Abstract
Metabolic syndrome (MetSyn) represents a clustering of different metabolic abnormalities. MetSyn prevalence is present in approximately 25% of all adults with increased prevalence in advanced ages. The presence of one component of MetSyn increases the risk of developing MetSyn later in life and likely represents a high lifetime burden of cardiovascular disease risk. Therefore we pooled data from multiple studies to establish the prevalence of MetSyn and MetSyn component prevalence across a broad range of ethnicities. PubMed, SCOPUS and Medline databases were searched to find papers presenting MetSyn and MetSyn component data for 18–30 year olds who were apparently healthy, free of disease, and MetSyn was assessed using either the harmonized, National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII), American Heart Association/National Heart, Blood and Lung Institute (AHA/NHBLI), or International Diabetes Federation (IDF) definitions of MetSyn. After reviewing returned articles, 26,609 participants' data from 34 studies were included in the analysis and the data were pooled. MetSyn was present in 4.8–7% of young adults. Atherogenic dyslipidaemia defined as low high density lipoprotein (HDL) cholesterol was the most prevalent MetSyn component (26.9–41.2%), followed by elevated blood pressure (16.6–26.6%), abdominal obesity (6.8–23.6%), atherogenic dyslipidaemia defined as raised triglycerides (8.6–15.6%), and raised fasting glucose (2.8–15.4%). These findings highlight that MetSyn is prevalent in young adults. Establishing the reason why low HDL is the most prevalent component may represent an important step in promoting primary prevention of MetSyn and reducing the incidence of subsequent clinical disease.
Highlights
-
•
MetSyn prevalence ranges from 4.8% to 7.0% in young adults.
-
•
Low HDL cholesterol is the most prevalent component of MetSyn in young adults.
-
•
Increased understanding of low HDL in young adults may better promote primary prevention efforts to reduce future disease.
1. Introduction
MetSyn is an asymptomatic, pathophysiological state characterised by obesity, insulin resistance, hypertension, dysglycaemia, and dyslipidaemia (Alberti et al., 2009). While several criteria and definitions have been used to identify MetSyn (Alberti et al., 2009, Grundy et al., 2005, Alberti et al., 2005, Anon, 2001); it is generally agreed that a combination of three or more of the following components must be present: large waist circumference, elevated triglycerides, low HDL-cholesterol, raised blood pressure, and elevated fasting blood glucose.
The International Diabetes Federation (IDF) estimates that ≈ 25% of the world's population has MetSyn (O'Neill and O'Driscoll, 2015) although this estimate varies widely due to the age, ethnicity, and gender of the population studied (Kaur, 2014). Having a slightly raised value of a MetSyn component at a younger age increases the future risk for MetSyn later in life (Gündogan et al., 2009). Therefore it is important to establish the prevalence of MetSyn components in young adults (18–30 years), as the presence of a MetSyn component could represent a lifetime of increased cardiovascular disease risk. Moreover, the early identification of MetSyn components could lead to targeted interventions to prevent the development of the syndrome, and thus reduce cardiovascular disease risk in later life.
Therefore, we performed a pooled analysis of previous literature that examined the prevalence of MetSyn and components of MetSyn in young adults with the purpose of determining: 1) the global prevalence of MetSyn in young adults, and 2) the most prevalent MetSyn component in this population.
2. Methods
PubMed, SCOPUS and Medline were searched using the terms “Metabolic Syndrome”, “Prevalence”, and “Young Adults” combined with the Boolean operator “AND”. The search was repeated using the term “College Students” instead of “Young Adults” and the results combined. Duplicates from the returned reference lists were discarded and the list was consolidated into one list from the three databases.
Abstracts from the returned references were downloaded and were kept if the abstract indicated that the article may contain data relating to MetSyn and apparently healthy young adults. The remaining articles were downloaded in full and analysed for specific data relating to MetSyn in young adults. Studies were included if 1) Participants were sampled on the basis of being apparently healthy, free of chronic conditions, or having specific anthropometric characteristics; 2) Data were available in the age range of 18–30 years old; 3) The prevalence for MetSyn was supplied or able to be calculated; 4) The NCEP-ATP III criteria (Anon, 2001), Revised NCEP-ATPIII (referred to here as AHA/NHBLI) criteria (Grundy et al., 2005), International IDF criteria (Alberti et al., 2005) or the harmonized criteria (Alberti et al., 2009) for MetSyn were used (Table 1). In addition, the article had to be written in English and accessible either through open access or our institution's library subscription that has access to 1200 + databases and 98,000 e-journals. All papers were cross checked to ensure that data were not used across multiple studies.
