Abstract
Objective
To determine the clinical significance of the polymerase chain reaction (PCR)-reverse dot blot (RDB) human papillomavirus (HPV) genotyping assay in cervical cancer screening.
Methods
A total of 10,442 women attending the Fujian Provincial Maternity and Children's Health Hospital were evaluated using the liquid-based cytology (thinprep cytologic test [TCT]) and the PCR-RDB HPV test. Women with HPV infection and/or abnormal cytology were referred for colposcopy and biopsy. For HPV DNA sequencing, 120 specimens were randomly selected. Pathological diagnosis was used as the gold standard.
Results
Using the PCR-RDB HPV test, overall HPV prevalence was 20.57% (2,148/10,442) and that of high-risk (HR)-HPV infection was 18.68% (1,951/10,442). There was 99.2% concordance between HPV PCR-RDB testing and sequencing. In this studied population, the most common HR-HPV types were HPV-16, -52, -58, -18, -53, -33, and -51, rank from high to low. HPV-16, -18, -58, -59, and -33 were the top 5 prevalent genotypes in cervical cancer but HPV-16, -18, -59, -45, and -33 were the top 5 highest risk factors for cancer (odds ratio [OR]=34.964, 7.278, 6.728, 6.101, and 3.658; all p<0.05, respectively). Among 10,442 cases, 1,278 had abnormal cytology results, of which, the HR-HPV positivity rate was 83.02% (1,061/1,278). To screen for cervical cancer by PCR-RDB HPV testing, when using CIN2+, CIN3+, and cancer as observed endpoints, the sensitivity was 90.43%, 92.61%, and 94.78% and the negative predictive value (NPV) was 99.06%, 99.42%, and 99.78%, respectively. PCR-RDB HPV and TCT co-testing achieved the highest sensitivity and NPV.
Conclusion
For cervical cancer screening, the PCR-RDB HPV test can provide a reliable and sensitive clinical reference.
Keywords: Papillomaviridae, Genotype, Cell Biology, Histology, Cancer Screening
INTRODUCTION
Cervical cancer is the second most frequent cancer among women globally, especially in developing countries. It is estimated that there were roughly 527,624 newly diagnosed cervical cancer cases and 265,653 related deaths in 2012 [1]. Despite the successful implementation of cytopathological screening programs, every year approximately 11,000 women are diagnosed with cervical cancer and 3,900 die from this disease in the United States of America [2]. In China, the age-standardized incidence and mortality rate is 7.5 and 3.4 per 100,000 women, respectively [3].
More than 150 different genotypes of human papillomavirus (HPV) have been identified thus far, and there are approximately 40 known genital HPVs [4,5]. Genital HPV genotypes are categorized according to their epidemiological association and ability of causing genital cancers. Infection with low-risk HPV (LR-HPV) types, such as HPV-6 and -11, can cause early or low-grade changes in cervical epithelial cells. However, substantial evidence has confirmed that persistent infection with high-risk HPV (HR-HPV) is a major causative factor for malignant transformation of cervical epithelial cells and for the development of cervical intraepithelial neoplasia (CIN) and invasive cervical cancer [5,6,7]. Although it is well known that HPV-16 and -18 are the predominant HR types found in cervical cancer tissue worldwide, the prevalence of the other HR-HPV types show geographical and regional variation [8,9,10]. The prevalence of HR-HPV in Fujian province, which lies in the south and east of China and has a population of 39 million, is still not clear yet.
The first HPV test approved for use in cervical screening programs was the Qiagen® Hybrid Capture 2 (HC2) assay (QIAGEN Inc., Gaithersburg, MD, USA), which employs an RNA probe cocktail to induce a chemiluminescent reaction upon HPV DNA binding with any of the 13 HR types. HC2 generally has good clinical utility for the detection of high-grade premalignant disease graded CIN2 or CIN3 [11,12], but there have been concerns about its low specificity and positive predictive value (PPV). Several reports have indicated that it erroneously detects LR-HPV types [13,14,15] and may even cross-react with non-HPV DNA [16]. This is seen more in older women, where polymerase chain reaction (PCR) fails to detect HR-HPV in as many as 50% of HC2-positive samples [17]. In 2011, the Cobas® HPV test (Roche Molecular Systems Inc., Branchburg, NJ, USA), a PCR-based genotyping method, received Food and Drug Administration (FDA) approval for screening of cervical cancer [18]. Moreover, in 2014, the FDA approved the Cobas® HPV test (Roche Molecular Systems Inc.) for use as a first-line primary screening test for cervical cancer in women aged 25 and older [19]. In 2015 the Society of Gynecologic Oncology (SGO)/American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines addressed the use of HR-HPV testing alone as a primary screening approach, which also suggest the use of genotyping for HPV-16 and -18 as a way to triage HR-HPV positive women [20].
In this study, we evaluated the PCR-reverse dot blot (PCR-RDB) Yaneng® Human Papillomavirus Genotyping kit (Yaneng Biotech, Shenzhen, China) for the detection of 23 types, 18 HR, and 5 LR-HPV types. RDB assays have been used to detect certain genetic disorders on the basis of technical simplicity. It is a well-developed method that can genotype multiple single nucleotide polymorphisms (SNPs) simultaneously, and can be used for the diagnosis of homozygous, hemizygous, and heterozygous-deficient patients [20]. It has been successfully used for several genetic disorders, including thalassemia and G6PD deficiency [21,22,23,24]. The purpose of this study was to estimate the overall HPV prevalence, type- and age-specific prevalence, extent of multiple infections in a hospital-based population from Fujian province. Furthermore, the clinical performance characteristics of the Yaneng® PCR-RDB assay (Yaneng Biotech), including the sensitivity, specificity, PPV, and negative predictive value (NPV) for the detection of CIN2 and greater (CIN2+) cervical lesions was evaluated.
