIα is known to function as a GDI for Rac and often controls all aspects of cellular motility, migration, and invasion, along with cellular polarity [14]. Our most recent studies demonstrate that RhoGDIα SUMOylation at lys-138 is crucial for its inhibition of colon cancer cell migration and invasion [15]. In the current study, we showed that RhoGDIα is p65 downstream mediator responsible for its promotion of BC migration.
F-box and WD repeat domain-containing 7 (FBW7) is a member of the F-box protein family, which constitutes one subunit of the Skp1, Cul1, and F-box protein (SCF) ubiquitin ligase complex [16]. The role of FBW7 is to target the degradation of critical cellular regulators, thereby controlling essential cellular processes including cell cycle, DNA damage response, cell differentiation, apoptosis, and tumorigenesis [17], [18]. Deletion and mutation of FBW7 have been frequently noticed in various human malignancies, such as gastric cancer [19], colon cancer [20], breast carcinoma [21], esophageal squamous cell carcinoma [22], and pancreatic cancer [23]. Since reduced FBW7 expression levels and loss-of-function mutations are found in a wide range of human cancers, FBW7 is generally considered as a tumor suppressor [24]. However, the function and regulation of FBW7 are poorly studied in the occurrence and progression of BC. Herein, we demonstrated that FBW7 directly interacts with RhoGDIα, which leads to the ubiquitination and protein degradation of RhoGDIα, thus facilitating the migration abilities of BC cells. These findings may lead to the change of the concept of nature of FBW7 in BCs.
In the current study, we identified a novel molecular mechanism responsible for the oncogenic role of p65, which promoted BC cell migration via FBW7 E3 ligase-dependent ubiquitin degradation of RhoGDIα protein. Subsequently, we also demonstrated that p65 overexpression decreased pten mRNA transcription, thereby stabilizing FBW7 protein. Taken together, our studies provided an important insight into understanding the nature of BC migration and revealed a significant potential for the development of p65-based specific therapeutic strategy for the treatment of human BC patients.
Methods
Reagents, Plasmids, and Antibodies
BBN was purchased from TCI AMERICAN (Cambridge, MA). Proteasome inhibitor MG132 and protein synthesis inhibitor cycloheximide (CHX) were purchased from Calbiochem (Billerica, MA). The dual luciferase assay kit was brought from Promega (Madison, WI). TRIzol reagent and the SuperScript First-Strand Synthesis system were acquired from Invitrogen (Grand Island, NY). PolyJet DNA In Vitro Transfection Reagent was purchased from SignaGen Laboratories (Rockville, MD). The constructs of short hairpin RNA specifically targeting p65 (shp65), RhoGDIα (shRhoGDIα), and their nonsense controls were purchased from Open Biosystems (Thermo Fisher Scientific, Pittsburgh, PA). Lentivirus and retrovirus plasmids specifically targeting mouse FBW7 (shFBW7) were kindly provided by Dr. Iannis Aifantis (New York University School of Medicine, New York, NY) [25]. The pEGFP-C3/RhoGDIα vector expressing green fluorescent protein (GFP)–tagged RhoGDIα was kindly provided by Dr. Mark R. Philips (New York University School of Medicine, New York, NY) and used in our published studies [26]. Plasmids encoding enhanced GFP-PTEN or His-FBW7, and PTEN promoter-driven luciferase reporter were described in our previous studies [4], [27], [28]. The antibodies specific against p65, Rac1, RhoA, RhoGDIα, GFP, PTEN, SKP1, SKP2, AKT, p-AKT(Thr308), p-AKT(Ser473), His, HA, and GAPDH were purchased from Cell Signaling Technology (Danvers, MA). The antibody for FBW7 was purchased from Aviva Systems Biology Corporation (San Diego, CA). Antibodies against β-Actin were bought from Sigma (St. Louis, MO).
Cell Lines, Cell Culture, and Transfection
The p65−/− murine embryonic fibroblasts (MEFs) and their corresponding wild-type (WT) MEFs were cultured as described in our previous studies [29]. The stable cell lines of p65−/−(p65) were established and described in our previous publications [4]. Human normal bladder epithelial cell line UROtsa was a gift from Dr. Scott Garrett (Department of Pathology School of Medicine and Health Sciences, University of North Dakota, Grand Forks, ND) and used in our published studies [30]. These cells were maintained at 37°C in a 5% CO2 incubator with RPMI medium 1640 supplemented with 10% FBS, 2 μM L-glutamine, and 25 μg/ml gentamycin. UMUC3 cells were used in our previous studies [31], [32]. The monolayer growth of human BC T24 cells andT24T cells was kindly provided by Dr. Dan Theodorescu (University of Colorado Comprehensive Cancer Center, Denver, CO) [33] and were used in our previous studies [34]. These cells were maintained in DMEM-F12 (1:1) (Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated FBS, 2 μM L-glutamine, and 25 μg/ml gentamycin. All cell lines were authenticated on the basis of viability, recovery, growth, morphology, and chemical response, as well as by testing STR loci and gender using the PowerPlex 16 HS System provided by Genetica DNA Laboratories (Burlington, NC).
Cell transfections were performed with PolyJet DNA In Vitro Transfection Reagent (SignaGen Laboratories, Rockville, MD) according to the manufacturer's instructions. For stable transfection, cell cultures were subjected to hygromycin B (200-400 μg/ml), G418 (500-1000 μg/ml), or puromycin (0.2-0.3 μg/ml), and cells surviving from the antibiotics selection were pooled as stable mass transfectants as described in our previous studies [35], [36].
Western Blot Analysis
Whole cell extracts were prepared with the cell lysis buffer (10 mM Tris-HCl, pH 7.4, 1% sodium dodecyl sulfate, and 1 mM Na3VO4) as described in our previous studies [34], [36]. Thirty micrograms of proteins was resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a membrane, probed with the indicated primary antibodies, and incubated with the AP-conjugated secondary antibody. Signals were detected by the enhanced chemifluorescence Western blotting system. The images were acquired by scanning with the phosphor imager (Typhoon FLA 7000 imager; Pittsburgh, PA) as described in a previous report [37], [38].
Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted by using the TRIzol reagent (Invitrogen, Grand Island, NY) as described in the manufacturer's instructions. Total RNA (5 mg) was used for first-strand cDNA synthesis with oligo (dT) 20 primer by SuperScript III First-Strand Synthesis system (Invitrogen). Specific primers (Invitrogen, Grand Island, NY) were used for PCR amplification. The primers used in this study were as followw: human RhoGDIα (forward: 5′-GAG CCT GCG AAA GTA CAA GG-3′ reverse: 5′-TCC TTC CAG CAC AAA CGA CTG-3′); human and mouse FBW7 (forward: 5′-ACT GAG CTG CAT TTG CCT TT-3′ reverse: 5′-CCT CAG AAC CAT GGT CCA AC-3′), PTEN (forward: 5′-ACA CCG CCA AAT TTA ACT GC -3′ reverse: 5′-TAC ACC AGT CCG TCC CTT TC-3′), mouse β-actin (forward: 5′-ATA TCG CTG CGC TGG TCG-3′ reverse: 5′-AGG ATG GCG TGA GGG AGA-3′), and human β-actin (forward: 5′-CCT GTG GCA TCC ATG AAA CT -3′ reverse: 5′-GTG CTA GGA GCC AGA GCA GT-3′).
Luciferase Reporter Assay
For the determination of PTEN promoter transcription activity, p65+/+, p65−/−, and p65−/−(p65) cells were transiently co-transfected with the PTEN promoter-driven luciferase reporter together with pRL-TK. Twenty-four hours after transfection, luciferase activity was determined using the luciferase Assay System kit (Promega, Madison, WI). The results were normalized by internal TK signal. All experiments were done in triplicates and the results expressed as mean ± S.D. (standard error).