Table 1.
Metabolic syndrome criteria according to Harmonized (Alberti et al., 2009), IDF (Alberti et al., 2005), NCEP-ATPII (Anon, 2001) and AHA/NHBLI criteria (Grundy et al., 2005).
| MetSyn Criteria | Harmonized |
IDF |
NCEP-ATPIII |
AHA/NHBLI |
|---|---|---|---|---|
| Any three or more of: | WC ≥ 94 cm (male) WC ≥ 80 cm (female) |
Any three or more of: | Any three or more of: | |
| WC | Ethnic specific cut points | And two or more of: | ≥ 102 cm (male) ≥88 cm (female) |
≥ 102 cm (male) ≥88 cm (female) |
| HDL | < 1.03 mmol/L (male) <1.29 mmol/L (female) OR taking medication for reduced HDL |
< 1.03 mmol/L (male) <1.29 mmol/L (female) OR taking medication for reduced HDL |
< 1.03 mmol/L (male) <1.29 mmol/L (female) OR taking medication for reduced HDL |
< 1.03 mmol/L (male) <1.29 mmol/L (female) OR taking medication for reduced HDL |
| TG | ≥ 1.7 mmol/L or medication for elevated TG | ≥ 1.7 mmol/L or medication for elevated TG | ≥ 1.7 mmol/L or medication for elevated TG | ≥ 1.7 mmol/L or medication for elevated TG |
| BP | ≥ 130 mm Hg Systolic BP or ≥ 85 mm Hg Diastolic BP or on BP-lowering medication | ≥ 130 mm Hg Systolic BP or ≥ 85 mm Hg Diastolic BP or on BP-lowering medication | ≥ 130 mm Hg Systolic BP or ≥ 85 mm Hg Diastolic BP or on BP-lowering medication | ≥ 130 mm Hg Systolic BP or ≥ 85 mm Hg Diastolic BP or on BP-lowering medication |
| FBG | ≥ 5.6 mmol/L or antidiabetic medication | ≥ 5.6 mmol/L or antidiabetic medication | ≥ 6.1 mmol/L or antidiabetic medication | ≥ 5.6 mmol/L or antidiabetic medication |
MetSyn – metabolic syndrome; WC – abdominal obesity; HDL - atherogenic dyslipidaemia (low HDL); TG - atherogenic dyslipidaemia (raised triglycerides); BP - raised blood pressure; FBG – raised fasting glucose.
Data relating to MetSyn and MetSyn components in 18–30 year old adults were extracted from the reviewed articles and MetSyn and MetSyn component prevalence was calculated using the four different definitions. The results were tabulated (Table 2).
Table 2.
Prevalence of metabolic syndrome and metabolic syndrome components in 26,609 young adults.
| Author | Country | Total (n) | MetSyn | WC | HDL | TG | BP | FBG | |
|---|---|---|---|---|---|---|---|---|---|
| Harmonized | Al Dhaheri et al. (2016) | UAE | 555 | 38 (6.8) | 101 (18.2) | 271 (48.8) | 8 (1.4) | 30 (5.4) | 54 (9.7) |
| Bennett et al. (2014) | Jamaica | 746 | 6 (0.8) | 108 (14.5) | 343 (46.0) | 4 (0.5) | 154 (20.6) | 8 (1.1) | |
| Ferguson et al. (2010) | Jamaica | 839 | 10 (1.2) | 134 (16.0) | 393 (46.8) | 5 (0.6) | 56 (6.7) | 10 (1.2) | |
| Gavrila et al. (2011)a | Spain | 292 | 18 (6.2) | 81 (27.7) | 49 (16.8) | 24 (8.2) | 48 (16.4) | 11 (3.8) | |
| Gupta et al. (2009) | India | 486 | 12(2.5) | 51 (10.5) | 150 (30.9) | 27 (5.6) | 15 (3.1) | 31 (6.4) | |