MATERIALS AND METHODS
1. Study population
All specimens were collected from women in the Fujian Provincial Maternity and Children's Health Hospital of Fujian Medical University (FMCH) by using plastic cervical swabs. The study was approved by the Hospital Ethics Committee of FMCH. The population eligible for this study included 10,442 women (age range: 18 to 82 years old), which involved 2 cohorts, one consisting of healthy patients undergoing routine physical examination, and another consisting of patients visiting the outpatient clinic for any gynecologic conditions. All women received PCR-RDB HPV genotyping and cytology screening by gynecological practitioners between July 2008 and August 2014. The population consisted of hospital staff, policewomen, teachers, workers, civil servants, and retirees. The participants were required to fulfill the following criteria: 1) sexually active women of age 18 years or older; 2) no previous histology of gynecological diseases; 3) have not participated in any previous cervical cancer screening project; and 4) willingness to undergo HPV testing. The study flowchart is shown in (Supplementary Fig. 1). All patients provided informed consent.
2. Cervical specimen collection
Cervical cells were collected from the cervical canal of all participants with plastic brushes and placed into 20-mL vials of ThinPrep® PreservCyt® solution (Hologic Inc., Waltham, MA, USA) for cytology and into 2-mL vials of preservation solution for HPV DNA testing. The samples for cytology were stored at 4℃. For HPV assays, 1 mL of the cells collected in the preservation solution was used to extract HPV DNA and stored at −20℃.
3. Liquid-based cytology
Cytological specimens were blinded and evaluated, independent from the results of the other assays by 2 experienced cytopathologists. If the diagnosis was different, the cervical samples were reviewed again and a consensus diagnosis was obtained. The results were evaluated using the Bethesda system. Samples were classified as: negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL), squamous cervical cancer (SCC), atypical glandular cells (AGC), and adenocarcinoma in situ (AIS).
4. PCR-RDB HPV genotyping for 23 types
The PCR-RDB HPV genotyping for 23 types (Yaneng Biotech) assay can identify 18 HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and 83) and 5 LR-HPV types (6, 11, 42, 43, and 81). The L1 consensus HPV PGMY09/PGMY11 primer set [10] was used to amplify 5 μL of the extracted HPV DNA or control (positive or negative) in the 24-μL reaction system. HPV was amplified in a thermal cycler under the following conditions: 50℃ for 15 minutes, 95℃ for 10 minutes, followed by denaturation at 94℃ for 10 seconds, annealing at 45℃ for 90 seconds, and extension at 72℃ for 30 seconds for a total of 40 cycles. After amplification, HPV genotyping was done by hybridization and RDB on the strips fixed with 23 different type-specific probes. The blue spots on the strip could be judged as positive by the naked eye.
5. Interpretation of HPV infection and HPV gene sequence analysis
The numbers on the strip represented the HPV genotypes, for example, “16” for HPV-16, and “IC” was the abbreviation for internal control (Supplementary Fig. 2A). A blue spot observed only on “IC” indicated no HPV infection or below the limit of detection (Supplementary Fig. 2B). If both “IC” and any one site of HPV types showed a blue-spot signal, the sample was considered positive specifically for a single HR-HPV infection (Supplementary Fig. 2C and D), while blue dot signals observed on multiple sites represented a multi-type HPV infection (Supplementary Fig. 2E and F). However, if the “IC” site and HPV genotype sites were negative, the test was considered invalid. Sixty cases that were HPV-positive by PCR-RDB and 60 cases that were HPV-negative were randomly chosen for gene sequencing by Sunbiotech Co., Ltd, Beijing, China.
6. Histology
Women who were HPV-positive and/or had an abnormal cytological result (with a grade higher than ASC-US) were referred for colposcopy and punch biopsy. Women with a punch biopsy diagnosis greater than HSIL received a loop electrosurgical excision procedure (LEEP) cone biopsy or conization by cold knife. Specimens were fixed in 10% formalin and routinely processed for paraffin embedding. Subsequently, 4-μm-thick histological sections were cut and stained with hematoxylin and eosin using the standard method. Cervical biopsy specimens were then histologically examined and classified according to the CIN system [16].
7. Statistical analysis
The performance characteristics of the screening tests were evaluated by calculating the sensitivity, specificity, PPV, and NPV according to the standard definitions for CIN2, CIN3, and invasive cervical cancer. All confidence intervals (CIs) were exact binomial CIs. Chi-square test, Fisher's exact test, and cumulative risk analysis were performed. In addition, an analysis exploring PCR-RDB test characteristics at different cut points was undertaken. These results were then plotted. All data analyses were performed using SPSS 17.0 (IBM, Chicago, IL, USA).
RESULTS
1. Consistency between PCR-RDB HPV test and HPV gene sequencing
There were 120 specimens that were chosen randomly to compare the result of PCR-RDB HPV genotyping and HPV gene sequencing (Supplementary Fig. 2G and H). Among them, only one case was not consistent with HPV-positive results, which was positive by PCR-RDB but negative in HPV DNA sequencing. Overall, there was 99.2% (119/120) concordance between the 2 methods.
