Abstract
Objective
Rikkunshito, an herbal medicine, is widely prescribed in Japan for the treatment of anorexia and functional dyspepsia, and has been reported to recover reductions in food intake caused by cisplatin. We investigated whether rikkunshito could improve chemotherapy-induced nausea and vomiting (CINV) and anorexia in patients treated with cisplatin.
Methods
Patients with uterine cervical or corpus cancer who were to receive cisplatin (50 mg/m2 day 1) and paclitaxel (135 mg/m2 day 0) as first-line chemotherapy were randomly assigned to the rikkunshito group receiving oral administration on days 0–13 with standard antiemetics, or the control group receiving antiemetics only. The primary endpoint was the rate of complete control (CC: no emesis, no rescue medication, and no significant nausea) in the overall phase (0–120 hours). Two-tailed p<0.20 was considered significant in the planned analysis.
Results
The CC rate in the overall phase was significantly higher in the rikkunshito group than in the control group (57.9% vs. 35.3%, p=0.175), as were the secondary endpoints: the CC rate in the delayed phase (24–120 hours), and the complete response (CR) rates (no emesis and no rescue medication) in the overall and delayed phases (63.2% vs. 35.3%, p=0.095; 84.2% vs. 52.9%, p=0.042; 84.2% vs. 52.9%, p=0.042, respectively), and time to treatment failure (p=0.059). Appetite assessed by visual analogue scale (VAS) appeared to be superior in the rikkunshito group from day 2 through day 6.
Conclusion
Rikkunshito provided additive effect for the prevention of CINV and anorexia.
Keywords: Anorexia, Antiemetics, Nausea, Vomiting, Rikkunshito
INTRODUCTION
International guidelines for the treatment of chemotherapy-induced nausea and vomiting (CINV) have recommended the use of serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist and neurokinin-1 (NK-1) receptor antagonist and corticosteroids in patients receiving highly emetogenic chemotherapy such as cisplatin [1,2]. However, the effect of this treatment is still unsatisfactory, because the complete response rate (CR: no emesis, no rescue medication) and complete control rate (CC: CR plus no significant nausea) are reported to be only 40%–75%, and anorexia is observed about 15% of patients [3,4,5]. In addition, food intake has been reported to decrease to 25% of baseline by 7 days after chemotherapy that included cisplatin [6]. Therefore, more effective treatment for CINV and anorexia is required.
Rikkunshito, a Japanese traditional herbal medicine known as Kampo [7], is composed of 8 herbs (Atractylodis lanceae rhizoma, Ginseng radix, Pinelliae tuber, Hoelen, Zizyphi fructus, Aurantii nobilis pericarpium, Glycyrrhizae radix, and Zingiberis rhizome). It is widely prescribed in Japan to treat gastrointestinal disorders such as anorexia, functional dyspepsia, and gastroesophageal reflux disease [8,9,10,11]. It has been demonstrated in mice that rikkunshito ameriolates the cisplatin-induced anorexia, and flavonoids in rikkunshito antagonized 5-HT2B and 5-HT2C receptors [12].
We, therefore, designed an exploratory trial to evaluate the additive effect of rikkunshito on CINV and anorexia in patients treated with cisplatin and paclitaxel.
MATERIALS AND METHODS
1. Study design
This study was a multicenter, randomized phase 2 study conducted at four institutions in Hokkaido, Japan. The protocol was reviewed and approved by the Japanese Organisation for Research and Treatment of Cancer (JORTC) Protocol Review Committee and the ethical review committee of each hospital. This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry as UMIN000011227.
Patients with histologically diagnosed uterine cervical or corpus cancer were eligible for inclusion if they were ≥20 years, no history of chemotherapy, and planned to receive cisplatin plus paclitaxel. They were also required to have the Eastern Cooperative Oncology Group (ECOG) performance status grade 0–2. Patients were not eligible if they suffered from brain metastasis, seizure, unconsciousness, gastrointestinal obstruction, vomiting, or nausea greater than grade 2 as described by the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0, or if they had undergone treatment within one month before enrollment with steroids, androgens, progesterones, other herbal medicines, other medicines with the potential to increase appetite, or opioids. Patients were not allowed to receive progesterones after day 0.