| Huang et al. (2015) | Taiwan | 355 | 24 (6.8) | 63 (17.7) | 46 (20.6) | 32 (9.0) | 123 (34.6) | 4 (1.1) | |
| Kaduka et al. (2012) | Kenya | 90 | 9 (10.0) | 17 (18.9) | 47 (52.2) | 3 (3.3) | 51 (56.7) | 1 (1.1) | |
| Lin et al. (2014) | China | 323 | 22 (6.8) | 180 (55.7) | 63 (19.5) | 40 (12.4) | 20 (6.2) | 22 (6.8) | |
| Martins et al. (2015)a | Brazil | 2031 | 242 (9.0) | 646 (31.8) | 851 (41.9) | 254 (12.5) | 465 (22.9) | 73 (3.6) | |
| Sy et al. (2014)a | Philippines | 861 | 108 (12.5) | 173 (20.1) | 467 (54.2) | 171 (19.9) | 127 (14.8) | 144 (16.7) | |
| Overall | 6578 | 430 (6.5%) | 1554 (23.6%) | 2707 (41.2%) | 568 (8.6%) | 1089 (16.6%) | 358 (5.4%) | ||
| NCEP –ATPIII | Erem et al. (2008) | Turkey | 1306 | 93 (7.1) | 182 (13.9) | 318 (21.3) | 183 (14.0) | 424 (32.5) | 25 (1.9) |
| Gündogan et al. (2009)a | Turkey | 84 | 10 (11.9) | 25 (29.8) | 19 (22.6) | 24 (28.6) | 20 (23.8) | 11 (13.1) | |
| Huang et al. (2004) | USA | 163 | 1 (0.6) | 3 (1.8) | 22 (13.5) | 4 (2.5) | 2 (1.2) | 3 (1.8) | |
| Li et al. (2010)a | China | 2532 | 101 (4.0) | 79 (3.1) | 742 (29.3) | 241 (9.5) | 519 (20.5) | 58 (2.3) | |
| Manjunath et al. (2014) | India | 473 | 41 (8.7) | 76 (16.1) | 184 (38.9) | 37 (7.8) | 123 (26.0) | 42 (8.9) | |
| Mikkola et al. (2007)a | Finland | 1099 | 38 (3.5) | 51 (4.6) | 212 (19.3) | 31 (2.8) | 565 (51.4) | 25 (2.3) | |
| Sidorenkov et al. (2010) | Russia | 862 | 23 (2.7) | 19 (2.2) | 266 (30.9) | 83 (9.6) | 149 (17.3) | 6 (0.7) | |
| Sinha et al. (2013) | India | 85 | 8 (9.4) | 18 (21.2) | 53 (62.4) | 18 (21.2) | 5 (5.9) | 7 (8.2) | |
| Soysal et al. (2005) | Turkey | 285 | 18 (6.3) | 14 (4.9) | 40 (14.0) | 115 (40.4) | 25 (8.8) | 13 (4.6) | |
| Overall | 6889 | 333 (4.8%) | 467 (6.8%) | 1856 (26.9%) | 736 (10.7%) | 1832 (26.6%) | 190 (2.8%) | ||
| IDF | Bener et al. (2009)a | Qatar | 203 | 16 (7.9) | 16 (7.9) | 43 (21.2) | 30 (14.8) | 34 (16.7) | 8 (3.9) |
| da Costa et al. (2011) | Brazil | 711 | 28 (3.9) | 90 (12.7) | 313 (44.0) | 35 (4.9) | 71 (10.0) | 35 (1.4) | |
| da Silveira et al. (2010)a | Brazil | 3599 | 240 (6.7) | 618 (17.2) | 694 (19.3) | 598 (16.6) | 883 (24.5) | 1322 (36.7) | |
| Gavrila et al. (2011)a | Spain | 292 | 18 (6.2) | 81 (27.7) | 49 (16.8) | 24 (8.2) | 48 (16.4) | 11 (3.8) | |
| Gündogan et al. (2009)a | Turkey | 84 | 16 (19.0) | 25 (29.8) | 19 (22.6) | 24 (28.6) | 20 (23.8) | 11 (13.1) | |
| Hildrum et al. (2007)a | Norway | 1615 | 19 (1.2) | 414 (25.6) | 459 (28.4) | 221 (13.7) | 499 (30.9) | 179 (11.1) | |
| Huang et al. (2007)a | USA | 300 | 2 (0.7) | 8 (2.7) | 73 (24.3) | 27 (9.0) | 11 (3.7) | 27 (9.0) | |
| Kanitkar et al. (2015) | India | 250 | 55 (22.0) | 139 (55.6) | 93 (37.2) | 71 (29.2) | 21 (8.4) | 44 (17.6) | |
