Abstract
Cholinergic function plays a role in a variant of context fear conditioning known as the context preexposure facilitation effect (CPFE; Robinson-Drummer, Dokovna, Heroux, & Stanton, 2016). In the CPFE, acquisition of a context representation, the context-shock association, and expression of context fear occur across successive phases, usually 24hr apart. Systemic administration of scopolamine, a muscarinic acetylcholine receptor antagonist, prior to each phase (context preexposure, immediate-shock training, and testing) disrupts the CPFE in juvenile rats (Robinson-Drummer et al., 2016). Dorsal hippocampal (dHPC) cholinergic function contributes significantly to this effect, as local infusion of scopolamine into the dHPC prior to any individual phase of the CPFE produces a disruption identical to systemic administration (Robinson-Drummer et al., 2016). The current experiment extended these findings to another forebrain region implicated in the CPFE, the medial prefrontal cortex (mPFC). Adolescent rats received bilateral infusions of scopolamine (35μg/side) or PBS 10 min before all three phases of the CPFE or only prior to a single phase. Intra-mPFC administration of scopolamine prior to all three phases significantly impaired fear conditioning suggesting that mPFC cholinergic function is necessary for successful CPFE performance. Analyses of the individual infusion days revealed a significant impairment of the CPFE when infusions occurred prior to preexposure or training (i.e. immediate footshock) but not prior to testing. In total, these findings suggests a role of mPFC cholinergic function in in the acquisition and/or consolidation of a contextual representation and the context-shock association but not to retrieval or expression of fear memory. Implications for mPFC involvement in contextual fear conditioning and neurological dysfunction following neonatal alcohol exposure are discussed.
Keywords: CPFE, fear, prefrontal, muscarinic, spatial learning
Introduction
Cholinergic function is crucial for performance of several forms of Pavlovian conditioning. Scopolamine, a muscarinic acetylcholine (mACh) receptor antagonist, administered during training can disrupt standard contextual fear conditioning (sCFC) to a background context (Anagnostaras, Maren, & Fanselow, 1995; Anagnostaras, Maren, Sage, Goodrich, & Fanselow, 1999; Gale, Anagnostaras, & Fanselow, 2001) as well as conditioning to a discrete cue (however see Hunt & Richardson, 2007). In addition, a variant of sCFC known as the context preexposure facilitation effect (CPFE; Fanselow, 1990) has been used to specify the particular psychological processes affected by cholinergic antagonism during fear conditioning (Brown, Kennard, Sherer, Comalli, & Woodruff-Pak, 2011; Chang & Liang, 2012; Robinson-Drummer et al., 2016). During the CPFE, learning about the context (preexposure), context-shock association (training), and retrieval and expression of the context-fear memory (testing) occur across three separate days. Relative to sCFC, the temporal separation of the learning experiences during the CPFE make it well suited to separately analyze the mechanisms of context learning vs. context-shock learning as determinants of conditioned fear performance.
Similar to sCFC, performance of the CPFE is significantly impaired by antagonizing cholinergic receptors. Prior to conditioning on any single phase of the CPFE, both systemic and intra-hippocampal scopolamine administration disrupts testing day performance (Brown et al., 2011; Robinson-Drummer et al., 2016). Furthermore, post-shock (but not post-preexposure) intra-hippocampal infusions of scopolamine significantly impairs CPFE performance (Chang & Liang, 2012). These results support previous reports that the hippocampus is critical for contextual conditioning during the CPFE (Matus-Amat, Higgins, Barrientos, & Rudy, 2004; Matus-Amat, Higgins, Sprunger, Wright-Hardesty, & Rudy, 2007) and extend those results by suggesting a specific role of the hippocampal cholinergic system in contextual conditioning using the CPFE. Although most CPFE research has focused on this region, the hippocampus is not the singular target of cholinergic projections, so other brain regions receiving these projections may also play a role in the CPFE.