Enrollment and randomization were processed at the central registration center. Patients were randomized by the minimization method balancing the arms with institution and treatment (adjuvant vs. neo-adjuvant chemotherapy vs. chemotherapy only).
2. Treatment
Patients were randomly assigned to the rikkunshito group or the control group. All patients received chemotherapy with intravenous (IV) paclitaxel 135 mg/m2 for 3 or 24 hours on day 0, and IV cisplatin 50 mg/m2 for 2 hours on day 1. They also received standard antiemetic therapy according to the Japanese antiemetic guideline of the Japan Society of Clinical Oncology [13]: IV granisetron 3 mg on days 0 and 1; oral aprepitant 125 mg on day 1 and 80 mg on days 2 and 3; and IV dexamethasone 9.9 mg on day 1 and 6.6 mg on days 2–4. The rikkunshito group received oral rikkunshito 7.5 g on days 0–13. It was permitted to administer paclitaxel 135 mg/m2 or 175 mg/m2 on day 1, in which case it was infused for 3 hours, and granisetron was administered only on day 1.
Rescue medication was allowed to treat refractory and persistent CINV; however, these treatments were considered treatment failure. The timing and choice of rescue treatment was decided by the investigator.
3. Assessments
Each patient fulfilled a diary from day 0 to day 13. The diary captured information about the timing of each emetic episode, while the severity of nausea and degree of appetite were evaluated by the patient everyday with a 100-mm visual analogue scale (VAS). The left end of the VAS (0 mm) was labeled as “no nausea”, and the right end (100 mm) was labeled as “nausea as bad as it could be.” A VAS score of <5 mm was considered to indicate no nausea, and that of 5–25 mm as no significant nausea. Concomitant medications including rescue medication were referred to the medical records.
The primary efficacy endpoint was the CC rate (no emesis, no rescue medication, no significant nausea) during the overall phase (0–120 hours). Secondary endpoints were the CR rate (no emesis, no rescue medication), total control rate (no emesis, no rescue medication, and no nausea), and time to treatment failure (i.e., time to the first emetic episode or time to rescue medication) during the overall phase, the CC rate and CR rate during the acute phase (0–24 hours) and delayed phase (24–120 hours), and the VAS score from day 0 through day 13. In addition, we assessed the patient's quality of life (QOL) using the Quality of Life Questionnaire (QLQ)-C30 of The European Organisation for Research for Treatment of Cancer (EORTC), and their anorexia-cachexia score (ACS) using The Functional Assessment of Anorexia/Cachexia Therapy (FAACT) on days 0, 6, and 13. We also measured serum levels of acyl ghrelin on days 0, 1, 2, 5, and 14 using an Active Ghrelin ELISA Kit (LSI Medience, Tokyo, Japan). All adverse events (AEs) and laboratory tests were graded according to the CTCAE (version 4.0) of the National Cancer Institute.