Cell Migration and Invasion Assay
The migration chambers (353097) and invasion chambers (354480) were purchased from Corning Incorporated (Corning, NY). The migration and invasion chambers were placed into wells containing 500 μl of the indicated cell culture medium. Cells suspended in 400 μl of 0.1% FBS medium were seeded onto the chambers in triplicate according to the manufacturer's instruction. After incubation for 24 hours, the cells on both the inside and outside of the chamber were fixed with 3.7% formalin for 2 minutes, washed with PBS twice, then transferred to 100% methanol for 20 minutes, and washed with PBS twice again, and then finally the cells were stained by Giemsa (1:20 diluted with PBS) at room temperature for 15 minutes in the dark. They were washed again with ddH2O twice; then the noninvaded or nonmigrated cells were scraped off with a cotton swab (PBS wetted) four times. The images were taken under a microscope, Olympus DP71 (Olympus America Inc., Center Valley, PA), and the number of the cells in each image was counted by the software “Image J.” The migration rate was normalized with the vector control cells based on the counted migrated cell number, while the invasion rate was calculated by divided with the migrated cell number and then normalized with the vector control cells according to the manufacturer's instruction. The data shown are representative of three independent experiments.
Immunoprecipitation
Cells were lysed in cell lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM Na3VO4, 0.5% NP-40, and complete protein cocktail inhibitors from Roche) on ice. Lysate (0.5 mg) was precleared by incubation with Protein A/G plus-agarose (Santa Cruz Biotechnology, Inc., Dallas, TX) and then incubated with specific antibody at 4°C for −12 hours. Protein A/G plus-agarose (40 μl) was added to the mixture and incubated with agitation for an additional 4 hours at 4°C. The immunoprecipitates were washed three times with cell lysis buffer and then subjected to Western blot.
BBN-Induced High Invasive BC in Mice
All procedures involving animals were conducted in compliance with guidelines for ethical animal research and approved by the New York University School of Medicine Institutional Animal Care and Use Committee (IACUC); the IACUC approval protocol number is 130212-03. The C57BL/6J mice at age of 3 to 4 weeks were randomly divided into 2 groups, including vehicle negative control group and BBN-treated group. In the BBN-treated group, each mouse was supplied ad libitum with tap water containing 0.05% BBN (TCI America, Portland, OR) in opaque bottles for 23 weeks and thereafter with tap water without BBN. The drinking water was prepared freshly twice a week, and consumption was recorded to estimate BBN intake. Negative control mice received regular tap water. The mice from the BBN-treated group and vehicle negative control group were then sacrificed at week 24 upon BBN exposure. The bladder tissues were removed for pathological analysis and evaluation of the expression of p65 with immunohistochemistry (IHC) staining.
Immunohistochemistry Paraffin (IHC-P)
The antibodies specific against p65 (Cell Signaling Technology, Danvers, MA) were used for IHC staining as described in our previous publications [39]. The resulting immunostaining images were captured using the AxioVision Rel.4.6 with computerized image analysis system (Carl Zeiss, Oberkochen, Germany). Protein expression levels were analyzed by calculating the integrated optical density per stained area (IOD/area) using Image-Pro Plus version 6.0 (Media Cybernetics, MD).
Statistical Analysis
The Student's t test was utilized to determine significant differences. The differences were considered to be significant at a P ≤ .05.
Results
p65 Was Overexpressed in BBN-Induced Mouse High Invasive BCs and Human BC Cell Lines, with Promotion of BC Migration
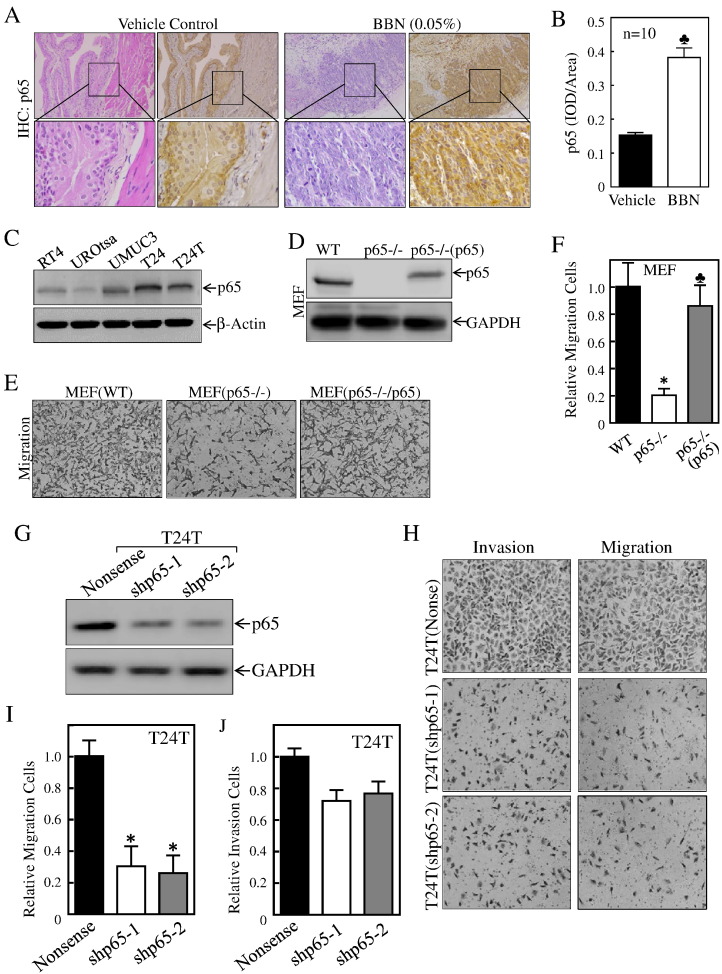
Expression of p65 had been reported previously in human BC tissues [7], [40], [41]. To elucidate the mechanism and role of this overexpression in BC development, we examined p65 abundance in both mouse bladder carcinogenic model and human BC cell lines. The results from the IHC staining revealed that p65 was remarkably overexpressed in vivo in mouse invasive BCs due to BBN exposure (n = 10) (Figure 1, A and B). Consistently, p65 expression in high-grade invasive BC cell lines UMUC3, T24, and T24T was also higher than that in immortalized normal epithelial UROtsa cells (Figure 1C). To determine whether p65 overexpression is critical for human cell migration, p65+/+, p65−/−, and p65−/−(p65) immortalized MEF cells were employed. As shown in Figure 1, D-F, p65−/− cell migration was attenuated compared to either p65+/+ cells or p65−/−(p65) cells. To test this observation in human BC cells, shp65 was stably transfected into T24T cells, and the stable transfectants were identified as shown in Figure 1G. Consistent with the result observed in MEF cell, the knockdown of p65 led to a significant inhibition of the migration with slight inhibition of invasion of T24T cells(Figure 1, H-J). These results strongly reveal that p65 overexpression plays a crucial role in the promotion of BC cell migration.
Figure 1.
p65 was overexpressed in BBN-induced high invasive mouse BCs and human BC cell lines and promoted BC cell migration.