| Li et al. (2010)a | China | 2532 | 147 (5.8) | 79 (3.1) | 742 (29.3) | 241 (9.5) | 519 (20.5) | 58 (2.3) | |
| Martins et al. (2015)a | Brazil | 2031 | 242 (11.9) | 646 (31.8) | 851 (41.9) | 254 (12.5) | 465 (22.9) | 73 (3.6) | |
| Mikkola et al. (2007)a | Finland | 1099 | 75 (6.8) | 134 (12.2) | 212 (19.3) | 31 (2.8) | 565 (51.4) | 221 (20.1) | |
| Sy et al. (2014)a | Philippines | 861 | 108 (9.1) | 173 (20.1) | 467 (54.2) | 171 (19.9) | 127 (14.8) | 144 (16.7) | |
| Tope et al. (2013)a | USA | 376 | 35 (9.3) | 43 (11.4) | 73 (19.4) | 21 (5.6) | 38 (10.1) | 42(11.2) | |
| Overall | 13,953 | 971 (7.0%) | 2466 (17.7%) | 4088 (29.3%) | 1750 (12.5%) | 3301 (23.7%) | 2150 (15.4%) | ||
| AHA/NHBLI | Bener et al. (2009)a | Qatar | 203 | 15 (7.4) | 16 (7.9) | 43 (21.2) | 30 (14.8) | 34 (16.7) | 8 (3.9) |
| Cheserek et al. (2014) | China | 200 | 1 (0.5) | 4 (2.0) | 19 (9.5) | 13 (6.5) | 24 (12.0) | 6 (3.0) | |
| da Silveira et al. (2010)a | Brazil | 3599 | 213 (5.9) | 269 (7.5) | 694 (19.3) | 598 (16.6) | 883 (24.5) | 610 (16.9) | |
| Dalleck and Kjelland (2012) | USA | 207 | 14 (6.8) | 12 (5.8) | 98 (47.3) | 28 (13.5) | 34 (16.4) | 15 (7.2) | |
| de Kroon et al. (2008) | Netherlands | 642 | 48 (7.5) | 78 (12.1) | 187 (29.1) | 50 (7.8) | 274 (42.7) | 75 (11.7) | |
| Fernandes and Lofgren (2011) | USA | 189 | 7 (3.7) | 14 (7.4) | 38 (20.1) | 33 (17.5) | 4 (2.1) | 14 (7.4) | |
| Gavrila et al. (2011)a | Spain | 292 | 10 (3.4) | 31 (10.6) | 49 (16.8) | 24 (8.2) | 48 (16.4) | 11 (3.8) | |
| Hildrum et al. (2007)a | Norway | 1615 | 19 (1.2) | 414 (25.6) | 459 (28.4) | 221 (13.7) | 499 (30.9) | 179 (11.1) | |
| Huang et al. (2007)a | USA | 300 | 4 (1.3) | 8 (2.7) | 73 (24.3) | 27 (9.0) | 11 (3.7) | 27 (9.0) | |
| Morrell et al. (2013) | USA | 1610 | 81 (5.0) | 209 (130) | 467 (29.0) | 258 (16.0) | 403 (25.0) | 64 (4.0) | |
| Morrell et al. (2012) | USA | 2103 | 103 (4.9) | 94 (4.5) | 538 (25.6) | 350 (16.6) | 681 (32.4) | 177 (8.4) | |
| Shahbazian et al. (2013) | Iran | 203 | 13 (6.4) | 26 (12.8) | 85 (41.9) | 47 (23.2) | 1 (0.5) | 35 (17.2) | |
| Sharifi et al. (2009) | Iran | 934 | 70 (7.5) | 31 (9.7) | 714 (76.4) | 246 (26.3) | 56 (6.0) | 74 (7.9) | |
| Tope et al. (2013)a | USA | 376 | 45 (12.0) | 43 (11.4) | 73 (19.4) | 21 (5.6) | 38 (10.1) | 42 (11.2) | |
| Overall | 12,473 | 643 (5.2%) | 1309 (10.5%) | 3537 (28.4%) | 1946 (15.6%) | 2990 (24.0%) | 1337 (10.7%) |
Data are expressed as n (%).
MetSyn – metabolic syndrome; WC – abdominal obesity; HDL - atherogenic dyslipidaemia (low HDL); TG - atherogenic dyslipidaemia (raised triglycerides); BP - raised blood pressure; FBG – raised fasting glucose.
Indicates study used more than one definition of MetSyn and is included multiple times.