2. Prevalence of HPV infection
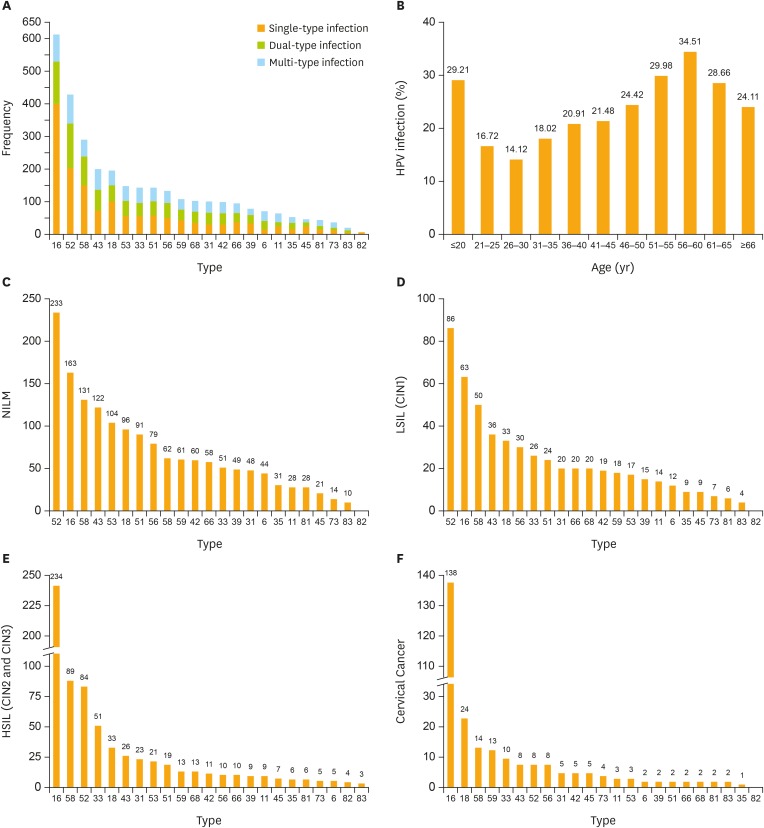
This study cohort consisted of 10,442 women who had undergone PCR-RDB HPV genotyping and cytology screening. The average age of participants in this study was 38.00±9.96 years old (range: 18 to 82 years old). All 23 HPV subtypes could be detected (Table 1, Fig. 1A), according to the results, the overall HPV infection rate in this population was 20.57% (2,148/10,442), and the positivity of HR-HPV was 18.68% (1,951/10,442). The positivity of single-type HPV infection was 13.88% (1,449/10,442), accounting for 67.46% of the positive population (1,449/2,148). The positivity of dual-type infection was 4.46% (466/10,442), accounting for 21.69% of the positive specimens (466/2,148). The HPV infection rates for different age groups were 29.21% (<20 years old), 16.72% (21–25 years old), 14.12% (26–30 years old), 18.02% (31–35 years old), 20.91% (36–40 years old), 21.48% (41–45 years old), 24.42% (46–50 years old), 29.98% (51–55 years old), 34.51% (56–60 years old), 28.66% (61–65 years old), and 24.11% (>65 years old) (Fig. 1B). The HPV infection rate showed a bi-modal trend, increasing with an increase in age, and the HPV infection rate of different age groups was statistically significant (χ2=272.740; p<0.001). The positivity of multi-type infection was 2.23% (233/10,442), accounting for 10.85% of the positive specimens (233/2,148). In the HPV infected population, HPV-16 was detected in 613 cases, accounting for 28.54% (613/2,148) of all positive specimens (including mixed infection) and 31.42% (613/1,951) of HR-HPV positive specimens. HPV-16 was the most prevalent genotype in the HPV-positive women, followed by HPV-52, -58, -43, -18, -53, -33, -51, -56, -59, -68, -31, -42, -66, -39, -6, -11, -35, -45, -81, -73, -83, and -82, from highest to lowest. The most prevalent HPV genotypes are variable in different population. The top 5 rates were HPV-52, -16, -58, -43, -53 in the NILM cases (Fig. 1C), and they are HPV-52, -16, -58, -43, -18 in the cases with pathologic diagnosis as LSIL (CIN1) (Fig. 1D). But they are HPV-16, -58, -52, -33, -18 in HSIL cases (CIN2 and CIN3) and HPV-16, -18, -58, -59, -33 in cervical cancer cases (Fig. 1E and F).
Table 1. HPV infection by age related distribution (n=10,442).
| Age (yr) | Negative (n=8,294) | Single-type HPV (n=1,449) | Dual-type HPV* (n=466) | Multi-type HPV† (n=233) | Total HPV infection (n=2,148)‡ |
|---|---|---|---|---|---|
| ≤20 (n=89) | 63 | 5 | 11 | 10 | 26 (29.21) |
| 21–25 (n=861) | 717 | 92 | 30 | 22 | 144 (16.72) |
| 26–30 (n=1,749) | 1,502 | 161 | 56 | 30 | 247 (14.12) |
| 31–35 (n=1,693) | 1,388 | 201 | 72 | 32 | 305 (18.02) |
| 36–40 (n=1,765) | 1,396 | 271 | 80 | 18 | 369 (20.91) |
| 41–45 (n=1,853) | 1,455 | 278 | 84 | 36 | 398 (21.48) |
| 46–50 (n=1,335) | 1,009 | 238 | 60 | 28 | 326 (24.42) |
| 51–55 (n=537) | 376 | 113 | 32 | 16 | 161 (29.98) |
| 56–60 (n=284) | 186 | 54 | 24 | 20 | 98 (34.51) |
| 61–65 (n=164) | 117 | 21 | 11 | 15 | 47 (28.66) |
| ≥66 (n=112) | 85 | 15 | 6 | 6 | 27 (24.11) |
Values are presented as number (%). Detected by PCR-RDB genotyping, 18 HR-HPV, and 5 LR-HPV subtypes could be analyzed in the population.
HPV, human papillomavirus; HR-HPV, high-risk human papillomavirus; LR-HPV, low-risk human papillomavirus; PCR-RDB, polymerase chain reaction-reverse dot blot.
*Dual-type infection: patients with 2 HPV subtypes infection simultaneously; †Multi-type infection: patients with 3 or more HPV subtypes infection simultaneously; ‡Represented the percent of HPV-positive patients in different age groups.
Fig. 1.
Prevalence of different HPV genotype infections. (A) HPV-16 is most prevalent genotype in HPV-positive women. (B) The HR-HPV infection rate showed a bimodal trend, and the rates of different age groups were statistically significant (χ2=272.740; p<0.001). Top 5 most frequent HPV genotypes in (C) NILM patients; (D) LSIL (CIN1) patients; (E) HSIL patients (CIN2 and CIN3); and (F) cervical cancer. Orange bar in (C-F) is the cumulative cases of HPV genotypes.