(A) HE staining and IHC staining were performed to evaluate morphology and p65 expression in BBN-induced mouse invasive BCs; the IHC images were captured using the AxioVision Rel.4.6 computerized image system as described in “Materials and Methods”. (B) The p65 protein expression levels were analyzed by calculating the integrated IOD/area using Image-Pro Plus version 6.0. The Student's t test was utilized to determine the P value; and the symbol (♣) indicates a significant increase from vehicle-treated mice (*P < .05) (n = 10). (C & D) Whole cell extracts from the indicated cells were subjected to Western blot for determination of p65 protein expression. (E & F) The migration abilities of MEF(WT), MEF(p65−/−), and MEF[p65−/−(p65)] were determined by using BD BioCoat Matrigel Migration Chamber without the Matrigel. The bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value. The asterisk (*) indicates a significant decrease as compared with MEF(WT) cells (*P < .05), and the symbol (♣) indicates a significant increase as compared with MEF(p65−/−) cells (♣P < .05). (G) p65 knockdown constructs were stably transfected into T24T cells. The knockdown efficiency of p65 protein was assessed by Western blotting. (H-J) The migration and invasion abilities of T24T(shp65) transfectants were evaluated in comparison to their scramble vector transfectant T24T(nonsense). (I) The bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value; the asterisk (*) indicates a significant decrease in comparison to scramble vector transfectants (*P < .05). (J) The invasion rate was normalized with the insert control according to the manufacturer's instruction. The bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, P > .05.
RhoGDIα Was a p65 Downstream Effector Mediating p65 Promotion of BC Cell Migration
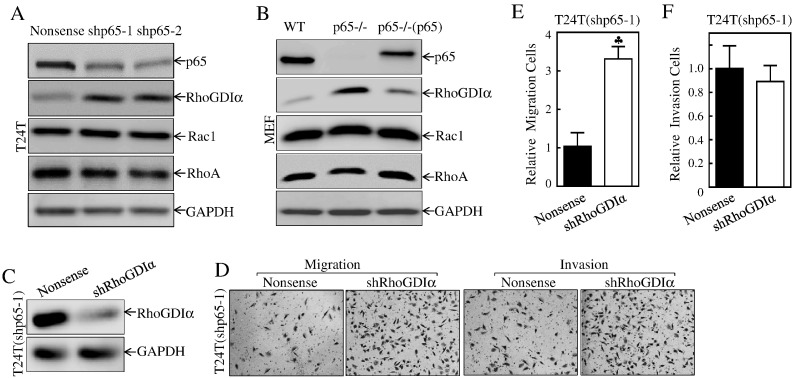
The RhoGDIα is a member of RhoGDI family and plays an essential role in modulation of small GTPase activity via regulating GDP/GTP exchange [42], thereby negatively regulating actin polymerization and cell migration [13], [43]. To elucidate the mechanism underlying the p65 regulation of BC cell migration, the expression levels of RhoGDIαand its regulated small GTPase Rac and RhoA were determined in p65 knockdown transfectants in comparison to their scramble nonsense transfectants. As shown in Figure 2A, impaired expression of p65 by either shRNA or gene deletion resulted in a dramatic increase in RhoGDIα protein in both MEF and T24T cells, while there was no consistent impact on Rac expression and a slight effect on RhoA level in both cells under the same experimental conditions (Figure 2, A and B). Consistently, restoration of p65 expression in p65-deficient cells impaired RhoGDIα protein abundance in p65−/− MEF cells (Figure 2B). Thus, we then stably transfected the shRNA targeting RhoGDIα into the T24T(shp65-1) transfectant (Figure 2C), and the effects of RhoGDIα in T24T cell migration and invasion were assessed. As shown in Figure 2, D-F, knockdown of RhoGDIα reversed the deficiency of migration with marginal effect on cell invasion in T24T(shp65-1) cells. Our results strongly demonstrate that RhoGDIα is a p65 downstream effector responsible for p65 specific promotion of cancer cell migration in BC cells.
Figure 2.
RhoGDIα mediated p65 promoting BC migration.
(A & B) The indicated cell extracts were subjected to Western blot to determine protein expression of p65, RhoGDIα, RhoA, and Rac1. GAPDH was used as a loading control. (C) RhoGDIα knockdown constructs were stably transfected into T24T(shp65-1) cells. The stable transfectants were identified by Western blotting. (D) The migration and invasion abilities of T24T(shp65/shRhoGDIα) and their nonsense control T24T(shp65/nonsense) were determined as described in “Materials and Methods” section. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, and the symbol (♣) indicates a significant increase as compared with nonsense control cells (P < .05) (E). (F) The invasion rate was normalized with the insert control according to the manufacturer's instruction. The bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, P > .05.
p65 Regulated RhoGDIα Protein Degradation
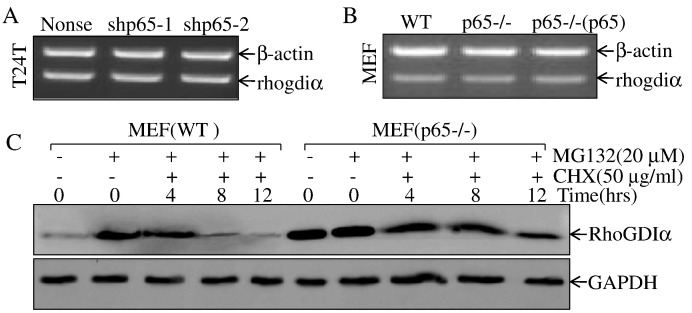
To understand the mechanisms underlying p65 downregulation of RhoGDIα expression, we first examined the potential regulation at mRNA levels. The results showed that there was no observable difference at RhoGDIα mRNA levels betweenT24T(shp65-1) cells and their scramble nonsense transfectants (Figure 3A). The results obtained from MEFs with either p65 deletion and its expression restoration also consistently showed that the p65 regulation of RhoGDIα expression did not occur at mRNA level (Figure 3B), excluding the possibility of p65 regulating RhoGDIα gene transcription or mRNA stability. Thus, we next assessed the potential effect of p65 knockdown on RhoGDIα protein degradation. As shown in Figure 3C, pretreatment of cells with proteasome inhibitor MG132 led to accumulation of RhoGDIα protein in both p65+/+ and p65−/− cells. The MG132 was then removed, and the protein synthesis inhibitor CHX was added for the indicated times. The effect of p65 on the dynamics of RhoGDIα protein degradation was determined. The deficiency of p65 profoundly slowed down RhoGDIα protein degradation rate in comparison to that observed in p65+/+ cells, indicating that p65 promotes RhoGDIα protein degradation.
Figure 3.
p65 promoted degradation of RhoGDIα protein.
(A & B) T24T(shp65-1), T24T(shp65-2) vs. T24T(nonsense) or MEF(p65−/−), MEF[p65−/−(p65)] vs. MEF(WT) cells were extracted for total RNA with Trizol reagent. RT-PCR assay was used to determine RhoGDiα mRNA expression. β-Actin was used as an internal control. (C) The indicated cells were pretreated with proteasome inhibitor MG132 for 10 hours and were then followed by treatment of cells with CHX for the indicated times. The cell extracts were subjected to Western blot, and GAPDH was used as a protein loading control.