3. Results
From the initial search, 1276 unique citations were returned, of which 992 studies were immediately discarded based on the title or abstract. The remaining 284 studies were evaluated against the inclusion criteria. Thirty-four papers were included in the final review with 11 studies (Bener et al., 2010, Silveira et al., 2010, Gavrila et al., 2011, Gündogan et al., 2009, Hildrum et al., 2007, Huang et al., 2007, Li et al., 2010, Martins et al., 2015, Mikkola et al., 2007, Sy et al., 2014, Tope et al., 2013) providing MetSyn data based on multiple definitions. Data from 26,609 different people aged between 18 and 30 years from 17 countries were available for analysis – see table for individual and grouped prevalence data. Please note that the combined number of observations for all MetSyn definitions is > 26,609 due to the inclusion of studies using multiple definitions.
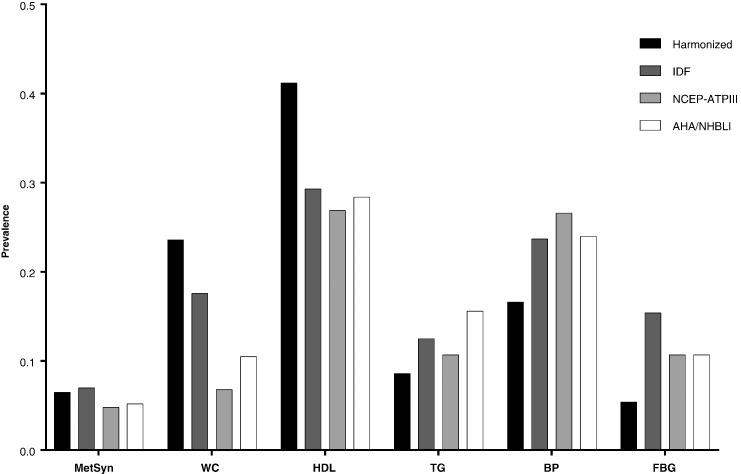
Overall MetSyn prevalence was 4.8% (NCEP-ATPIII, n = 333/6889) 5.2% (AHA/NHBLI, n = 643/12473), 7.0% (IDF, n = 971/13953) and 6.5% (harmonized, n = 430/6578). Atherogenic dyslipidaemia defined as low HDL was the most prevalent MetSyn component regardless of the criteria used (26.9–41.2%) followed by raised blood pressure (16.6–26.6%), abdominal obesity (6.8–23.6%), atherogenic dyslipidaemia defined as raised triglycerides (8.6–15.6%), and raised fasting glucose (2.8–15.4%) (Fig. 1).
Fig. 1.
Prevalence of metabolic syndrome and metabolic syndrome components in 26,609 young adults according to four metabolic syndrome criteria.
MetSyn – metabolic syndrome; WC – abdominal obesity; HDL - atherogenic dyslipidaemia (low HDL); TG - atherogenic dyslipidaemia (raised triglycerides); BP - raised blood pressure; FBG – raised fasting glucose.
4. Discussion
This study provides new information about MetSyn in young adults in two important ways. First, pooled analysis of a large sample suggests that 5–7% of young adults have MetSyn. While the prevalence is less than the IDF estimated prevalence in all adults of 25% worldwide (O'Neill and O'Driscoll, 2015), the development of MetSyn early in adulthood can lead to an elevated lifetime burden of cardiovascular disease risk. Second, one third of all participants had at least one component of MetSyn with low HDL being the most prevalent component. This latter finding raises the possibility that low HDL may be a key marker identifying early pathology associated with the development of MetSyn.
Prevention of the development of the first MetSyn component may have significant public health benefits as the presence of one component is predictive of the development of MetSyn (Cheung et al., 2008). Low HDL cholesterol occurs primarily due to increased triglyceride formation reducing cholesterol content of the lipoprotein core (Eckel et al., 2004). Accordingly, it was expected that a higher prevalence of raised triglyceride levels would be observed in the current findings; however, this did not occur. We speculate that this could reflect currently unknown mechanisms regarding HDL metabolism or a triglyceride cut-off point not calibrated to changes in HDL levels in young adults. Regardless, while low HDL is not universally exhibited in all young adults with at least one MetSyn component, our findings demonstrate that low HDL is the most frequently exhibited MetSyn component regardless of MetSyn definition and may indicate the initiation of pathphysiological processes that underpin the development of MetSyn for many young adults. Further research should be undertaken to identify why low-HDL is the most common component of MetSyn in young adults.
MetSyn component prevalence was lower than reported for European adults from a more diverse and older aged population than the current study (Vishram et al., 2014). Approximately 45% of 19–39 year old adults had the BP component, 25% the WC component, 25% TG component, and 20% with HDL component of MetSyn (Vishram et al., 2014). Vishram et al. also report an increased prevalence of BP and WC in males with increased age with a peak prevalence of elevated TGs and reduced HDL in the 40–49 year age bracket with a subsequent decline in older age ranges (50–59, 60–78 years). A similar pattern was observed in females except TG prevalence increased with age and only HDL prevalence is decreased in ages above 40–49 years. Therefore, prevalence of MetSyn components in young adults is expected to increase up to the age of 50.