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
3. HPV infections in patients with different cytology results
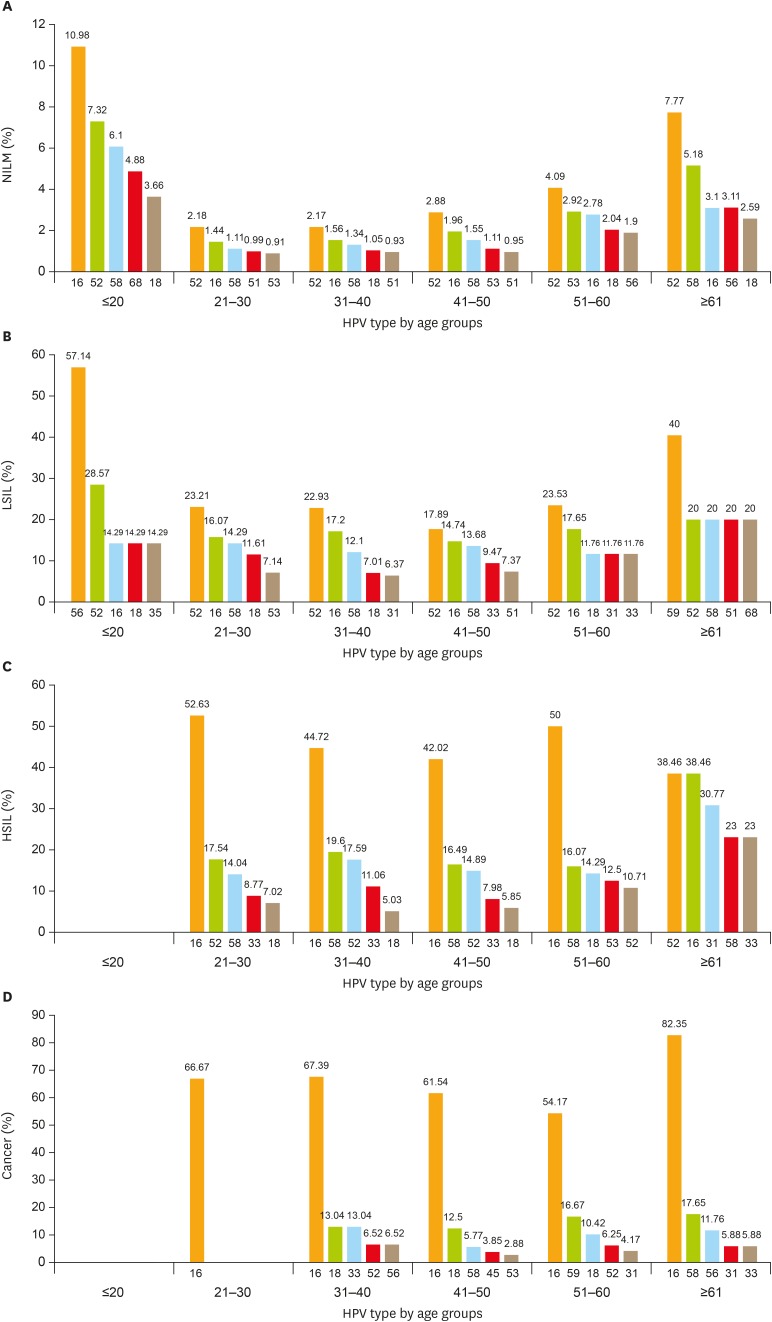
Out of 10,442 cases, cytological examination was normal in 87.76%. Abnormal cytology results were detected in 1,278 patients, including 5.62% (587/10,442) with ASC-US and ASC-H, 2.99% (312/10,442) with LSIL, 3.04% (317/10,442) with HSIL or cervical squamous carcinoma, and 0.59% (62/10,442) with AGC or cervical adenocarcinoma. Comparing the overall HR-HPV infection rate, the HR-HPV positivity rate was 11.86% in women with NILM and 83.02% in women with abnormal cytological results (χ2=2,022.140; p<0.010). HR-HPV prevalence was 74.45% in patients diagnosed with ASC-US, 90.71% in patients with LSIL, 94.64% in patients with HSIL and cervical squamous carcinoma, and 66.13% in patients with AGC and AIS, which were significantly higher than in the NILM population (all p<0.010). The top 5 most frequent HR-HPV types in different age groups were also analyzed in patients with different cervical lesion (Fig. 2).
Fig. 2.
Age-related distribution of HPV infection. Top 5 most frequent HPV genotypes in different age groups of (A) NILM patients; (B) LSIL (CIN1) patients; (C) HSIL patients (CIN2 and CIN3); and (D) cervical cancer.
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
4. HPV infections in patients with different cervical lesions
Overall, 8,077 patients were negative for both cytology and HPV infection, and 2,365 cases with either HR-HPV positive or abnormal cytology were referred for colposcopy and biopsy. The pathology result was used as a final diagnosis standard. The proportion of HPV infection was 12.41% (1,155/9,309) in patients with cervicitis or NILM, 84.30% (333/395) in patients with CIN1, 86.78% (197/227) in patients with CIN2, 89.73% (262/292) in patients with CIN3, and 91.78% (201/219) in patients with cervical cancer (Table 2). When comparing different cervical lesions, the difference in HPV infection rate was statistically significant (χ2=3,507.293; p<0.010). Additionally, the difference in the proportion of HPV subtypes was statistically significant (χ2=48.184; p<0.010).
Table 2. Compare HPV infection and TCT with pathological diagnosis (n=10,442).
| Subjects | Pathologic diagnosis | χ2* | p-value | χ2† | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NILM (n=9,309) | CIN1 (n=395) | CIN2 (n=227) | CIN3 (n=292) | Cancer (n=219) | |||||||
| TCT (n=10,442) | |||||||||||
| HPV-positive (n=2,148) | 1,155 | 333 | 197 | 262 | 201 | ||||||
| HPV-negative (n=8,294) | 8,154 | 62 | 30 | 30 | 18 | ||||||
| Cytological diagnosis | |||||||||||
| NILM (n=9,164) | 743.729 | 0.001 | 1,125.568 | 0.001 | |||||||
| HPV-positive (n=1,087) | 890 | 63 | 52 | 49 | 33 | ||||||
| HPV-negative (n=8,077) | 8,027 | 14 | 19 | 12 | 5 | ||||||
| ASC (n=587) | 22.125 | 0.001 | 38.252 | 0.001 | |||||||
| HPV-positive (n=437) | 168 | 142 | 41 | 57 | 29 | ||||||
| HPV-negative (n=150) | 99 | 36 | 6 | 6 | 3 | ||||||
| LSIL (n=312) | 0.584 | 0.540 | 3.103 | 0.524 | |||||||
| HPV-positive (n=283) | 68 | 118 | 47 | 39 | 11 | ||||||
| HPV-negative (n=29) | 6 | 11 | 4 | 5 | 3 | ||||||
| ≥HSIL (n=317)‡ | 10.688 | 0.011 | 10.741 | 0.020 | |||||||
| HPV-positive (n=300) | 10 | 4 | 53 | 111 | 122 | ||||||
| HPV-negative (n=17) | 4 | 0 | 1 | 7 | 5 | ||||||
| ≥AGC (n=62)§ | 5.866 | 0.019 | 9.103 | 0.038 | |||||||
| HPV-positive (n=41) | 19 | 6 | 4 | 6 | 6 | ||||||
| HPV-negative (n=21) | 18 | 1 | 0 | 0 | 2 | ||||||
ASC, atypical squamous cells; AGC, atypical glandular cells; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy; TCT, thinprep cytologic test.