Upregulated FBW7 Mediated p65 Promoting RhoGDIα Protein Degradation
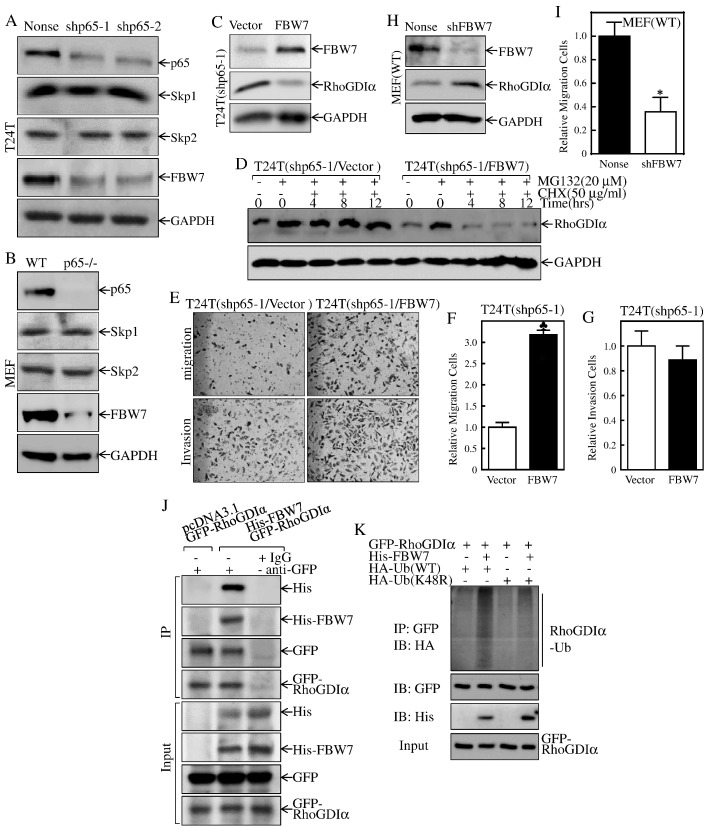
E3 ligase-mediated protein ubiquitination and degradation are the mechanisms responsible for most of protein degradation through a proteasome-dependent manner [44]. FBW7 is a well-characterized E3 ubiquitin ligase and a key substrate recognition component for the SCF ubiquitin ligase complex [45], therefore being responsible for the regulation of the ubiquitination of several proteins [17] and targeting many oncoproteins for their degradation [46], [47], [48]. Deletion and mutation of FBW7 have been frequently identified in many cancers, including gastric cancer, colon cancer, hepatocellular carcinoma, and breast carcinoma [17], [49]. However, the exact role of FBW7 in BC cell migration has never been explored. To investigate the mechanisms underlying the p65 promotion of RhoGDIα protein degradation, we firstly examined several E3 ligase expressions, including FBW7, Skp1, and Skp2. Surprisingly, the abundance of FBW7 protein was remarkably impaired in T24T due to knockdown of p65 (Figure 4A). Consistently, FBW7 expression was much lower in p65−/− cells than that in p65+/+ cells (Figure 4B). In contrast, Skp1 and Skp2 expression was comparable between either T24T(shp65) and T24T(Nonsense) or p65+/+ and p65−/− cells (Figure 4, A and B). To provide the definitive evidence regarding role of FBW7 in p65 mediating RhoGDIα protein degradation, we stably transfected the FBW7 overexpressed plasmid into T24T(shp65-1) cells. As shown in Figure 4, C and D, the overexpression of FBW7 alone dramatically attenuated RhoGDIα expression and promoted RhoGDIα protein degradation as compared with its scramble vector transfectants under the same experimental conditions. These results depicted a critical role and mechanism of FBW7 promotion of RhoGDIα protein degradation. Intriguingly, we also performed the cell migration assay in T24T(shp65/vector) cells and T24T(shp65/FBW7) cells and found that cell migratory activity was increased in T24T(shp65/FBW7) cells without showing significant effect on cell invasion (Figure 4, E-G). Consistently, knockdown of FBW7 also increased RhoGDIα expression and decreased the migration activity compared to their scramble vector transfectants (Figure 4, H and I). Our results validate the conclusion that FBW7 plays an essential role in p65 mediating degradation of RhoGDIα protein and thereby promoting cell migration activity.
Figure 4.
FBW7 was a p65 downstream effector and promoted RhoGDIα protein degradation.
(A & B) The indicated cell extracts were subjected to Western blot to determine protein expression of p65, Skp1, Skp2, and FBW7. GAPDH was used as a loading control. (C) The FBW7 overexpression constructs were stably transfected into T24T(shp65-1) cells. The cell extracts were subjected to Western blot to determine the expression of FBW7 and RhoGDIα. GAPDH was used as a protein loading control. (D) The indicated cells were pretreated with proteasome inhibitor MG132 for 10 hours and were then followed by treatment of cells with CHX for the indicated times. Then, cell extracts were subjected to Western blotting, and GAPDH was used as a protein loading control. (E-G) The migration and invasion abilities of T24T(shp65/FBW7) and their vector control T24T(shp65/vector) cells were determined as described in “Materials and Methods” section. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, and the symbol (♣) indicates a significant increase as compared with scramble control vector transfectants (P < .05) (E). (F) The invasion rate was normalized with the insert control according to the manufacturer's instruction. The bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, P > .05. (H) FBW7 knockdown constructs were stably transfected into MEF(WT) cells. The FBW7 knockdown efficiency was evaluated, and the expression of RhoGDIα was determined by Western blotting. GAPDH was used as a protein loading control. (I) The migration abilities of MEF(shFBW7) and their nonsense control transfectants were determined by using the BD BioCoat Matrigel Migration Chamber without the Matrigel. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, and the asterisk (*) indicates a significant decrease as compared with nonsense control cells (*P < .05). (J) 293T cells were transfected with GFP-RhoGDIα together with pcDNA3.1 or His-FBW7 constructs. Co-immunoprecipitation was performed with anti-GFP antibody-conjugated agarose beads. Immunoprecipitates were then subjected to immunoblotting for the detection of FBW7 using His-antibody. (K) 293T cells were transfected with constructs of GFP-RhoGDIα in combination with His-FBW7 and ubiquitin-WT, or ubiquitin-K48R as indicated. Forty-eight hours after transfection, cells were lysed and co-immunoprecipitated with anti-GFP antibody-conjugated agarose beads and then immunoblotted with anti-HA, anti-GFP, and anti-His antibodies for detection of RhoGDIα ubiquitination.
To determine whether FBW7 acted as the E3 ligase mediating RhoGDIα protein degradation, we co-transfected GFP-RhoGDIα construct with His-FBW7 into 293T cells and performed the immunoprecipitation assay to verify the interaction of FBW7 with RhoGDIα protein. As shown in Figure 4J, His-taged FBW7 was specifically presented in the immune complex pulled down of GFP-RhoGDIα by using the anti-GFP antibody in transfectants harboring His-FBW7, revealing that FBW7 interacts with RhoGDIα protein. Moreover, conjugation of ubiquitin to RhoGDIα was detected in the presence of exogenous WT ubiquitin in the immune complex pulled down of GFP-RhoGDIα by anti-GFP antibody, whereas expressing mutant of ubiquitin (K48R) impaired RhoGDIα ubiquitination (Figure 4K), suggesting that the FBW7 E3 ligase was required for p65-mediated RhoGDIα ubiquitination and protein degradation, as well as cell migration.
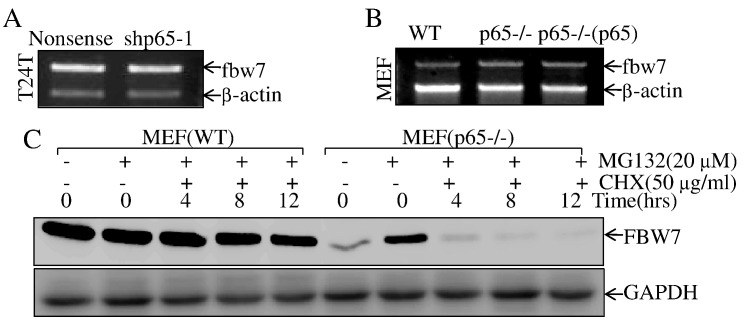
p65 Was Required for FBW7 Protein Stabilization
To elucidate the mechanisms underlying p65 upregulation of FBW7 E3 ligase, we examined FBW7 mRNA levels by RT-PCR in either p65 knockdown or knockout cells in comparison to their scramble cells. The results showed that FBW7 mRNA level was comparable in T24T(nonsense) vs. T24T(shp65) cells or p65+/+ vs. p65−/− (Figure 5, A and B), while restoration of p65 expression in p65−/−cells did not show any observable effect on FBW7 mRNA abundance (Figure 5B). We next tested whether p65 was able to inhibit FBW7 protein degradation. As shown in Figure 5C, the FBW7 protein degradation rate was markedly increased in p65−/− cells in comparison to that in p65+/+ cells, strongly suggesting that p65 mediates FBW7 protein stabilization.