4. Statistical analysis
To calculate sample size, we assumed an overall CC rate of 75% in the rikkunshito group, and 50% in the control group. A sample size of 20 evaluable patients in each group was thus needed to ensure 60% power with a significance level of 0.2 (2-tailed) for comparison. For the primary endpoint, superiority of the rikkunshito group vs. the control group was evaluated using χ2 tests, with 2-tailed p<0.200 considered to indicate a significant difference in the planned analysis. We set a significant level relatively lenient as 0.20 by considering the exploratory nature and feasibility of this study. Point estimates and 80% confidence interval (CI) of each group are calculated respectively. The difference between the groups in time to treatment failure was analyzed using Kaplan-Meier estimates and the log-rank test. Analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
1. Patients
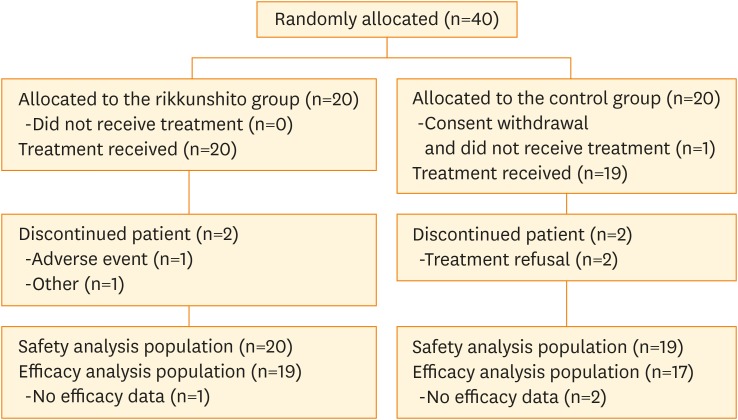
Forty patients were enrolled from 4 institutions from July 2013 to December 2015; 36 patients (19 in the rikkunshito group, 17 in the control group) were included in the efficacy analysis (Fig. 1), and 39 patients (20 in the rikkunshito group, 19 in the control group) were included in the safety analysis. Baseline characteristics of enrolled subjects are shown in Table 1. Although the median age in the rikkunshito group was slightly higher than that of the control group, other baseline characteristics were comparable between the groups.
Fig. 1.
CONSORT diagram.
CONSORT, Consolidated Standards of Reporting Trials.
Table 1. Patient baseline and disease characteristics.
| Characteristics | Rikkunshito group (n=20) | Control group (n=20) | |
|---|---|---|---|
| Age (yr) | 51.5 (14.0) | 43.1 (11.1) | |
| Weight (kg) | 56.0 (10.9) | 53.7 (7.9) | |
| ECOG PS | |||
| 0 | 19 (95.0) | 20 (100.0) | |
| 1 | 1 (5.0) | 0 (0.0) | |
| Cancer type | |||
| Corpus | 3 (15.0) | 2 (10.0) | |
| Cervical | 17 (85.0) | 18 (90.0) | |
| No distant metastasis | 17 (85.0) | 17 (85.0) | |
| No ascites | 17 (85.0) | 18 (90.0) | |
| Chemotherapy | |||
| Preoperative | 9 (45.0) | 10 (50.0) | |
| Postoperative | 9 (45.0) | 8 (40.0) | |
| No operation plan | 2 (10.0) | 2 (10.0) | |
| Grade of nausea | |||
| 0 | 20 (100) | 20 (100) | |
| 1 | 0 | 0 | |
Data are presented as mean (SD) or number of patients (%).