The medial prefrontal cortex (mPFC) is involved in the top down control of cognitive function (Dalley, Cardinal, & Robbins, 2004), in systems consolidation, and in behavioral expression of context conditioning (Frankland & Bontempi, 2005; Wiltgen & Tanaka, 2013). However, recently its role has been extended to include the initial acquisition of context memories (for review see Giustino & Maren, 2015). Following the training phase of the CPFE, the mPFC shows learning-related increases in immediate early gene expression in both adult (Chakraborty, Asok, Stanton, & Rosen, 2016) and developing rats (Asok, Schreiber, Jablonski, Rosen, & Stanton, 2013; Schreiber, Asok, Jablonski, Rosen, & Stanton, 2014) and after hippocampal lesions or inactivation, compensatory mechanisms in the mPFC subserve fear conditioning to contextual stimuli (Zelikowsky et al., 2013). Additionally, the mPFC receives rich innervation from the basal forebrain cholinergic system (Henny & Jones, 2008) making it a likely contributor to the disruptive effects of cholinergic antagonism on contextual fear conditioning.
Although many studies have explored the importance of mPFC cholinergic function to attention and working memory tasks (Broersen, Heinsbroek, de Bruin, Uylings, & Olivier, 1995; Chen, Baxter, & Rodefer, 2004; Chudasama, Dalley, Nathwani, Bouger, & Robbins, 2004; McGaughy, Ross, & Eichenbaum, 2008; Newman & McGaughy, 2008), the neuromodulatory role of the mPFC cholinergic system in (contextual) fear conditioning is largely unexplored. The current study investigated the effect of intra-mPFC antagonism of cholinergic function during all three conditioning phases of the CPFE in 31-day-old rats, a period that marks the transition from juvenile to adolescent stages of development (Spear, 2000). In Experiment 1, scopolamine was administered prior to all three phases of the CPFE to broadly implicate the mPFC cholinergic system in the CPFE. Experiments 2–4 each examined cholinergic antagonism on only a single day of the CPFE (i.e. preexposure, training or testing day only) in order to more precisely identify the psychological processes that may be impaired by mPFC scopolamine infusions. Results of the current study support a role for the mPFC cholinergic system in context learning and context-shock association but not retrieval or expression of context fear.
General Methods
Subjects
Time-mated females were housed with breeder males overnight and were examined for an ejaculatory plug the following day and, if found, that day was designated as gestational day (GD) 0. Dams were housed in clear polypropylene cages measuring 45 × 24 × 21 cm with standard bedding and access to ad libitum water and rat chow. Animals were maintained on a 12:12h light/dark cycle with lights on at 7:00 am. Date of birth (GD22) was designated as postnatal day (PD) 0. Litters were culled on PD3 to eight pups (usually 4 males and 4 females) and were paw-marked with subcutaneous injections of non-toxic black ink for identification. Pups were weaned from their mother on PD21 and housed with same-sex litter mates in 45 × 24 × 17 cm cages. On PD29 animals were individually housed in small white polypropylene cages (24 × 18 ×13 cm) with ad libitum access to water and rat chow for the remainder of the experiment. All subjects were treated in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Delaware following guidelines established by the National Institute of Health.
Apparatus and Stimuli
Fear conditioning occurred in four clear Plexiglas chambers designated as Context A as described previously (Heroux, Robinson-Drummer, Rosen, & Stanton, 2016; Murawski & Stanton, 2010; Robinson-Drummer et al., 2016). The chambers measured 16.5 × 12.1 × 21.6 cm and were arranged in a 2 × 2 formation on a Plexiglas stand within a fume hood which provided ambient light and background noise. Each chamber had a grid floor made of 9 stainless steel bars, 0.5 cm in diameter and spaced 1.25 cm apart. The unconditioned stimulus (US), two 1.5mA, 2s foot shocks, was delivered using a shock scrambler (Med Associates, Georgia, VT ENV-414S) connected to the grid floor. Video of each session (preexposure, training, testing) was recorded using FreezeFrame software (Actimetrics, Wilmette IL), which measures change in pixilation, with freezing defined as a bout of 0.75s or longer without a change in pixels. The FreezeFrame software recorded video from the four chambers simultaneously. Context B consisted of the same Plexiglas chambers used in Context A with modifications, which have been described previously (Asok et al., 2013; Murawski & Stanton, 2010; Robinson-Drummer et al., 2016; Schreiber et al., 2014). Wire mesh inserts, which protruded into the chambers, changed both the texture of the floor and the dimensions of chamber. In addition, white opaque coverings were added such that only the wall facing the camera remained unobscured.
