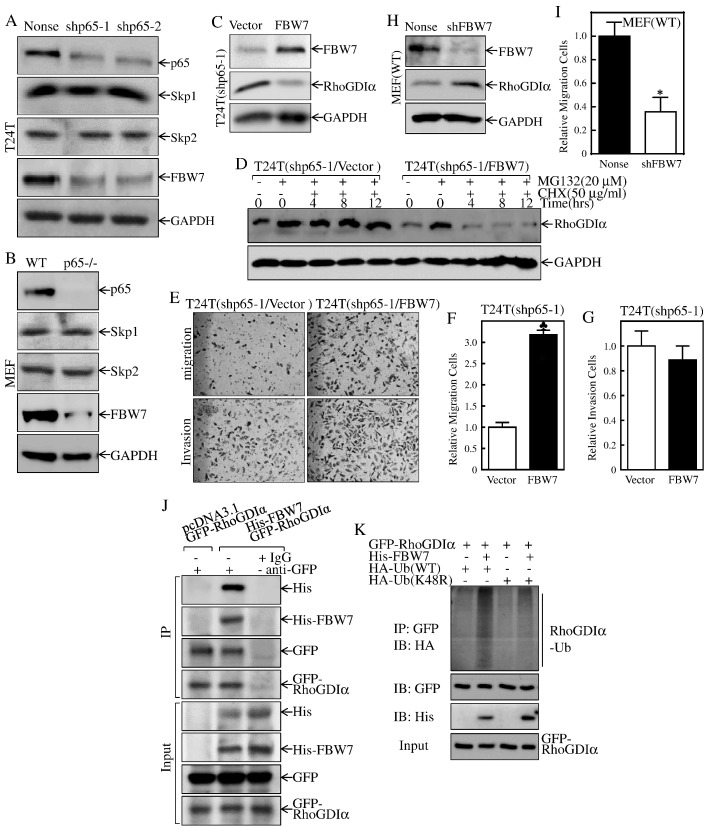
Figure 4.
FBW7 was a p65 downstream effector and promoted RhoGDIα protein degradation.
(A & B) The indicated cell extracts were subjected to Western blot to determine protein expression of p65, Skp1, Skp2, and FBW7. GAPDH was used as a loading control. (C) The FBW7 overexpression constructs were stably transfected into T24T(shp65-1) cells. The cell extracts were subjected to Western blot to determine the expression of FBW7 and RhoGDIα. GAPDH was used as a protein loading control. (D) The indicated cells were pretreated with proteasome inhibitor MG132 for 10 hours and were then followed by treatment of cells with CHX for the indicated times. Then, cell extracts were subjected to Western blotting, and GAPDH was used as a protein loading control. (E-G) The migration and invasion abilities of T24T(shp65/FBW7) and their vector control T24T(shp65/vector) cells were determined as described in “Materials and Methods” section. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, and the symbol (♣) indicates a significant increase as compared with scramble control vector transfectants (P < .05) (E). (F) The invasion rate was normalized with the insert control according to the manufacturer's instruction. The bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, P > .05. (H) FBW7 knockdown constructs were stably transfected into MEF(WT) cells. The FBW7 knockdown efficiency was evaluated, and the expression of RhoGDIα was determined by Western blotting. GAPDH was used as a protein loading control. (I) The migration abilities of MEF(shFBW7) and their nonsense control transfectants were determined by using the BD BioCoat Matrigel Migration Chamber without the Matrigel. Bars represent mean ± SD from three independent experiments. Student's t test was utilized to determine the P value, and the asterisk (*) indicates a significant decrease as compared with nonsense control cells (*P < .05). (J) 293T cells were transfected with GFP-RhoGDIα together with pcDNA3.1 or His-FBW7 constructs. Co-immunoprecipitation was performed with anti-GFP antibody-conjugated agarose beads. Immunoprecipitates were then subjected to immunoblotting for the detection of FBW7 using His-antibody. (K) 293T cells were transfected with constructs of GFP-RhoGDIα in combination with His-FBW7 and ubiquitin-WT, or ubiquitin-K48R as indicated. Forty-eight hours after transfection, cells were lysed and co-immunoprecipitated with anti-GFP antibody-conjugated agarose beads and then immunoblotted with anti-HA, anti-GFP, and anti-His antibodies for detection of RhoGDIα ubiquitination.