Figure 5.
p65 inhibited E3 ligase FBW7 degradation.
(A & B) T24T(shp65-1) vs. T24T(nonsense) or MEF(p65−/−), MEF[p65−/−(p65)] vs. MEF(WT) cells were extracted for total RNA with Trizol reagent. RT-PCR assay was used to determine fbw7 mRNA expression. β-Actin was used as an internal control. (C) The indicated cells were pretreated with proteasome inhibitor MG132 for 10 hours, and the cells were then treated with CHX for the indicated times. Then cell extracts were subjected to Western blot, and GAPDH was used as a protein loading control.
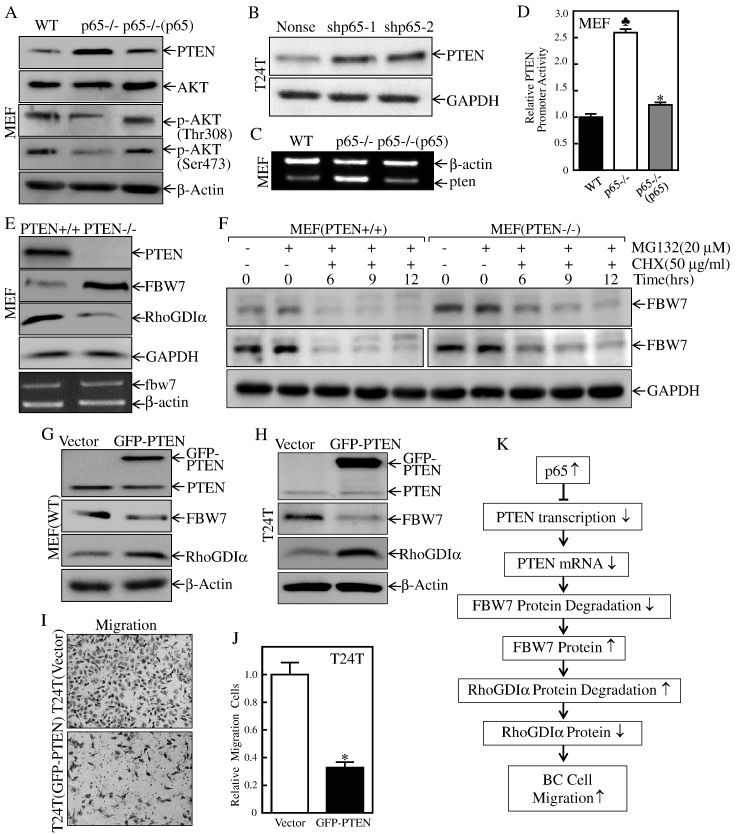
PTEN Was a p65 Downstream Mediator Responsible for Stabilization of FBW7 Protein
PTEN can interact with both amplified in breast 1 (AIB1) and FBW7α by enhancing the FBW7 ubiquitin-mediated degradation of AIB1 [50], while loss of PTEN results in the stabilization of Aurora-A by cooperating with and attenuating FBW7-dependent degradation of Aurora-A through the Akt/GSK3β pathway [51]. PTEN expression can be suppressed by tumor necrosis factor-α through the activation of the NF-κB [52]. Therefore, it can be speculated that PTEN is critical for p65 upregulation of FBW7 expression. Our results showed that p65 deficiency led to a remarkable increase in PTEN expression, while a PTEN downstream substrate, Akt phosphorylation at Thr308/Ser473, was dramatically downregulated in p65−/− cells, which could be completely reversed upon introduction of p65 into p65−/− cells (Figure 6A). Consistently, similar inhibitory effect of p65 on PTEN protein expression was also observed in T24T(shp65) vs. T24T(nonsense) cells (Figure 6B). The results obtained from RT-PCR and PTEN promoter-driven luciferase reporter assays also showed a consistent effect of p65 on PTEN mRNA abundance and promoter-driven reporter transcription activity (Figure 6, C and D), revealing that p65 is a negative regulator for PTEN transcription. Although the reciprocal relationship of FBW7 with PTEN has been reported [24], the mechanism underlying PTEN regulation of FBW7 remains unexplored. To determine the role of PTEN in regulation of FBW7 and its regulated RhoGDIα expression, we compared the expression of FBW7 and RhoGDIα between PTEN+/+ and PTEN−/− cells. As shown in Figure 6E, knockout of PTEN remarkably increased FBW7 level and impaired RhoGDIα protein expression without affecting FBW7 mRNA abundance, suggesting that PTEN inhibits FBW7 protein abundance without affecting mRNA level. Therefore, we next tested whether PTEN was able to promote FBW7 protein degradation. As shown in Figure 6F, the knockout of PTEN resulted in the slowdown of FBW7 protein degradation, suggesting that PTEN expression increases in FBW7 protein degradation. This notion was strongly supported by the results showing that ectopic overexpression of GFP-PTEN led to the reduction of FBW7 abundance accompanied with RhoGDIα upregulation in both T24T and p65+/+ cells (Figure 6, G and H). Consistently, overexpression of GFP-PTEN inhibited cell migration in T24T cells. Our results demonstrate that p65 overexpression leads to downregulating PTEN transcription, which further inhibits FBW7 degradation, consequently promoting FBW7 mediated RhoGDIα protein degradation and thereby releasing RhoGDIα inhibition of small GTPase activity and promoting BC cell migration as illustrated in Figure 6K.
Figure 6.
PTEN was p65 a downstream effector and promoted FBW7 protein degradation and inhibited BC migration.
(A) The indicated cell extracts were subjected to Western blot to determine protein expression of PTEN, AKT, p-AKT(Thr308), and p-AKT(Ser473). (B) The indicated cell extracts were subjected to Western blot to determine protein expression of PTEN. GAPDH was used as a protein loading control. (C) MEF(p65−/−), MEF[p65−/−(p65)] vs. MEF(WT) cells were extracted for total RNA with Trizol reagent. RT-PCR assay was used to determine pten mRNA expression. β-Actin was used as an internal control. (D) Human PTEN promoter-driven luciferase activity was evaluated in MEF(p65−/−), MEF[p65−/−(p65)] and MEF(WT) cells. The results were normalized by internal TK activity. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value. The symbol (♣) indicates a significant increase as compared with MEF(WT) cells (♣P < .05), and the asterisk (*) indicates a significant decrease as compared with MEF(p65−/−) cells, *P < .05. (E) Cell lysates and total RNAs extracted from the indicated cells were subjected to either Western blot (top panel) for determination of PTEN, FBW7, and RhoGDIα or RT-PCR (bottom panel) for determination of fbw7 mRNA expression, respectively. (F) The indicated cells were pretreated with proteasome inhibitor MG132 for 10 hours, and the cells were then treated with CHX for the indicated times. Then cell extracts were subjected to Western blot, and GAPDH was used as a protein loading control. (G & H) PTEN overexpression constructs were stably transfected into MEF(WT) cells and T24T cells. The stable cell extracts were subjected to Western blot to determine protein expression of GFP-PTEN, PTEN, FBW7, and RhoGDIα. β-Actin was used as a protein loading control. (I-J) The migration abilities of T24T(GFP-PTEN) and the vector control cells were determined as described in “Materials and Methods” section. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, and the asterisk (*) indicates a significant decrease as compared with vector control cells (*P < .05) (J). (K) The proposed mechanisms underlying p65 overexpression in the promotion of human BC cell migration: p65 overexpression inhibits pten mRNA transcription, which further stabilizes the protein expression of ubiquitin E3 ligase FBW7, in turn increasing the degradation of RhoGDIα protein, finally promoting BC migration.