Surgery
On PD29, juvenile rats were taken from post-weaning group housing and anesthetized with an i.p. ketamine/xylazine injection and subcutaneous buprenorphine near the incision site to reduce post-operative discomfort. A fused double-guide cannula (Plastics One, Roanoke, VA) was implanted bilaterally to terminate above the prelimbic region of medial prefrontal cortex using the following coordinates: anteroposterior (AP) +9.0mm and mediolateral (ML) ±0.6mm relative to interaural midline and dorsoventral (DV) −2.3mm relative to the top of the skull. Cannula were fixed in placed using dental acrylic and curved “skull hooks” (Schiffino, Murawski, Rosen, & Stanton, 2011; Watson & Stanton, 2009). Following surgery, dummy internals and dust caps were inserted in the guide cannula to reduce occlusion of the guide cannula and rats were allowed to recover in individual white cages with electric heating pads placed under half of the cage floor. Animals were allowed to recover for approximately 24hr until their cannula were cleared the following day. For each animal, 0.25μL of the vehicle phosphate buffered saline (PBS; Fisher Scientific, Waltham, MA) was infused per side to ensure that no cannulas were occluded.
Drug Infusion
Depending on their drug condition (see Behavioral Procedures and Experimental Design below) rats received microinjections of either PBS or scopolamine hydrobromide (Scop; Sigma Aldrich, St. Louis, MO) dissolved in PBS approximately ten minutes before behavioral training. Animals were hand held while scopolamine (140μg/μL dissolved in PBS) was infused at a rate of 0.25μL per minute for a single minute, administering 35μg of scopolamine per side per animal. This dose has been used previously in our lab (Brito, Davis, Stopp, & Stanton, 1983; Robinson-Drummer et al., 2016) and similar doses of scopolamine have been infused intra-cranially in other labs (Chang & Liang, 2012; Gale et al., 2001; Rogers & Kesner, 2004). Drug injectors were left in place for an additional minute to allow diffusion of drug before removal. PBS control animals were administered the same volume of PBS at the same rate as scopolamine animals. A 0.25μL infusion diffuses about 1mm from the cannula tip ensuring that the spread of the drug is restricted to the prefrontal cortex. This is based on our other studies using injected dyes (Jablonski, Watson, & Stanton, 2010) or labelled muscimol (Heroux et al., submitted). After infusions, animal were returned to their home cage until conditioning.
Behavioral Procedure
Behavioral training occurred over three days from PD31-33 (±1d) using the previously described multiple preexposure procedure (Dokovna, Jablonski, & Stanton, 2013; Robinson-Drummer et al., 2016). On the preexposure day, (PD31) pups were weighed, and then placed in transport boxes of clear Lexan (11 × 11 × 18 cm) covered with orange construction paper to obscure visual cues during transport. Rats were brought in sets of 4 to the hallway immediately adjacent to the training room while the chambers were cleaned with 5% ammonium hydroxide solution. Each rat was placed in its designated chamber and allowed to explore the context for 5min. They were then removed, and placed back in their respective transport boxes for approximately 1min, brought back over and placed in the chamber for a 1min exposure. This was repeated 4 times for a total of five 1-minute exposures. Animals were then removed and returned to their home cage, ending the preexposure session. Pre group animals were exposed to the Context A chamber configuration while a second group of animals were treated identically to the Pre group however the chambers were arranged in the Context B configuration (see Apparatus and Stimulus above) and comprised the alternate exposure condition (Alt-Pre group)
On PD32, animals from all groups were trained in Context A. Weighing, transport and chamber cleaning was identical to that performed on the preexposure day. Rats were brought from the waiting area one at a time, placed in their respective training chamber, and received two immediate (<5s) 1.5mA, 2s foot shock. Animals were immediately removed from the chamber following the foot shock, returned to their transport cages and, following training of the last animal, all 4 animals were taken back to their home cages.