ECOG, Eastern Cooperative Oncology Group; PS, performance status; SD, standard deviation.
2. Efficacy
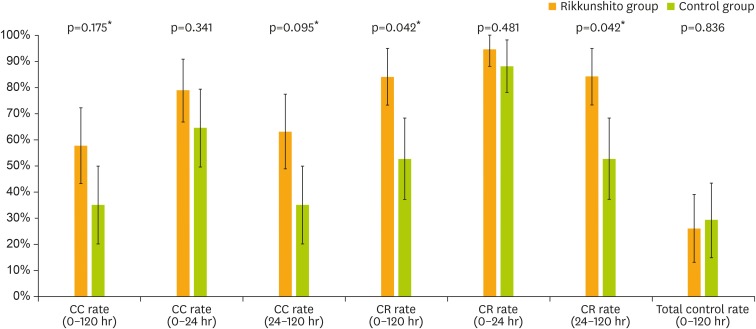
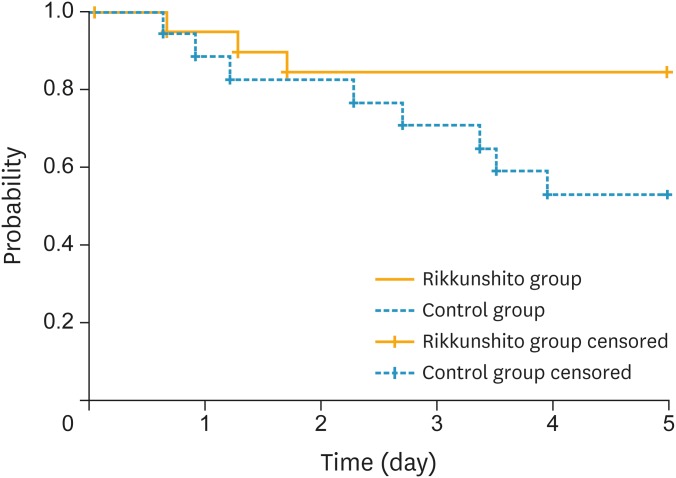
The primary endpoint, CC rate during the overall phase, was superior in the rikkunshito group compared to the control group (57.9% [80% CI=43.4–72.4] vs. 35.3% [80% CI=20.4–50.2]; p=0.175) (Fig. 2). The CR rate during the overall phase was also superior in the rikkunshito group (84.2% [80% CI=73.5–94.9] vs. 52.9% [80% CI=37.4–68.5]; p=0.042). In addition, CC and CR rates in the delayed phase were superior in the rikkunshito group (63.2% [80% CI=49.0–77.3] vs. 35.3% [80% CI=20.4–50.2]; p=0.095, and 84.2% [80% CI=73.5–94.9] vs. 52.9% [80% CI=37.4–68.5]; p=0.042, respectively). However, the CC and CR rates in the acute phase and the total control rate in the overall phase did not differ significantly between the groups. The time to treatment failure was significantly longer in the rikkunshito group than in the control group (p=0.059, Fig. 3).
Fig. 2.
Proportion of subjects with primary and secondary endpoints. The primary endpoint was CC rate in the overall phase (0–120 hours), while others were included in the secondary endpoints. Data are presented as point estimates and 80% CI.
CC, complete control; CI, confidence interval; CR, complete response.
*p<0.200 from 2-tailed χ2 test vs. control group.
Fig. 3.
Kaplan-Meier plot of time to treatment failure (i.e., to first emesis or use of rescue medication).
p=0.059 (log-rank test).
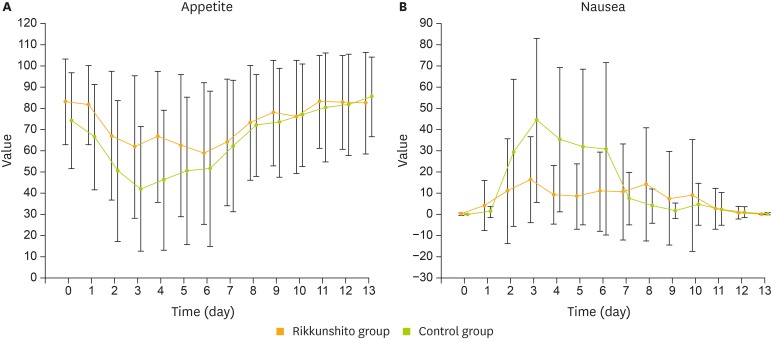
Appetite and nausea, as assessed by VAS, appeared to be superior in the rikkunshito group from day 2 through day 6, and were comparable between the groups from day 7 through day 13 (Fig. 4). Changes in serum acyl ghrelin levels were not observed after cisplatin administration in the control group; rikkunshito appeared to have no influence on serum acyl ghrelin levels (Supplementary Table 1).
Fig. 4.
VAS results for degree of appetite (A) and severity of nausea (B).
VAS, visual analogue scale.
The other secondary endpoints, EORTC QLQ-C30 score and FAACT ACS, did not differ significantly between the groups, although the QLQ-C30 score for nausea and vomiting appeared to be superior on day 6 in the rikkunshito group (treatment difference 9.3 points, Supplementary Table 2).