Discussion
Bladder cancer is the 3rd most common cancer in men and the 11th most common cancer in women [53]. The depth of invasion of the bladder wall is closely associated with clinical treatment of BCs [54]. Since high-grade muscle invasive BC can progress to life-threatening metastases and the 5-year overall survival rate in patients with distant metastasis is 6%, elucidation of mechanisms for the regulation of motility of human BC is of importance for reducing the mortality of this disease [55], [56], [57]. Cellular migration is a key property of live cells, including normal and malignant cells, and is critical for many cellular functions, such as proper immune response and tissue homeostasis [58], whereas cell invasion is common in only malignant cancer cells and can be defined as the ability of cells to migrate through the extracellular matrices and to penetrate into tissues or infiltrate into neighboring new tissues [59]. Invasive abilities of cancer cells are related to cell migration and many other factors, including MMP-2, MMP-9, Rac1, RhoA, and SOD2 [30]. Learning more about the cellular and molecular basis of these different migration/invasion programs of BC will help us to develop new treatment strategies. A recent IHC analysis of human bladder paraffin-embedded tissue from 116 patients showed that the majority (113 of 116) of patients display concurrent cytoplasmic and nuclear expression of p65/RelA [41]. Our current studies also found that p65 protein overexpression was observed in 100% of BBN-induced high invasive BCs and in three human BC cell lines compared to normal UROtsa cells. Our studies also indicated that p65 overexpression is tightly associated with BC cell migration ability, which led us to explore the mechanism of p65-mediated BC migration, and the results revealed that overexpressed p65 downregulated PTEN transcription/expression, which further resulted in inhibition of FBW7 degradation, in turn promoting FBW7-mediated RhoGDIα degradation and increasing BC migration. Our studies reveal an important aspect of the oncogenic p65 overexpression in BC motility and may further contribute to designing new therapeutic strategies to BC treatment.
Rho family GTPases control a wide range of cellular processes, including cell adhesion, migration, and proliferation [60]. Rho GDP dissociation inhibitor α (RhoGDI) is a key downregulator of the biological activities of small Rho family GTPases [61], which are directly linked to its primary function in the regulation of actin cytoskeleton reorganization and migration [42]. RhoGDIα is also crucial for cancer cell migration and invasion [13], [62]. Herein, we observed that RhoGDIα protein expression was upregulated in p65-deficient cells, and knockdown of RhoGDIα promoted the cell migration in p65 overexpressed BC T24T cells, suggesting that RhoGDIα downregulation by p65 plays a critical role in p65-mediated BC migration. Previous studies have shown that regulation of RhoGDIα is mainly restricted at the posttranslational level by phosphorylation [63] and is also regulated at the posttranscriptional level by miRNA [64]. In the current study, we uncovered that the FBW7 E3 ligase was able to directly use RhoGDIα as its substrate and ubiquitinized and degraded via proteasome-dependent fashion.
FBW7 is the substrate-recognition component of a specific ubiquitin ligase complex [17]. The tumor suppressor function of FBW7 has been previously reported in several types of cancers by targeting multiple well-known onco-proteins for degradation [46], [47], [48]. It has been reported that FBW7 is significantly mutated (>10% of samples) in at least five tumor types including bladder carcinomas [65], but nothing is known about the biological significance of those mutations in BCs. A recent study reports that FBW7 targets myeloid cell leukemia-1 for its degradation and is responsible for pretubulysin (a potent microtubule-binding agent produced by mycobacteria) induction of metastasis cell death in human BC T24 cells and MDA-MB-231 breast cancer cells [66]. However, the function and regulation of FBW7 are still poorly studied in human BCs. KLF5, one of the FBW7 substrates for ubiquitination and degradation, has been reported to promote cell proliferation and tumorigenesis of BC [67], while KLF5 also acts as a tumor suppressor in breast cancers [68], acute myeloid leukemia [69], and prostate cancer [69], suggesting that FBW7’s function depends on certain cellular contexts of its substrates in the intact cells. Our current study showed that p65 overexpression was essential for FBW7 protein expression via inhibition of PTEN transcription. By overexpressing FBW7 in p65 knockdown T24T cells, we observed a remarkable downregulation of RhoGDIα and rescued cell migration as compared with the T24T(shp65/vector) cells, suggesting that RhoGDIα acts as a potential new substrate of FBW7, facilitating the p65-dependent migration abilities of BC cells and revealing the potential promotive effect of FBW7 in promotion of BC migration. Thus, more investigations are needed to delineate the exact role of FBW7 in BC progression and invasion.
FBW7 is regulated by multiple protein factors, such as p53, Pin1, Hes-5 Numb4, and microRNAs [70]. A recent study has also shown that PTEN interacted with FBW7α via its C2 domain, thereby acting as a bridge between AIB1 and FBW7α, and enhancing degradation of AIB1, which eventually accounts for its decreased transcriptional activity [50]. In this study, we showed that PTEN deletion increased in FBW7 protein stability, suggesting that PTEN promotes FBW7 protein degradation. This notion was greatly supported by the findings that ectopic expression of GFP-PTEN attenuated FBW7 abundance and T24T cell migration. Our results demonstrate a novel regulatory axis responsible for modulation of FBW7 protein degradation. Although PTEN inhibition of cancer cell migration has been reported in previous study [71], the mechanism underlying PTEN inhibition of cancer cell migration is never explored. We uncovered here that downregulation of PTEN by overexpressed p65 or loss of function of PTEN by mutation in cancer cells could result in elevation of FBW7, which in turn promotes its substrate RhoGDIα protein degradation and BC cell migration. These findings have deepened our understanding the nature of PTEN and FBW7 in regulation of BC migration.
Conclusions
In summary, our results have shown that p65 is significantly upregulated in BBN-induced high invasive BCs and human BC cell lines. Our studies have also uncovered a new PTEN/FBW7/RhoGDIα axis, which is responsible for the oncogenic role of RelA p65 in promotion of human BC cell migration. The overexpression of p65 inhibits PTEN mRNA transcription, thereby leading to promotion of FBW7 protein degradation. More importantly, we have identified a physical interaction between FBW7 and RhoGDIα, which is critical for RhoGDIα ubiquitination and degradation, revealing that RhoGDIα is an FBW7 new substrate and mediates its protein degradation. Thus, the novel overexpressed p65-initiated PTEN/FBW7/RhoGDIα migration regulatory axis provides new insights into the mechanisms of the underlying nature of p65 in regulation of BC cell motility.
Funding
This work was partially supported by grants from NIH/NCI (CA165980, CA177665, CA112557) and NIH/NIEHS (ES000260), the Natural Science Foundation of China (NSFC81229002, NSFC31301881, NSFC81372946), and Key Project of Science and Technology Innovation Team of Zhejiang Province (2013TD10).
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article.
Authors' Contributions
Chuanshu Huang, Haishan Huang, and Junlan Zhu designed the studies; Yang Li and Junlan Zhu detected the cells' biological function, conducted the RT-PCR assays, carried out the Western blot assays and luciferase reporter assays, and performed the statistical analysis; Caiyi Chen, Jiugao Ma, Wenrui Sun, Zhongxian Tian, and Jiheng Xu detected the IHC staining assays; Dongyun Zhang carried the animal studies; Haishan Huang, Chuanshu Huang, Yang Li, Junlan Zhu, and Claire S. Liu drafted the manuscript. Junlan Zhu and Haishan Huang helped to acquire the experimental data. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
All procedures involving animals were conducted in compliance with guidelines for ethical animal research and approved by the New York University School of Medicine IACUC; the IACUC approval protocol number is 130212-03.