Testing occurred 24hr following training in Context A. Weighing, transport and chamber cleaning was identical to that performed on the preexposure and training days. All animals were returned to the chambers in which they were trained 24hr earlier and tested, for 5min, under identical circumstances as previously described for preexposure (in Context A).
Experimental Design
For all studies, animals were randomly assigned to receive either PBS or Scop prior to behavioral conditioning on either all three days of training (Experiment 1) or prior to only the preexposure (Experiment 2), training (Experiment 3) or testing day (Experiment 4). Pre group animals were split between drug (PBS or Scop) and infusion day (all days, pre-only, training-only or test-only) while the Alt-Pre group was pooled across drug (except where noted). The Alt-Pre group has historically performed similarly regardless of drug treatment (Dokovna et al., 2013; Jablonski, Schiffino, & Stanton, 2012; Robinson-Drummer et al., 2016; Schiffino et al., 2011) and pooling reduces the number of animals needed for completion of the study. Sex was counterbalanced within litters for each preexposure, drug and infusion day group and was collapsed within an experimental group (except where noted).
Data & Statistical Analysis
Data and statistical analyses were as described previously (Murawski & Stanton, 2010; Robinson-Drummer et al., 2016). All collected data were analyzed using FreezeFrame software (Acimetrics, Wilmette IL) with a bout of freezing (the cessation of all movement except breathing) set to 0.75 seconds. The software program computes a “motion index” that was adjusted to set a freezing threshold separately for each animal (per software instructions) by a blind observer who verified from the video record that small movements were not recorded as freezing. Once set, the threshold did not change during a session. We have validated this procedure against other scoring methods (e.g., hand scoring of video records by blind observers) and found that it is very reliable (r = 0.976; p < .01; unpublished observations). Freezing behavior was scored as the total percent time spent freezing over a 5min testing session. Animal data was imported into Statistica 10 data analysis software. Statistical significance was set to p < 0.05. Outliers were removed from test day freezing scores to reduce statistical variability. They were defined a priori as having a score ±1.96 standard deviations from the mean of all other rats in their respective groups. The typical outlier greatly exceeded the 1.96 threshold. Across this entire study, the mean (± SE) outlier z-score value was 3.39 (± 0.58). Planned comparisons and post-hoc Neuman-Keuls tests were used to assess any significant effects revealed by ANOVA.
Experiment 1
mPFC muscarinic cholinergic function is necessary for successful performance of the CPFE (Figure 2)
Figure 2.