*χ2: the difference of patients with CIN2+ between the HPV-positive and -negative groups; †χ2: the difference between the HPV-positive and -negative groups in patients with different pathologic diagnosis and cytological diagnosis; ‡≥HSIL: HSIL and cervical squamous carcinoma; §≥AGC: AGC and cervical adenocarcinoma.
In patients with abnormal cytology, the detection rate of CIN2+ lesions in the HPV-positive group was significantly higher than that in the HPV-negative group (49.6% [526/1,061] vs. 19.4% [42/217]; p<0.010) (Table 2). It was similar in patients with ASC (29.1% [127/437] vs. 10% [15/150]; p<0.010), ≥HSIL (95.3% [286/300] vs. 76.5% [13/17]; p<0.050), and AGC and AIS (39.0% [16/41] vs. 9.5% [2/21]; p<0.05). However, the difference was not significant in patients with LSIL (34.3% [97/283] vs. 41.3% [12/29]; p=0.540) (Table 2). In respect to different pathological changes, with an advance in the grade of lesion, the HR-HPV detection rate also increased, while there was no apparent change in the LR-HPV detection rate for these same categories (NILM, 23.14%; CIN1, 24.02%; CIN2, 14.72%; CIN3, 9.54%; and cervical cancer, 9.45%; p>0.050).
5. The cumulative risk of HR-HPV genotype for cervical cancer
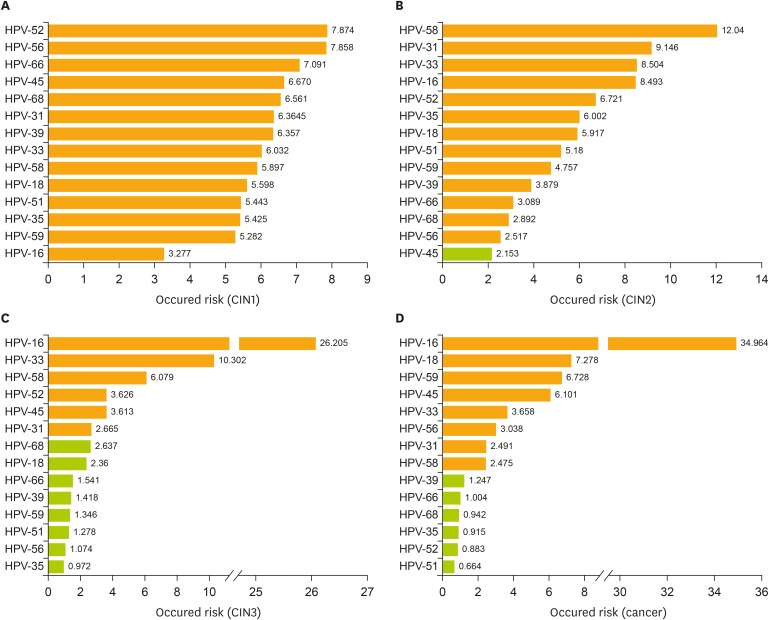
Infection with different HR-HPV subtypes showed a different risk for cervical lesions. In the detection of HPV subtypes, the detection rate of HPV-16 rose gradually along with an increase in the level of cervical lesions (χ2=1,332.268; p<0.05) and it is similarly for HPV-18 (χ2=758.834; p<0.05). Risk assessment of CIN1 showed the following: HPV-52 (odds ratio [OR]=7.874; 95% CI=6.059–10.234), HPV-56 (OR=9.146; 95% CI=5.166–11.952), HPV-66 (OR=7.091; 95% CI=4.284–11.737), HPV-45 (OR=6.670; 95% CI=3.184–13.973), HPV-68 (OR=6.561; 95% CI=3.979–10.189), and all p<0.05 (Fig. 3A). Risk assessment of CIN2 showed the following: HPV-58 (OR=12.04; 95% CI=8.598–16.869), HPV-31 (OR=9.146; 95% CI=5.267–15.879), HPV-33 (OR=8.504; 95% CI=5.244–13.791), HPV-16 (OR=8.493; 95% CI=6.344–11.369), HPV-52 (OR=6.721; 95% CI=4.799–9.414), and all p<0.05 (Fig. 3B). Risk assessment of CIN3 showed the following: HPV-16 (OR=26.205; 95% CI=20.544–33.830), HPV-33 (OR=10.302; 95% CI=6.735–15.637), HPV-58 (OR=6.079; 95% CI=4.266–8.675), HPV-52 (OR=3.626; 95% CI=2.522–5.173), and all p<0.05(Fig. 3C). Infection with HPV-16 was the most important risk factor for cervical cancer occurrence (OR=34.964; 95% CI=26.178–46.898), followed by HPV-18 (OR=7.278; 95% CI=4.640–12.418), HPV-59 (OR=6.728; 95% CI=3.708–12.207), HPV-45 (OR=6.101; 95% CI=2.381–15.631), HPV-33 (OR=3.658; 95% CI=1.896–7.058), and all p<0.05 (Fig. 3D).
Fig. 3.
Cumulative occurred risk of HR-HPV genotype for cervical lesions. Cumulative occurred risk of each HPV genotype: in patients with (A) a pathologically diagnosed CIN1; (B) CIN2; (C) CIN3; and (D) invasive cervical cancer.