No patient sample or tissue was used in this study.
Competing Interests
The authors declare that they have no competing interests.
Consent for Publication
Not applicable.
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgements
We thank Dr. Iannis Aifantis (New York University School of Medicine, New York, NY) for the kind gift of lentivirus and retrovirus plasmid specifically targeting mouse fbw7 (shFbw7). We thank Dr. Mark R. Philips (New York University School of Medicine, New York) for the kind gift of pEGFP-C3/RhoGDIα vector expressing GFP-tagged RhoGDIα.
Contributor Information
Chuanshu Huang, Email: Chuanshu.huang@nyumc.org.
Haishan Huang, Email: haishan_333@163.com.
References
- 1.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 3.Wang SL, Liu ZJ, Wang LS, Zhang XR. NF-kappa B signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–334. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Ma L, Li J, Yu Y, Zhang D, Wei J, Jin H, Xu D, Gao J, Huang C. NF-κB1 inhibits c-Myc protein degradation through suppression of FBW7 expression. Oncotarget. 2014;5:493. doi: 10.18632/oncotarget.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun F, Qu Z, Xiao Y, Zhou J, Burns TF, Stabile LP, Siegfried JM, Xiao G. NF-kappaB1 p105 suppresses lung tumorigenesis through the Tpl2 kinase but independently of its NF-kappaB function. Oncogene. 2015 doi: 10.1038/onc.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Liu C, Chen D, Yang WH, Liu X, Liu CG, Dugas CM, Tang F, Zheng P, Liu Y. FOXP3 controls an miR-146/NF-kappaB negative feedback loop that inhibits apoptosis in breast cancer cells. Cancer Res. 2015;75:1703–1713. doi: 10.1158/0008-5472.CAN-14-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee N, Houston TJ, Cardenas E, Ghosh R. To be an ally or an adversary in bladder cancer: the NF-kappaB story has not unfolded. Carcinogenesis. 2015;36:299–306. doi: 10.1093/carcin/bgu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netto GJ. Clinical applications of recent molecular advances in urologic malignancies: no longer chasing a "mirage"? Adv Anat Pathol. 2013;20:175–203. doi: 10.1097/PAP.0b013e3182863f80. [DOI] [PubMed] [Google Scholar]
- 9.Adra CN, Iyengar AR, Syed FA, Kanaan IN, Rilo HL, Yu W, Kheraj R, Lin SR, Horiuchi T, Khan S, Weremowicz S, Lim B, Morton CC, Higgs DR. Human ARHGDIG, a GDP-dissociation inhibitor for Rho proteins: genomic structure, sequence, expression analysis, and mapping to chromosome 16p13.3. Genomics. 1998;53:104–109. doi: 10.1006/geno.1998.5482. [DOI] [PubMed] [Google Scholar]
- 10.Rong F, Li W, Chen K, Li DM, Duan WM, Feng YZ, Li F, Zhou XW, Fan SJ, Liu Y, Tao M. Knockdown of RhoGDIalpha induces apoptosis and increases lung cancer cell chemosensitivity to paclitaxel. Neoplasma. 2012;59:541–550. doi: 10.4149/neo_2012_070. [DOI] [PubMed] [Google Scholar]
- 11.Kasper B, Tidow N, Grothues D, Welte K. Differential expression and regulation of GTPases (RhoA and Rac2) and GDIs (LyGDI and RhoGDI) in neutrophils from patients with severe congenital neutropenia. Blood. 2000;95:2947–2953. [PubMed] [Google Scholar]
- 12.Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, Mansel RE. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9:6432–6440. [PubMed] [Google Scholar]
- 13.Wang H, Wang B, Liao Q, An H, Li W, Jin X, Cui S, Zhao L. Overexpression of RhoGDI, a novel predictor of distant metastasis, promotes cell proliferation and migration in hepatocellular carcinoma. FEBS Lett. 2014;588:503–508. doi: 10.1016/j.febslet.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Zhang D, Liu J, Li J, Yu Y, Wu XR, Huang C. RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility. J Biol Chem. 2012;287:13752–13760. doi: 10.1074/jbc.M111.337469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 17.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Taranets L, Popov N. Regulating Fbw7 on the road to cancer. Semin Cancer Biol. 2016;36:62–70. doi: 10.1016/j.semcancer.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Yokobori T, Mimori K, Iwatsuki M, Ishii H, Onoyama I, Fukagawa T, Kuwano H, Nakayama KI, Mori M. p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res. 2009;69:3788–3794. doi: 10.1158/0008-5472.CAN-08-2846. [DOI] [PubMed] [Google Scholar]
- 20.Iwatsuki M, Mimori K, Ishii H, Yokobori T, Takatsuno Y, Sato T, Toh H, Onoyama I, Nakayama KI, Baba H. Loss of FBXW7, a cell cycle regulating gene, in colorectal cancer: clinical significance. Int J Cancer. 2010;126:1828–1837. doi: 10.1002/ijc.24879. [DOI] [PubMed] [Google Scholar]
- 21.Ibusuki M, Yamamoto Y, Shinriki S, Ando Y, Iwase H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer Sci. 2011;102:439–445. doi: 10.1111/j.1349-7006.2010.01801.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurashige J, Watanabe M, Iwatsuki M, Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K, Baba H. Overexpression of microRNA-223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer. 2012;106:182–188. doi: 10.1038/bjc.2011.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H, Gao J, Zhang B, Xu W, Liu J. ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell Res. 2015;25:561–573. doi: 10.1038/cr.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reavie L, Della Gatta G, Crusio K, Aranda-Orgilles B, Buckley SM, Thompson B, Lee E, Gao J. Regulation of hematopoietic stem cell differentiation by a single ubiquitin ligase-substrate complex. Nat Immunol. 2010;11:207–215. doi: 10.1038/ni.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhang D, Luo W, Yu Y, Yu J, Li J, Zhang X, Zhang B, Chen J, Wu XR. X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J Biol Chem. 2011;286:15630–15640. doi: 10.1074/jbc.M110.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, Tong Q, He J, Huang C. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res. 2005;65:6601–6611. doi: 10.1158/0008-5472.CAN-04-4184. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Xu J, Gao G, Li J, Huang H, Jin H, Zhu J, Che X, Huang C. Tumor-suppressor NFkappaB2 p100 interacts with ERK2 and stabilizes PTEN mRNA via inhibition of miR-494. Oncogene. 2015 doi: 10.1038/onc.2015.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, Shen HM, Whiteman M, Huang C. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol. 2006;175:607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, Zhang L, Huang H, Zhang D, Wu XR. Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines. Oncotarget. 2015;6:522–536. doi: 10.18632/oncotarget.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, Zhu J, Huang H, Xiang D, Li Y, Zhang D, Li J, Wang Y, Jin H, Jiang G. SESN2/sestrin 2 induction-mediated autophagy and inhibitory effect of isorhapontigenin (ISO) on human bladder cancers. Autophagy. 2016;12:1229–1239. doi: 10.1080/15548627.2016.1179403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Y, Cao Z, Hou Q, Ma C, Yao C, Li J, Wu XR, Huang C. Cyclin d1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells. Mol Cancer Ther. 2013;12:1492–1503. doi: 10.1158/1535-7163.MCT-12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gildea JJ, Golden WL, Harding MA, Theodorescu D. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes Chromosomes Cancer. 2000;27:252–263. doi: 10.1002/(sici)1098-2264(200003)27:3<252::aid-gcc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Che X, Zhang J, Li Y, Li J, Deng X, Zhu J, Jin H, Zhao Q, Huang C. Cheliensisin A (Chel A) induces apoptosis in human bladder cancer cells by promoting PHLPP2 protein degradation. Oncotarget. 