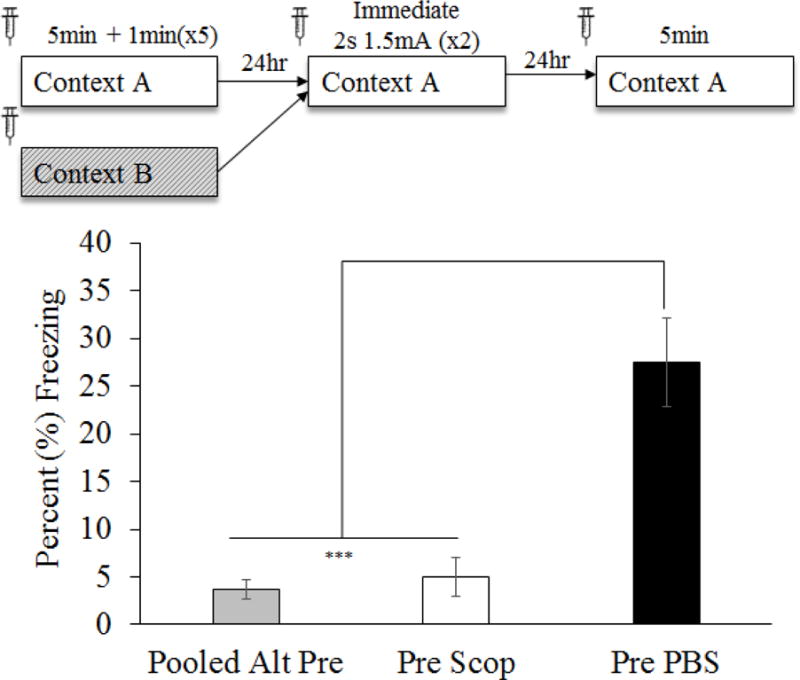
Mean (±SEM) percent time freezing during a 5min test of conditioned fear. Animals were given either PBS or scopolamine prior to all three conditioning phases of the CPFE. Comparisons reflect a one-way ANOVA for group (Pooled Alt-Pre, Pre Scop and Pre PBS). Scopolamine significantly impaired CPFE performance on the testing day. Asterisks indicate significance relative to Pre PBS group. *** = p < .001
Methods and Results
The current experiment administered 0.25μL vehicle PBS or 35μg scopolamine into the mPFC (see Surgery and Drug infusions sections above) 10 min prior to all three phases of the CPFE (i.e. preexposure, training and testing). Thirty-five Long-Evans rats from 22 litters were assigned to groups by drug (PBS v Scop) and preexposure condition (Pre v Alt-Pre) with testing day freezing being statistically compared across groups. There was no effect of drug observed in the Alt-Pre group [F(1,9) = 2.10, p = 0.18] so this variable was pooled across drug. Four subjects were removed due to misplaced cannula (Pre PBS n = 1, Pre Scop n = 3; Figure 1). A 2 (Sex: Male, Female) × 3 (Condition: Pooled Alt-Pre, PBS, Scop) factorial ANOVA revealed no significant main effect of Sex [F(1,22) = 1.42, p = .25] or Sex by Condition interaction [F(2, 22) = 1.92, p = .32] so all analyses were collapsed across this variable. Subsequent analyses are the result of a three group one-way ANOVA (Pooled Alt-Pre, Pre Scop, and Pre PBS). A single outlier was removed from each of the three experimental groups and final group sizes were as follows: Pooled Alt-Pre n = 11, Pre Scop n = 8, and Pre PBS n = 9. ANOVA revealed a significant effect of condition [F(1,25) = 22.26, p < .001] such that freezing in the Pre PBS group was significantly elevated above both Pre Scop and Pooled Alt-Pre (p’s < .001) however there was no difference between Pre Scop and Alt-Pre (p = .75), indicating that the drug abolished the CPFE. These results suggest a significant role for mPFC cholinergic function in successful performance of the CPFE but they do not indicate whether a particular phase of the CPFE is critical for this effect.
Figure 1.