3. Safety
AEs were reported by 95% of subjects, and this was similar to a population receiving cisplatin (Table 2). The most frequent all-grade AEs for the rikkunshito and control groups were a decrease in neutrophil count, hypoalbuminemia, and nausea. Most AEs were grade 1–2, and a decrease in neutrophil count was the most frequent AE above grade 3 in both the rikkunshito and control groups. AEs were generally comparable between the groups, although the ≥grade 3 AEs of increases in alanine aminotransferase (ALT, 2 cases), aspartate aminotransferase (AST, 1 case), and gamma-glutamyl transferase (GGT, 1 case) were observed only in the rikkunshito group.
Table 2. Summary of AEs.
| Events | Rikkunshito group (n=20) | Control group (n=19) | ||
|---|---|---|---|---|
| All | ≥Grade 3 | All | ≥Grade 3 | |
| Neutrophil count decreased | 15 (75.0) | 8 (40.0) | 9 (47.4) | 8 (42.1) |
| Hypoalbuminemia | 15 (75.0) | 0 (0.0) | 14 (73.7) | 0 (0.0) |
| Nausea | 13 (65.0) | 0 (0.0) | 13 (68.4) | 1 (5.3) |
| ALT increased | 12 (60.0) | 2 (10.0) | 11 (57.9) | 0 (0.0) |
| WBC decreased | 11 (55.0) | 2 (10.0) | 6 (31.6) | 3 (15.8) |
| Hyponatremia | 11 (55.0) | 0 (0.0) | 11 (57.9) | 0 (0.0) |
| Anorexia | 7 (35.0) | 0 (0.0) | 8 (42.1) | 0 (0.0) |
| Hypocalcemia | 6 (30.0) | 0 (0.0) | 7 (36.8) | 0 (0.0) |
| Malaise | 6 (30.0) | 0 (0.0) | 5 (26.3) | 0 (0.0) |
| Constipation | 5 (25.0) | 0 (0.0) | 4 (21.1) | 0 (0.0) |
| AST increased | 4 (20.0) | 1 (5.0) | 5 (26.3) | 0 (0.0) |
| Arthralgia | 4 (20.0) | 0 (0.0) | 4 (21.1) | 0 (0.0) |
| ALP increased | 3 (15.0) | 0 (0.0) | 3 (15.8) | 0 (0.0) |
| Diarrhea | 3 (15.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
| Creatinine increased | 2 (10.0) | 0 (0.0) | 3 (15.8) | 0 (0.0) |
| Vomiting | 2 (10.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
| Myalgia | 2 (10.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
| Back pain | 2 (10.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Platelet count decreased | 2 (10.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Oral mucositis | 2 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| GGT increased | 1 (5.0) | 1 (5.0) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 1 (5.0) | 0 (0.0) | 3 (15.8) | 0 (0.0) |
| Headache | 1 (5.0) | 0 (0.0) | 3 (15.8) | 0 (0.0) |
| Bloating | 1 (5.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
| Dysgeusia | 1 (5.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
| Hypokalemia | 1 (5.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Cheilitis | 1 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fever | 1 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dizziness | 1 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypercalcemia | 1 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Allergic reaction | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Hemoglobin increased | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Tinnitus | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Hyperkalemia | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
Values are presented as number of patients (%).
AEs, adverse events; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; WBC, white blood cell.
DISCUSSION
In this exploratory study, we investigated the efficacy of rikkunshito on CINV and anorexia in patients treated with cisplatin and paclitaxel. As we planned that p<0.20 is significant in our primary analysis, CC rate during the overall phase was considered superior in the rikkunshito group compared with the control group. The rikkunshito group also showed superior results for the CC rate in the delayed phase, the CR rate in the overall and delayed phases, time to treatment failure, and the VAS scores for appetite and nausea from day 2 through day 6.