Orange bar represents statistically significant (p<0.05) green bar represents not statistically significant (p>0.05).
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HR-HPV, high-risk human papillomavirus.
6. HPV, thinprep cytologic test (TCT), and TCT combined with HPV (co-testing) in screening for cervical cancer
The sensitivity, specificity, PPV, NPV, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were analyzed for different screening methods by HR-HPV, TCT, or co-testing (TCT+HR-HPV) when used to predict CIN2+, CIN3+, and cervical cancer as critical endpoints (Table 3). The results show that the sensitivity of co-testing and HR-HPV were higher than that of TCT. With the increase in cervical lesions, the sensitivity of the HPV, TCT, and co-testing rose, but the specificity was reduced.
Table 3. Compare cytology and HPV in the different degree of cervical lesion detection (n=10,442).
| Variable | Sensitivity | Specificity | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|
| CIN2+* | |||||||
| HPV | 90.43 (88.21–92.65) | 84.83 (84.12–85.55) | 31.02 (29.05–32.98) | 99.06 (98.85–99.27) | 5.8977 (5.5919–6.2202) | 0.1246 (0.1010–0.1537) | |
| TCT | 76.96 (73.93–80.00) | 92.73 (92.21–93.25) | 44.65 (41.92–47.39) | 98.14 (97.86–98.42) | 10.5837 (9.7569–11.4807) | 0.2484 (0.2177–0.2835) | |
| HPV+TCT | 95.26 (93.72–96.79) | 83.04 (82.29–83.79) | 29.98 (28.12–31.83) | 99.57 (99.42–99.71) | 5.6162 (5.3588–5.8861) | 0.0571 (0.0413–0.0789) | |
| CIN3+† | |||||||
| HPV | 92.61 (89.08–93.64) | 83.20 (82.46–83.93) | 21.76 (20.00–23.51) | 99.42 (99.26–99.58) | 5.3918 (5.1188–5.6793) | 0.1129 (0.0862–0.1478) | |
| TCT | 80.63 (77.20–84.05) | 91.32 (90.77–91.87) | 32.39 (29.82–34.96) | 98.92 (98.71–99.13) | 9.2889 (8.6029–10.0296) | 0.2122 (0.1777–0.2532) | |
| HPV+TCT | 96.67 (95.12–98.23) | 81.32 (80.55–82.09) | 21.07 (19.75–22.72) | 99.79 (99.69–99.89) | 5.1747 (4.9514–5.4081) | 0.0409 (0.0256–0.0653) | |
| Cervical cancer | |||||||
| HPV | 94.78 (91.14–95.42) | 81.11 (80.35–81.87) | 9.45 (8.27–10.69) | 99.78 (99.68–99.88) | 4.8581 (4.5915–5.1403) | 0.1013 (0.0651–0.1578) | |
| TCT | 82.65 (77.63–87.66) | 89.30 (88.70–89.90) | 14.23 (12.31–16.15) | 99.58 (99.45–99.72) | 7.7270 (7.1142–8.3925) | 0.1943 (0.1455–0.2594) | |
| HPV+TCT | 97.72 (95.74–99.70) | 79.11 (78.31–79.90) | 9.13 (8.65–10.29) | 99.94 (99.88–99.99) | 4.6772 (4.4810–4.8820) | 0.0289 (0.0121–0.0686) | |
Values are presented as value (95% CI).
CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; TCT, thinprep cytologic test.
*CIN2+: included CIN2, CIN3, and cervical carcinoma; †CIN3+: included CIN3 and cervical cancer.
DISCUSSION
Limited epidemiologic data are available for the national incidence of cervical cancer in China. The reported rate of abnormal cytology with ASC-US or greater varies from 8.9% to 25.7%, and the HPV infection rate varies from 6.93% to 26.0% [17,25,26]. In a cervical screening program of 8,556 healthy volunteers in Shenzhen, the abnormal cytology rate was 12.1% and HPV prevalence was 13.6% by the HC2 method and 11.1% by the Cervista® method (Hologic Inc., Madison, WI, USA) [26]. As for HR-HPV infection prevalence, a survey based on 13 Chinese medical centers with 30,207 cases showed an HPV infection rate of 17.7%, while the age-standardized prevalence was 16.8% [17]. Possible reasons for this inconsistency include sampling error, different study populations, geographical prevalence, racial differences, and differences in detection methods. In our study, based on a local center hospital population, the rate of abnormal cervical cytology was 12.24%, and the HPV infection rate was 20.57% when detected by PCR-RDB testing. Compared with the community population, the HPV infection rate was higher in our study, while it was consistent with the data from single-center or multicenter studies in China. The reason for this may be that hospital population-based surveys are opportunistic screenings with a selection bias [27].
Our results showed that the detection rate of CIN2+ by TCT in patients with abnormal cytology was 44.44% (568/1,278); in particular, the detection rate of CIN2+ was 24.19% (142/587) in patients with ASC, and 29.03% (18/62) in patients with ≥AGC. These results are consistent with the FDA reports. The category of “atypical squamous/glandular epithelial cells” refers to the existence of atypical cervical cells, not satisfying the diagnostic criteria of CIN. It may be associated with inflammation, intrauterine devices, poor slide preparation, age-related cellular changes, CIN, or cancer [28]. Several studies have suggested that abnormal cytological findings in the normal population can lead to erroneous diagnosis of ASC, AGC, or even LSIL [29]. Therefore, some more effective methods such as HPV assays and immunocytochemistry are needed to complement cytology for cervical cancer screening.
In our study, the most common types of HPV in Chinese patients were HPV-16, -52, -58, -43, -18, -53, -33, and -51. The prevalence of HR-HPV subtypes in different population showed a great variety. According to our data, the HPV-52 is the most frequent in the pathologic diagnosis as NILM and LSIL (CIN1) groups but not HPV-16. However, the top 5 genotypes in cervical cancer are HPV-16, -18, -58, -59, and -33. Interesting, the HPV-43 is the most common mixed infection LR-HPV. Infection by different HPV subtypes possesses potentially different risks for developing cervical cancer. The risk of HSIL lesions increase with age. It may be associated with the most infection in young women is HPV-52, a relative LR-HR-HPV to cancer. Some HR-HPV infection in young women can be spontaneously cleared within 12 months of infection [30]. But the elder women are most infected by HPV-16, the highest risk for cancer. Other explanations for the observed higher frequency of infection in older women can be the cohort effect or the reactivation of a latent HPV infection in the setting of age-related declining immunity [31].