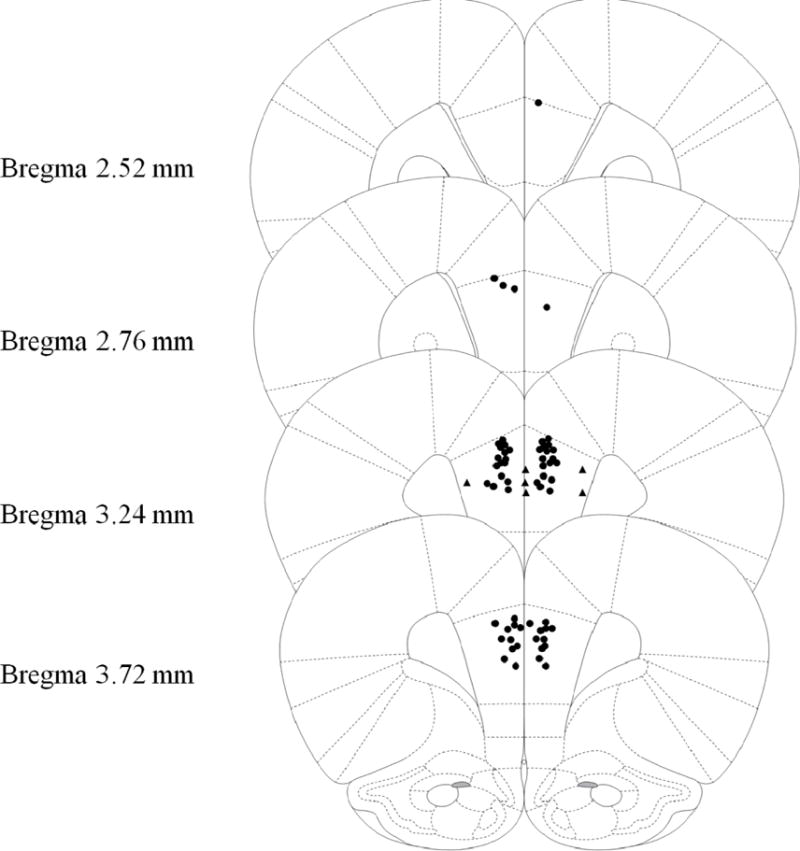
Schematic representation of injection cannula tip placement in the mPFC for Experiment 1. Animals included in final analyses are represented by filled black circle while animals excluded as mPFC placement misses are filled black triangle. Extremely anterior (Bregma 4.68mm or more) or posterior (Bregma 2.28 or less) were automatically excluded and are not represented in the following figure. Coronal brain images are adapted from the rat brain atlas of Paxinos and Watson (2007).
Experiment 2
Scopolamine infusions into the mPFC prior to the Preexposure day impairs contextual learning during the CPFE (Figure 4)
Figure 4.
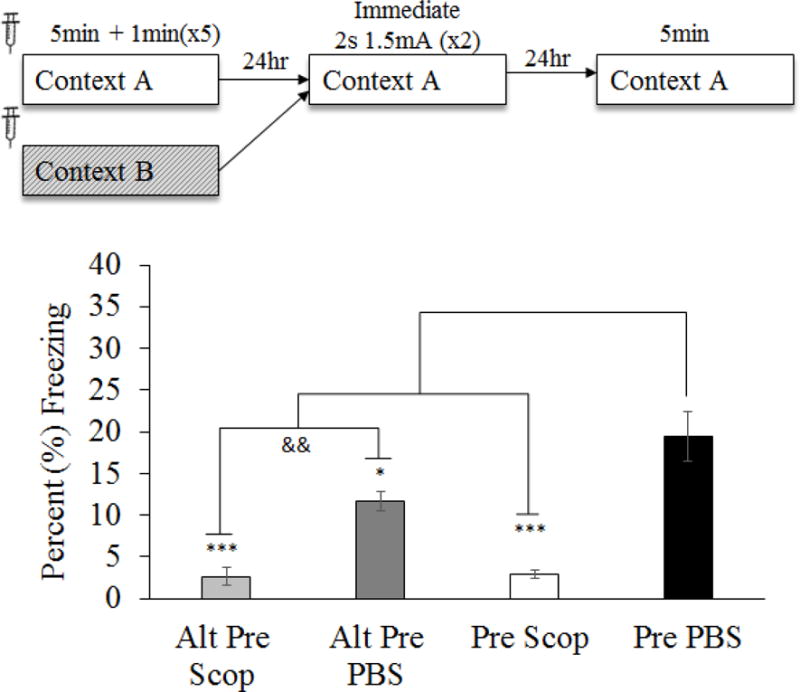
Mean (±SEM) percent time freezing during a 5min test of conditioned fear following pre-preexposure drug infusion (Experiment 2). Comparisons reflect an ANOVA for group (Alt- Pre PBS, Alt-Pre Scop, Pre PBS and Pre Scop) with planned comparisons. Scopolamine administered prior to context preexposure significantly impaired CPFE performance on the testing day. Asterisks indicate significance relative to Pre PBS group. Ampersand indicates significance within exposure condition. && = p < .01; * = p < .05; ** = p < .01; *** = p < .001.