While overall MetSyn prevalence was similar between the four MetSyn definitions, a wide range of prevalence was present for each MetSyn component. Differences in WC prevalence can be partially explained by the use of ethnic specific thresholds in the harmonized and IDF definitions but the difference in HDL, BP, and FBG prevalence, cannot be explained by the definition. Therefore, it is possible that these observations indicate young adults from different ethnicities are more prone to develop different components of MetSyn. Therefore, a possibility worth exploring is that all MetSyn component thresholds may be ethnic specific and thus specific ethnic thresholds for each MetSyn component may need to be developed to accurately assess MetSyn. This is similar to current recommendations of using ethnic specific thresholds for WC.
5. Conclusion
MetSyn prevalence ranges from 5 to 7% in young adults. Low HDL is the most prevalent component of MetSyn in young adults and thus may also be the first detectable component of MetSyn in many young adults. Exploring the importance and significance of low HDL in young adults may have considerable public health benefit as interventions aimed at improving low HDL cholesterol levels could reduce future incidence of MetSyn and subsequent clinical disease.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None of the authors report any conflict of interests.
Contributor Information
Paul B. Nolan, Email: p.nolan@auckland.ac.nz.
Graeme Carrick-Ranson, Email: g.ranson@auckland.ac.nz.
James W. Stinear, Email: j.stinear@auckland.ac.nz.
Stacey A. Reading, Email: s.reading@auckland.ac.nz.
Lance C. Dalleck, Email: ldalleck@western.edu.
References
- Al Dhaheri A.S., Mohamad M.N., Jarrar A.H. In: A Cross-Sectional Study of the Prevalence of Metabolic Syndrome among Young Female Emirati Adults. Ahmad R., editor. 11(7) 2016. (PLoS One). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti K.G.M.M., Zimmet P., Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Alberti K.G., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Anon Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults E and T of HBC in A. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA, J. Am. Med. Assoc. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Bener A., Zirie M., Musallam M., Khader Y.S., Al-Hamaq A.O. a a. Prevalence of metabolic syndrome according to Adult Treatment Panel III and International Diabetes Federation criteria: a population-based study. Metab. Syndr. Relat. Disord. 2009;7(3):221–229. doi: 10.1089/met.2008.0077. [DOI] [PubMed] [Google Scholar]
- Bener A., Mohammad A.G., Ismail A.N., Zirie M., Abdullatef W.K., Al-Hamaq A.O.A.A. Gender and age-related differences in patients with the metabolic syndrome in a highly endogamous population. Bosn. J. Basic Med. Sci. 2010;10(3):210–217. doi: 10.17305/bjbms.2010.2687. http://www.scopus.com/inward/record.url?eid=2-s2.0-77957107541&partnerID=40&md5=170c0d2a38201ecf068a85bb6cee2a33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N.R., Ferguson T.S., Bennett F.I. High-sensitivity C-reactive protein is related to central obesity and the number of metabolic syndrome components in Jamaican young adults. Front Cardiovasc Med. 2014;1 doi: 10.3389/fcvm.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheserek M.J., Wu G.-R., Shen L.-Y., Shi Y.-H., Le G.-W. Disparities in the prevalence of metabolic syndrome and its components among university employees by age, gender and occupation. J. Clin. Diagn. Res. 2014;8(2):65–69. doi: 10.7860/JCDR/2014/6515.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung B.M.Y., Wat N.M.S., Tam S. Components of the metabolic syndrome predictive of its development: a 6-year longitudinal study in Hong Kong Chinese. Clin. Endocrinol. 2008;68(5):730–737. doi: 10.1111/j.1365-2265.2007.03110.x. [DOI] [PubMed] [Google Scholar]
- Costa F.F. da, Montenegro V.B., Lopes T.J.A., Costa E.C. Combinação de fatores de risco relacionados à síndrome metabólica em militares da Marinha do Brasil. Arq. Bras. Cardiol. 2011;97(6):485–492. doi: 10.1590/s0066-782x2011005000113. [DOI] [PubMed] [Google Scholar]