Our study showed that the PCR-RDB HPV assay and cervical tissue pathology were positively correlated, indicating that the PCR-RDB technology can accurately detect HPV infection in cervical lesions. The success of HPV testing in clinical practice is largely dependent on its high sensitivity and NPV [28]. Therefore, false-negative results should be particularly worrying. To avoid this problem, an internal quality control was used in the PCR-RDB method to evaluate the occurrence of false negatives. In the detection of CIN2+ and CIN3+ by the PCR-RDB HPV test, the sensitivity was 90.43% and 92.61%, and the NPV was 99.06% and 99.42%, respectively. A prospective study showed that women with HPV-positive and cytology-negative findings have a higher risk of cervical lesions than those with HPV-negative and cytology-negative results in the following 5 years [32]. Detection of HR-HPV DNA can help physicians to stratify women in different risk categories.
Our results confirmed that in the cases of normal cytology, the PCR-RDB HPV test could help reduce the rate of misdiagnosis of CIN2+ lesions. Among the patients with normal cytology and HR-HPV positivity, 12.33% (134/1,087) were eventually diagnosed with CIN2+ and 3.04% (33/1,087) with cervical cancer. In the 2012 ASCCP guidelines, in the cases of negative cytology but positive HPV infection, 2 possible management strategies are recommended: either repeating cytological examination after 12 months, or performing HPV genotyping to exclude a HPV-16 or -18 type infections. Detection by PCR-RDB HPV genotyping can identify the genital HPV subtypes. Our data showed that of the patients with cytological results of NILM, ASC-US, and AGC, the HR-HPV positive women had a higher incidence of CIN2+ cervical lesions than HR-HPV negative women. Thus, those women may require more aggressive clinical intervention as opposed to watchful waiting. Meanwhile, HPV-negative patients have better clinical outcomes and they can be followed up for longer observation periods, avoiding overtreatment. HR-HPV detection using a standardized kit is more objective than cytological diagnosis [28,32]. Combination of HPV detection and cytology can improve the sensitivity and the NPV of screening for cervical cancer and CIN. According to the ASCCP guidelines, for women with a cytological diagnosis of ASC-US, the best clinical treatment should be supplemented feedback HPV detection to further triage and manage the disease. Moreover, the guidelines also recommend that women over the age of 30 receive combined cytology and HR-HPV detection as preliminary screening [20].
This is the first big study regarding the clinical application of the PCR-RDB HPV test in China. Our results show that the PCR-RDB HPV test is a reliable, sensitive, and accurate method, which can provide more clinical reference value. This clinical study confirmed that both the PCR-RDB HPV test alone or in combination with cytology was easy to use as a primary screening method.
ACKNOWLEDGMENTS
The authors would like to thank Miss Guifen Liu, Miss Lili Chen, and Miss Meimei Huang for their excellent assistance in this work.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.P., S.Y.
- Data curation: S.P., R.G., M.X., K.Y., D.B., L.F.
- Formal analysis: S.P., R.G., K.Y.
- Funding acquisition: S.P., S.Y.
- Investigation: S.P., S.Y., M.X., D.B., L.F.
- Methodology: S.P., S.Y., R.G.
- Project administration: S.P., S.Y.
- Resources: S.P., S.Y.
- Software: R.G., M.X.
- Supervision: S.P., S.Y.
- Validation: S.P., R.G., M.X., L.F.
- Visualization: K.Y.
- Writing - original draft: S.P., R.G., M.X., K.Y.
- Writing - review & editing: S.P., R.G., D.B.
Supplementary Material
HPV testing flowchart.
ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; ICC, invasive cervical cancer; LEEP, loop electrosurgical excision procedure; NILM, negative for intraepithelial lesion or malignancy; PCR-RDB, polymerase chain reaction-reverse dot blot; TCT, thinprep cytologic test.
Test for identifying HPV infection and HPV gene sequence.
HPV, human papillomavirus.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 2.Lewis DR, Chen HS, Midthune DN, Cronin KA, Krapcho MF, Feuer EJ. Early estimates of SEER cancer incidence for 2012: approaches, opportunities, and cautions for obtaining preliminary estimates of cancer incidence. Cancer. 2015;121:2053–2062. doi: 10.1002/cncr.29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan XF, Zhao ZM, Sun J, Chen F, Wen QL, Liu K, et al. Acceptability and correlates of primary and secondary prevention of cervical cancer among medical students in southwest China: implications for cancer education. PLoS One. 2014;9:e110353. doi: 10.1371/journal.pone.0110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 5.Meijer CJ, Snijders PJ, Castle PE. Clinical utility of HPV genotyping. Gynecol Oncol. 2006;103:12–17. doi: 10.1016/j.ygyno.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101:475–487. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AlObaid A, Al-Badawi IA, Al-Kadri H, Gopala K, Kandeil W, Quint W, et al. Human papillomavirus prevalence and type distribution among women attending routine gynecological examinations in Saudi Arabia. BMC Infect Dis. 2014;14:643. doi: 10.1186/s12879-014-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HY, Fei MD, Jiang Y, Fei QY, Qian H, Xu L, et al. The diversity of human papillomavirus infection among human immunodeficiency virus-infected women in Yunnan, China. Virol J. 2014;11:202. doi: 10.1186/s12985-014-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moosa K, Alsayyad AS, Quint W, Gopala K, DeAntonio R. An epidemiological study assessing the prevalence of human papillomavirus types in women in the Kingdom of Bahrain. BMC Cancer. 2014;14:905. doi: 10.1186/1471-2407-14-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poljak M, Marin IJ, Seme K, Vince A. Hybrid Capture II HPV Test detects at least 15 human papillomavirus genotypes not included in its current high-risk probe cocktail. J Clin Virol. 2002;25(Suppl 3):S89–S97. doi: 10.1016/s1386-6532(02)00187-7. [DOI] [PubMed] [Google Scholar]