2016 doi: 10.18632/oncotarget.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che X, Liu J, Huang H, Mi X, Xia Q, Li J, Zhang D, Ke Q, Gao J, Huang C. p27 suppresses cyclooxygenase-2 expression by inhibiting p38beta and p38delta-mediated CREB phosphorylation upon arsenite exposure. Biochim Biophys Acta. 2013;1833:2083–2091. doi: 10.1016/j.bbamcr.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Zhang J, Huang H, Li J, Yu Y, Jin H, Li Y, Deng X, Gao J, Zhao Q, Huang C. Crucial role of c-Jun phosphorylation at Ser63/73 mediated by PHLPP protein degradation in the cheliensisin a inhibition of cell transformation. Cancer Prev Res. 2014;7:1270–1281. doi: 10.1158/1940-6207.CAPR-14-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Li J, Wan Y, Lu J, Gao J, Huang C. GADD45alpha induction by nickel negatively regulates JNKs/p38 activation via promoting PP2Calpha expression. PLoS One. 2013;8:e57185. doi: 10.1371/journal.pone.0057185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, Zhu J, Li Y, Zhang L, Gu J, Xie Q, Jin H, Che X, Li J, Huang C, Chen LC, Lyu J, Gao J, Huang C. Upregulation of SQSTM1/p62 contributes to nickel-induced malignant transformation of human bronchial epithelial cells. Autophagy. 2016:1–17. doi: 10.1080/15548627.2016.1196313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin H, Xu J, Guo X, Huang H, Li J, Peng M, Zhu J, Tian Z, Wu XR, Tang MS. XIAP RING domain mediates miR-4295 expression and subsequently inhibiting p63alpha protein translation and promoting transformation of bladder epithelial cells. Oncotarget. 2016 doi: 10.18632/oncotarget.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozbek E, Otunctemur A, Calik G, Aliskan T, Cakir S, Dursun M, Somay A. Comparison of p38MAPK (mitogene activated protein kinase), p65 NFkappaB (nuclear factor kappa b) and EMMPRIN (extracellular matrix metalloproteinase inducer) expressions with tumor grade and stage of superficial and invasive bladder tumors. Arch Ital Urol Androl. 2011;83:181–187. [PubMed] [Google Scholar]
- 41.Levidou G, Saetta AA, Korkolopoulou P, Papanastasiou P, Gioti K, Pavlopoulos P, Diamantopoulou K, Thomas-Tsagli E, Xiromeritis K. Clinical significance of nuclear factor (NF)-kappaB levels in urothelial carcinoma of the urinary bladder. Virchows Arch. 2008;452:295–304. doi: 10.1007/s00428-007-0560-y. [DOI] [PubMed] [Google Scholar]
- 42.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrader EK, Harstad KG, Matouschek A. Targeting proteins for degradation. Nat Chem Biol. 2009;5:815–822. doi: 10.1038/nchembio.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji S, Qin Y, Liang C, Huang R, Shi S, Liu J, Jin K, Liang D, Xu W, Zhang B. FBW7 (F-box and WD repeat domain-containing 7) negatively regulates glucose metabolism by targeting the c-Myc/TXNIP (thioredoxin binding protein) axis in pancreatic cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2380. [DOI] [PubMed] [Google Scholar]
- 46.Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, Elledge SJ. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 47.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A. 2004;101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nateri AS, Riera-Sans L, Da Costa C, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303:1374–1378. doi: 10.1126/science.1092880. [DOI] [PubMed] [Google Scholar]
- 49.Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, Yao Y, Liu Q. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer. 2014;13:110. doi: 10.1186/1476-4598-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang C, Li S, Wang M, Chang AK, Liu Y, Zhao F, Xiao L, Han L, Wang D, Li S. PTEN suppresses the oncogenic function of AIB1 through decreasing its protein stability via mechanism involving Fbw7 alpha. Mol Cancer. 2013;12:21. doi: 10.1186/1476-4598-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon YW, Kim IJ, Wu D, Lu J, Stock WA, Jr, Liu Y, Huang Y, Kang HC, DelRosario R, Jen KY. Pten regulates Aurora-A and cooperates with Fbxw7 in modulating radiation-induced tumor development. Mol Cancer Res. 2012;10:834–844. doi: 10.1158/1541-7786.MCR-12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du C, Yi X, Liu W, Han T, Liu Z, Ding Z, Zheng Z, Piao Y, Yuan J, Han Y. MTDH mediates trastuzumab resistance in HER2 positive breast cancer by decreasing PTEN expression through an NFkappaB-dependent pathway. BMC Cancer. 2014;14:869. doi: 10.1186/1471-2407-14-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel R, Jiemin M, Zhaohui Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:20. [Google Scholar]
- 54.Cowan NC, Crew JP. Imaging bladder cancer. Curr Opin Urol. 2010;20:409–413. doi: 10.1097/MOU.0b013e32833cbcb9. [DOI] [PubMed] [Google Scholar]
- 55.Al-Samawi AS, Aulaqi SM. Urinary bladder cancer in yemen. Oman Med J. 2013;28:337–340. doi: 10.5001/omj.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF, Perrotte P. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37:219–225. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 58.Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014 doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. 2013. Cancer Invasion and Metastasis: Molecular and Cellular perspective. [Google Scholar]
- 60.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 61.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 62.Xiao Y, Lin VY, Ke S, Lin GE, Lin FT, Lin WC. 14-3-3tau promotes breast cancer invasion and metastasis by inhibiting RhoGDIalpha. Mol Cell Biol. 2014;34:2635–2649. doi: 10.1128/MCB.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee HS, Cheerathodi M, Chaki SP, Reyes SB, Zheng Y, Lu Z, Paidassi H, DerMardirossian C, Lacy-Hulbert A, Rivera GM. Protein tyrosine phosphatase-PEST and beta8 integrin regulate spatiotemporal patterns of RhoGDI1 activation in migrating cells. Mol Cell Biol. 2015;35:1401–1413. doi: 10.1128/MCB.00112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen J, Zhang Y, Lai P, Fan X, Zhou X. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 65.Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell. 2014;26:455–464. doi: 10.1016/j.ccell.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braig S, Wiedmann RM, Liebl J, Singer M, Kubisch R, Schreiner L, Abhari BA, Wagner E, Kazmaier U, Fulda S. Pretubulysin: a new option for the treatment of metastatic cancer. Cell Death Dis. 2014;5:e1001. doi: 10.1038/cddis.2013.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong XY, Bao Y, Zhou Z, Cheng X, Simons JW. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006;118:1346–1355. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 68.Chen C, Bhalala HV, Qiao H, Dong JT. A possible tumor suppressor role of the KLF5 transcription factor in human breast cancer. Oncogene. 2002;21:6567–6572. doi: 10.1038/sj.onc.1205817. [DOI] [PubMed] [Google Scholar]
- 69.Chen C, Bhalala HV, Vessella RL, Dong JT. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate. 2003;55:81–88. doi: 10.1002/pros.10205. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Ye X, Liu Y, Wei W, Wang Z. Aberrant regulation of FBW7 in cancer. Oncotarget. 2014;5:2000–2015. doi: 10.18632/oncotarget.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mense SM, Barrows D, Hodakoski C, Steinbach N, Schoenfeld D, Su W, Hopkins BD, Su T, Fine B, Hibshoosh H. PTEN inhibits PREX2-catalyzed activation of RAC1 to restrain tumor cell invasion. Sci Signal. 2015;8:ra32. doi: 10.1126/scisignal.2005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.