Although 5-HT3 receptor antagonist and NK-1 receptor antagonist are widely used for the prevention of CINV, it has been reported that other 5-HT receptors are also involved in anorexia; in animal experiments, agonists for 5-HT2B and 5-HT2C decreased the food intake [14,15]. Indeed, through animal experiments, it has been demonstrated that rikkunshito recovers the decrease in food intake and plasma ghrelin levels caused after cisplatin treatment, and that flavonoids in rikkunshito antagonizes the receptors for 5-HT2B and 5-HT2C [12]. Furthermore, it has been demonstrated that cisplatin suppresses the ghrelin receptor expression and ghrelin secretion in the hypothalamus through the 5-HT2C receptor, and that rikkunshito attenuates this effect [16,17]. In addition, it has also been demonstrated that rikkunshito antagonizes the 5-HT3 receptor [18]. Rikkunshito may therefore have improved CINV and anorexia through an effect on these pathways.
The effect of rikkunshito on CINV and anorexia has been investigated in several clinical trials. For example, Ohno et al. [19] reported the results of a cross-over trial of rikkunshito in gastric cancer patients who received cisplatin and S-1: rikkunshito ameliorated the decrease in plasma acyl ghrelin levels and increased food intake, and decreased the degree of anorexia after chemotherapy. Seike et al. [20] also investigated the efficacy of rikkunshito in esophageal cancer patients who received cisplatin, docetaxel and 5-fluorouracil (5-FU), and showed that rikkunshito improved the CTCAE grade of nausea on day 14 and the QOL score. In the present study, serum acyl ghrelin level was not decreased after cisplatin administration in the control group, and there was great variability among the patients with no significant difference between the groups. Thus, it is possible that serum acyl ghrelin levels had already recovered on the next day after cisplatin administration, or that rikkunshito may potentiate ghrelin receptor signaling [21].
In this study, patients formed a highly homogeneous population; all were women, all received the same amount of cisplatin and paclitaxel, all received the same antiemetics, and the vast majority had cervical cancer. Although the CC and CR rates in our control group were relatively low compared with other studies [3,4,22,23], it has been shown that CINV is worse in women and in younger patients [24,25].
This study had limitations: the sample size was small and the study was conducted in an unblind manner. A further confirmatory study using placebo with a larger sample size is required.
In conclusion, rikkunshito significantly improved CINV and anorexia in patients who were receiving cisplatin and paclitaxel. Based on these findings, we are planning a randomized, double-blind confirmatory trial of rikkunshito for the prevention of CINV and anorexia, especially during the delayed phase.
ACKNOWLEDGMENTS
We thank the patients, their family and clinical investigators participated in this study. We also thank Japanese Organisation for Research and Treatment of Cancer (JORTC) Protocol Review Committee and Independent Data Monitoring Committee for reviewing our protocol and third party monitoring throughout the trial.
Footnotes
Funding: This work was supported by the Third-term Comprehensive 10-year Strategy for Cancer Control (H22-General-035) from the Ministry of Health, Labour and Welfare, Japan, and by the Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and Development (AMED).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
This work was presented at the European Society for Medical Oncology 2016 (Copenhagen, October 2016).
- Conceptualization: O.S., W.H., K.H., Y.T., U.Y., I.S.
- Data curation: K.M., O.Y., T.S., M.T., N.E., K.H., S.T.
- Formal analysis: O.S., Y.T.
- Funding acquisition: U.Y., I.S.
- Methodology: O.S., W.H., K.H., Y.T., U.Y., I.S.
- Project administration: M.T.
- Supervision: A.M., S.N., U.Y., I.S.
- Writing - original draft: O.S.
- Writing - review & editing: W.H.