- 12.Einstein MH, Martens MG, Garcia FA, Ferris DG, Mitchell AL, Day SP, et al. Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol. 2010;118:116–122. doi: 10.1016/j.ygyno.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Schutzbank TE, Jarvis C, Kahmann N, Lopez K, Weimer M, Yount A. Detection of high-risk papillomavirus DNA with commercial invader-technology-based analyte-specific reagents following automated extraction of DNA from cervical brushings in ThinPrep media. J Clin Microbiol. 2007;45:4067–4069. doi: 10.1128/JCM.01833-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castle PE, Solomon D, Saslow D, Schiffman M. Predicting the effect of successful human papillomavirus vaccination on existing cervical cancer prevention programs in the United States. Cancer. 2008;113:3031–3035. doi: 10.1002/cncr.23762. [DOI] [PubMed] [Google Scholar]
- 15.Day SP, Hudson A, Mast A, Sander T, Curtis M, Olson S, et al. Analytical performance of the investigational use only Cervista HPV HR test as determined by a multi-center study. J Clin Virol. 2009;45(Suppl 1):S63–S72. doi: 10.1016/S1386-6532(09)70010-1. [DOI] [PubMed] [Google Scholar]
- 16.Waxman AG, Chelmow D, Darragh TM, Lawson H, Moscicki AB. Revised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervix. Obstet Gynecol. 2012;120:1465–1471. doi: 10.1097/aog.0b013e31827001d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan QJ, Hu SY, Guo HQ, Zhang WH, Zhang X, Chen W, et al. Liquid-based cytology and human papillomavirus testing: a pooled analysis using the data from 13 population-based cervical cancer screening studies from China. Gynecol Oncol. 2014;133:172–179. doi: 10.1016/j.ygyno.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Jun SY, Park ES, Kim J, Kang J, Lee JJ, Bae Y, et al. Comparison of the cobas 4800 HPV and HPV 9G DNA chip tests for detection of high-risk human papillomavirus in cervical specimens of women with consecutive positive HPV tests but negative Pap smears. PLoS One. 2015;10:e0140336. doi: 10.1371/journal.pone.0140336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham J, Stenger M. Cobas HPV test for first-line screening for cervical cancer. J Community Support Oncol. 2014;12:156–157. doi: 10.12788/jcso.0039. [DOI] [PubMed] [Google Scholar]
- 20.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Chen Z, Zhao L, Wang L, Tian M, Huang H, et al. Molecular diagnosis for a novel deletion mutation of α thalassemia. Zhonghua Xue Ye Xue Za Zhi. 2014;35:724–727. doi: 10.3760/cma.j.issn.0253-2727.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Hao Y, Xu ZY, Jin Q, Wu WQ, Cai J, Luo CQ, et al. Molecular and prenatal diagnosis for a Chinese pregnant woman with a novel mutation of β thalassemia. Zhonghua Xue Ye Xue Za Zhi. 2011;32:245–248. [PubMed] [Google Scholar]
- 23.Yang G, Cui JH, Chen S, Si JH, Tan JJ, Li PY, et al. Establishment of a new HBV genotyping method with PCR-RBD and its application. Zhonghua Gan Zang Bing Za Zhi. 2004;12:677–680. [PubMed] [Google Scholar]
- 24.Peng Q, Li S, Ma K, Li W, Ma Q, He X, et al. Large cohort screening of G6PD deficiency and the mutational spectrum in the Dongguan District in Southern China. PLoS One. 2015;10:e0120683. doi: 10.1371/journal.pone.0120683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan QJ, Hu SY, Zhang X, Ci PW, Zhang WH, Guo HQ, et al. Pooled analysis of the performance of liquid-based cytology in population-based cervical cancer screening studies in China. Cancer Cytopathol. 2013;121:473–482. doi: 10.1002/cncy.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belinson JL, Wu R, Belinson SE, Qu X, Yang B, Du H, et al. A population-based clinical trial comparing endocervical high-risk HPV testing using hybrid capture 2 and Cervista from the SHENCCAST II Study. Am J Clin Pathol. 2011;135:790–795. doi: 10.1309/AJCPKA6ATAPBZ6JQ. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Yu YH, Shen K, Xiao L, Luan F, Mi XJ, et al. Cervical cancer screening and analysis of potential risk factors in 43,567 women in Zhongshan, China. Asian Pac J Cancer Prev. 2014;15:671–676. doi: 10.7314/apjcp.2014.15.2.671. [DOI] [PubMed] [Google Scholar]
- 28.Stoler MH, Austin RM, Zhao C. Point-counterpoint: cervical cancer screening should be done by primary human papillomavirus testing with genotyping and reflex cytology for women over the age of 25 years. J Clin Microbiol. 2015;53:2798–2804. doi: 10.1128/JCM.01087-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burki TK. Atypical glandular cells and risk of cervical cancer. Lancet Oncol. 2016;17:e96. doi: 10.1016/S1470-2045(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 30.Ishida K, Araki A, Kobayashi M, Taniyama K, Nabika T, Nagasaki M. An evaluation of the diagnostic and prognostic significance of p16(INK4a) /p21(WAF1/Cip1) immunostaining in squamous intraepithelial lesions of the uterine cervix using liquid-based cytology specimens. Diagn Cytopathol. 2014;42:125–133. doi: 10.1002/dc.23008. [DOI] [PubMed] [Google Scholar]
- 31.Skinner SR, Wheeler CM, Romanowski B, Castellsagué X, Lazcano-Ponce E, Del Rosario-Raymundo MR, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: analysis of the control arm of the VIVIANE study. Int J Cancer. 2016;138:2428–2438. doi: 10.1002/ijc.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis. 2013;17:S56–S63. doi: 10.1097/LGT.0b013e318285437b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPV testing flowchart.
ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; ICC, invasive cervical cancer; LEEP, loop electrosurgical excision procedure; NILM, negative for intraepithelial lesion or malignancy; PCR-RDB, polymerase chain reaction-reverse dot blot; TCT, thinprep cytologic test.
Test for identifying HPV infection and HPV gene sequence.
HPV, human papillomavirus.