- Dalleck L.C., Kjelland E.M. The prevalence of metabolic syndrome and metabolic syndrome risk factors in college-aged students. Am. J. Health Promot. 2012;27(1) doi: 10.4278/ajhp.100415-QUAN-116. [DOI] [PubMed] [Google Scholar]
- Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2004;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Erem C., Hacihasanoglu A., Deger O. Prevalence of metabolic syndrome and associated risk factors among Turkish adults: Trabzon MetS study. Endocrine. 2008;33(1):9–20. doi: 10.1007/s12020-008-9044-3. [DOI] [PubMed] [Google Scholar]
- Ferguson T.S., Tulloch-Reid M.K., Younger N.O. Prevalence of the metabolic syndrome and its components in relation to socioeconomic status among Jamaican young adults: a cross-sectional study. BMC Public Health. 2010;10(1):307. doi: 10.1186/1471-2458-10-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J., Lofgren I. Prevalence of metabolic syndrome and individual criteria in college students. J. Am. Coll. Heal. 2011;59(4):313–321. doi: 10.1080/07448481.2010.508084. [DOI] [PubMed] [Google Scholar]
- Gavrila D., Salmerón D., Egea-Caparrós J.-M. Prevalence of metabolic syndrome in Murcia Region, a southern European Mediterranean area with low cardiovascular risk and high obesity. BMC Public Health. 2011;11(1):562. doi: 10.1186/1471-2458-11-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112(17) doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Gündogan K., Bayram F., Capak M. Prevalence of metabolic syndrome in the mediterranean region of Turkey: evaluation of hypertension, diabetes mellitus, obesity, and dyslipidemia. Metab. Syndr. Relat. Disord. 2009;7(5):427–434. doi: 10.1089/met.2008.0068. [DOI] [PubMed] [Google Scholar]
- Gupta R., Misra A., Vikram N.K. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc. Disord. 2009;9 doi: 10.1186/1471-2261-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildrum B., Mykletun A., Hole T. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7(1):220. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.T.-K., Kempf A.M., Strother M.L. Overweight and components of the metabolic syndrome in college students. Diabetes Care. 2004;27(12):3000–3001. doi: 10.2337/diacare.27.12.3000. [DOI] [PubMed] [Google Scholar]
- Huang T.T.-K., Shimel A., Lee R.E., Delancey W., Strother M.L. Metabolic risks among college students: prevalence and gender differences. Metab. Syndr. Relat. Disord. 2007;5(4):365–372. doi: 10.1089/met.2007.0021. [DOI] [PubMed] [Google Scholar]
- Huang C.-Y., Chang H.-H., Lu C.-W., Tseng F.-Y., Lee L.-T., Huang K.-C. Vitamin D status and risk of metabolic syndrome among non-diabetic young adults. Clin. Nutr. 2015;34(3):484–489. doi: 10.1016/j.clnu.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Kaduka L.U., Kombe Y., Kenya E. Prevalence of metabolic syndrome among an urban population in Kenya. Diabetes Care. 2012;35(4):887–893. doi: 10.2337/dc11-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanitkar S.A., Kalyan M., Diggikar P. Metabolic syndrome in medical students. J. Int. Med. Sci. Acad. 2015;28(1):14–15. http://www.scopus.com/inward/record.url?eid=2-s2.0-84931038720&partnerID=40&md5=6ce8860447410513c0207492e062ee73 [Google Scholar]
- Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014:1–21. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- de Kroon M.L. a, Renders C.M., Kuipers E.C.C. Identifying metabolic syndrome without blood tests in young adults—the Terneuzen Birth Cohort. Eur. J. Pub. Health. 2008;18(6):656–660. doi: 10.1093/eurpub/ckn056. [DOI] [PubMed] [Google Scholar]
- Li G., de Courten M., Jiao S., Wang Y. Prevalence and characteristics of the metabolic syndrome among adults in Beijing, China. Asia Pac. J. Clin. Nutr. 2010;19(1):98–102. http://www.scopus.com/inward/record.url?eid=2-s2.0-77952316082&partnerID=40&md5=c3cce8c2c5c0d2c166de8b35a51f7f6d [PubMed] [Google Scholar]
- Lin K.P., Liang T.L., Liao I.C., Tsay S.L. Associations among depression, obesity, and metabolic syndrome in young adult females. Biol. Res. Nurs. 2014;16(3):327–334. doi: 10.1177/1099800413500138. [DOI] [PubMed] [Google Scholar]