To examine prefrontal cholinergic involvement in context learning, intra-mPFC infusions of scopolamine for the current experiment were administered only prior to the preexposure day of the CPFE. Forty-one rats from 26 litters were grouped by drug (PBS v Scop) and preexposure condition (Pre v Alt-Pre). Four animals were removed from analyses due to misplaced cannula (Figure 3; Alt-Pre Scop n = 1, Pre PBS n = 2, Pre Scop n = 1). Again, lack of Sex [F(1,35) = 0.67, p = .42] or Sex × Condition interaction [F(2,35) = 2.32, p = .11] effects led us to pool subsequent analyses across this factor. There was a significant effect of drug in the Alt-Pre group [F(1,11) = 30.60, p < .001] so pooling across drug was not possible in this experiment. A single outlier was removed from Alt-Pre PBS, Alt-Pre Scop and Pre Scop groups and two outliers were removed from Pre PBS group. Final group sizes were as follows: Alt-Pre PBS n = 7, Alt-Pre Scop n = 6, Pre PBS n = 16, Pre Scop n = 12. The four experimental conditions were analyzed using ANOVA and planned comparisons. ANOVA revealed a significant effect of condition [F(3,37) = 13.21 p < .001]. Planned comparisons revealed a significant CPFE as measured by an increase in freezing in Pre PBS relative to Alt-Pre PBS [F(1,37) = 5.05, p = .03] and Alt-Pre Scop [F(1,37) = 20.74, p < .001]. In addition, Pre PBS freezing was significantly elevated above Pre Scop [F(1,37) = 31.70, p < .001] whereas there was no difference in freezing observed between Alt-Pre Scop and Pre Scop [F(1,37) = 0.003, p = .95] suggesting an elimination of the CPFE following mAChr antagonism prior to context preexposure in the Pre Scop group. These results indicate that muscarinic-type cholinergic function is necessary for context learning (or possibly consolidation of this learning) on the preexposure day of the CPFE.
Figure 3.
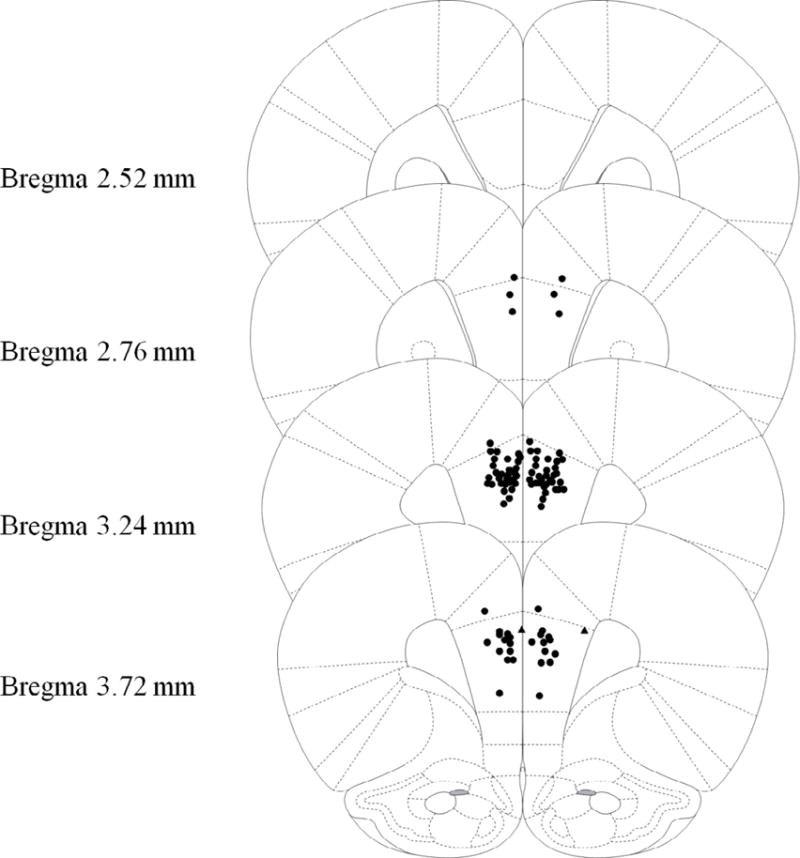
Schematic representation of injection cannula tip placement in the mPFC for Experiment 2. Animals included in final analyses are represented by filled black circle while animals excluded as mPFC placement misses are filled black triangle. Extremely anterior (Bregma 4.68mm or more) or posterior (Bregma 2.28 or less) were automatically excluded and are not represented in the following figure. Coronal brain images are adapted from the rat brain atlas of Paxinos and Watson (2007).