Supplementary Materials
Serum acyl ghrelin levels
Comparison between groups for EORTC QLQ-C30 and FAACT ACS scores
References
- 1.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Somerfield MR, Lyman GH. Antiemetic use in oncology: updated guideline recommendations from ASCO. Am Soc Clin Oncol Educ Book. 2012:532–540. doi: 10.14694/EdBook_AM.2012.32.230. [DOI] [PubMed] [Google Scholar]
- 2.Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 3.Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000–1006. doi: 10.1093/annonc/mdl019. [DOI] [PubMed] [Google Scholar]
- 4.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 6.Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer. 2012;118:4785–4794. doi: 10.1002/cncr.27430. [DOI] [PubMed] [Google Scholar]
- 7.Fuyuno I. Japan: Will the sun set on Kampo? Nature. 2011;480:S96. doi: 10.1038/480S96a. [DOI] [PubMed] [Google Scholar]
- 8.Tatsuta M, Iishi H. Effect of treatment with liu-jun-zi-tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther. 1993;7:459–462. doi: 10.1111/j.1365-2036.1993.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 9.Yagi M, Homma S, Kubota M, Iinuma Y, Kanada S, Kinoshita Y, et al. The herbal medicine rikkunshi-to stimulates and coordinates the gastric myoelectric activity in post-operative dyspeptic children after gastrointestinal surgery. Pediatr Surg Int. 2004;19:760–765. doi: 10.1007/s00383-003-1053-y. [DOI] [PubMed] [Google Scholar]
- 10.Mogami S, Hattori T. Beneficial effects of rikkunshito, a Japanese kampo medicine, on gastrointestinal dysfunction and anorexia in combination with Western drug: a systematic review. Evid Based Complement Alternat Med. 2014;2014:519035. doi: 10.1155/2014/519035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51:751–767. doi: 10.1007/s00535-016-1227-8. [DOI] [PubMed] [Google Scholar]
- 12.Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, et al. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–2013. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi H, Saeki T. An antiemetic guideline for patients with malignancies in Japan. Gan To Kagaku Ryoho. 2010;37:976–979. [PubMed] [Google Scholar]
- 14.De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24:341–353. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi A, Suzuki M, Sasamata M, Miyata K. Agonist diversity in 5-HT(2C) receptor-mediated weight control in rats. Psychopharmacology (Berl) 2005;178:241–249. doi: 10.1007/s00213-004-2019-z. [DOI] [PubMed] [Google Scholar]
- 16.Yakabi K, Kurosawa S, Tamai M, Yuzurihara M, Nahata M, Ohno S, et al. Rikkunshito and 5-HT2C receptor antagonist improve cisplatin-induced anorexia via hypothalamic ghrelin interaction. Regul Pept. 2010;161:97–105. doi: 10.1016/j.regpep.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, et al. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151:3773–3782. doi: 10.1210/en.2010-0061. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga K, Kido T, Ochi M, Sadakane C, Mase A, Okazaki H, et al. The traditional Japanese medicine rikkunshito promotes gastric emptying via the antagonistic action of the 5-HT(3) receptor pathway in rats. Evid Based Complement Alternat Med. 2011;2011:248481. doi: 10.1093/ecam/nep173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno T, Yanai M, Ando H, Toyomasu Y, Ogawa A, Morita H, et al. Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin Exp Gastroenterol. 2011;4:291–296. doi: 10.2147/CEG.S26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seike J, Sawada T, Kawakita N, Yamamoto Y, Yuasa Y, Yamai H, et al. A new candidate supporting drug, rikkunshito, for the QOL in advanced esophageal cancer patients with chemotherapy using docetaxel/5-FU/CDDP. Int J Surg Oncol. 2011;2011:715623. doi: 10.1155/2011/715623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1:e23. doi: 10.1038/tp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Cheng Y, Zhang H, Zhou C, Han B, Zhang Y, et al. Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer. 2014;22:979–987. doi: 10.1007/s00520-013-2043-9. [DOI] [PubMed] [Google Scholar]
- 23.Oyama K, Fushida S, Kaji M, Takeda T, Kinami S, Hirono Y, et al. Aprepitant plus granisetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients with gastric cancer treated with S-1 plus cisplatin. J Gastroenterol. 2013;48:1234–1241. doi: 10.1007/s00535-012-0746-1. [DOI] [PubMed] [Google Scholar]
- 24.Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107–117. doi: 10.1007/s00520-010-1073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol. 2015;26:1081–1090. doi: 10.1093/annonc/mdv138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum acyl ghrelin levels
Comparison between groups for EORTC QLQ-C30 and FAACT ACS scores