- Manjunath D., Uthappa C.K., Kattula S.R., Allam R.R., Chava N., Oruganti G. Metabolic syndrome among urban Indian young adults: prevalence and associated risk factors. Metab. Syndr. Relat. Disord. 2014;12(7):381–389. doi: 10.1089/met.2014.0003. [DOI] [PubMed] [Google Scholar]
- Martins M.L.B., Kac G., Silva R.A. Dairy consumption is associated with a lower prevalence of metabolic syndrome among young adults from Ribeirão Preto, Brazil. Nutrition. 2015;31(5):716–721. doi: 10.1016/j.nut.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Mikkola I., Keinänen-Kiukaanniemi S., Laakso M. Metabolic syndrome in connection with BMI in young Finnish male adults. Diabetes Res. Clin. Pract. 2007;76(3):404–409. doi: 10.1016/j.diabres.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Morrell J.S., Lofgren I.E., Burke J.D., Reilly R.A. Metabolic syndrome, obesity, and related risk factors among college men and women. J. Am. Coll. Heal. 2012;60(1):82–89. doi: 10.1080/07448481.2011.582208. [DOI] [PubMed] [Google Scholar]
- Morrell J.S., Cook S.B., Carey G.B. Cardiovascular fitness, activity, and metabolic syndrome among college men and women. Metab. Syndr. Relat. Disord. 2013;11(5):370–376. doi: 10.1089/met.2013.0011. [DOI] [PubMed] [Google Scholar]
- O'Neill S., O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- Shahbazian H., Latifi S.M., Jalali M.T. Metabolic syndrome and its correlated factors in an urban population in South West of Iran. J. Diabetes Metab. Disord. 2013;12(1) doi: 10.1186/2251-6581-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi F., Mousavinasab S.N., Saeini M., Dinmohammadi M. Prevalence of metabolic syndrome in an adult urban population of the west of Iran. Exp. Diabetes Res. 2009;2009:136501. doi: 10.1155/2009/136501. http://www.scopus.com/inward/record.url?eid=2-s2.0-74049095614&partnerID=40&md5=f9a269443bafd456e2cf26bf7e8244cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenkov O., Nilssen O., Brenn T., Martiushov S., Arkhipovsky V.L., Grjibovski A.M. Prevalence of the metabolic syndrome and its components in Northwest Russia: the Arkhangelsk study. BMC Public Health. 2010;10 doi: 10.1186/1471-2458-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira V.M.F. da, Horta B.L., Gigante D.P., Azevedo Junior M.R. Metabolic syndrome in the 1982 Pelotas cohort: effect of contemporary lifestyle and socioeconomic status. Arq. Bras. Endocrinol. Metabol. 2010;54(4):390–397. doi: 10.1590/s0004-27302010000400008. [DOI] [PubMed] [Google Scholar]
- Sinha S., Misra P., Kant S., Krishnan A., Nongkynrih B., Vikram N.K. Prevalence of metabolic syndrome and its selected determinants among urban adult women in South Delhi, India. Postgrad. Med. J. 2013;89(1048):68–72. doi: 10.1136/postgradmedj-2012-130851. [DOI] [PubMed] [Google Scholar]
- Soysal A., Demiral Y., Soysal D., Uçku R., Köseoğlu M., Aksakoğlu G. The prevalence of metabolic syndrome among young adults in Izmir, Turkey. Anadolu Kardiyol. Derg. 2005;5(3):196–201. http://www.ncbi.nlm.nih.gov/pubmed/16140651 [PubMed] [Google Scholar]
- Sy R.G., Llanes E.J.B., Reganit P.F.M. Socio-demographic factors and the prevalence of metabolic syndrome among Filipinos from the LIFECARE cohort. J. Atheroscler. Thromb. 2014;21(Suppl. 1):S9–S17. doi: 10.5551/jat.21_sup.1-s9. http://www.scopus.com/inward/record.url?eid=2-s2.0-84893136447&partnerID=40&md5=ca7c6446b5076198df77e0365826e864 [DOI] [PubMed] [Google Scholar]
- Tope A.M., Rogers P.F., Topè A.M., Rogers P.F. Metabolic syndrome among students attending a historically black college: prevalence and gender differences. Diabetol. Metab. Syndr. 2013;5(1):2. doi: 10.1186/1758-5996-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishram J.K.K., Borglykke A., Andreasen A.H. In: Impact of Age and Gender on the Prevalence and Prognostic Importance of the Metabolic Syndrome and Its Components in Europeans. The MORGAM Prospective Cohort Project. Lazzeri C., editor. 9(9) 2014. (PLoS One). [DOI] [PMC free article] [PubMed] [Google Scholar]