Experiment 3
Training day processes are significantly impaired by mPFC muscarinic antagonism during the CPFE (Figure 6)
Figure 6.
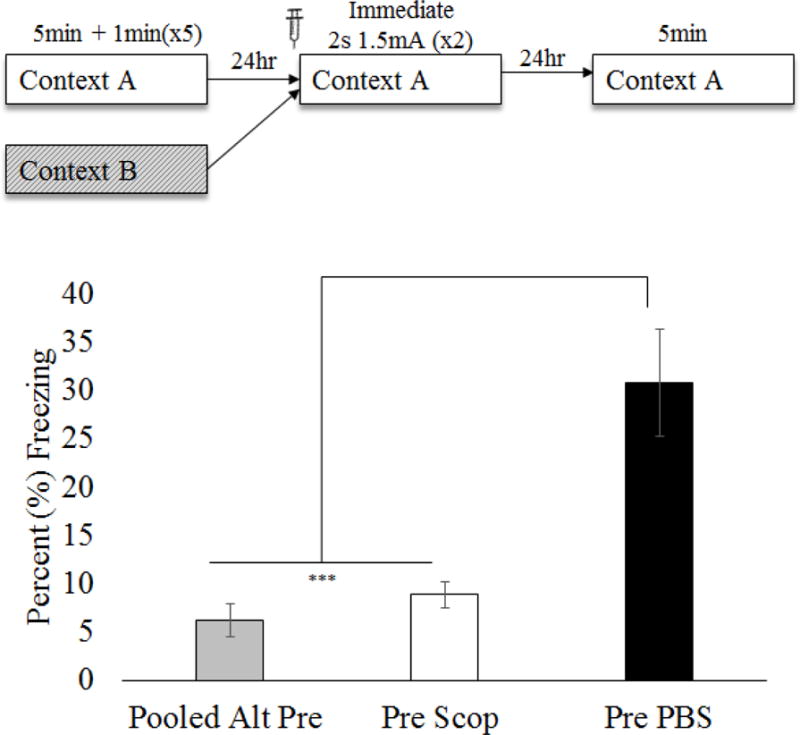
Mean (±SEM) percent time freezing during a 5min test of conditioned fear following pre-training drug infusion (Experiment 3). Comparisons reflect a one-way ANOVA for group (Pooled Alt-Pre, Pre Scop and Pre PBS). Pre-training scopolamine significantly impaired CPFE performance on the testing day. Asterisks indicate significance relative to Pre PBS group. *** = p < .001
Methods and Results
To examine prefrontal cholinergic involvement in context-shock learning, scopolamine was infused bilaterally into the mPFC only prior to immediate foot shock (two 2s, 1.5mA) training. Forty Long-Evans rats from 26 litters were grouped by drug (PBS v Scop) and preexposure condition (Pre v Alt-Pre). Three animals were removed from analyses due to misplaced cannula (Figure 5; Alt-Pre Scop n = 1, Pre PBS n = 1, Pre Scop n = 1). There was no effect of drug in the Alt-Pre groups [F(1,11) = 1.32, p = .27] so a Pooled Alt-Pre group was used. Data were also pooled across sex because a 2 (Sex: Male, Female) × 3 (Condition: Pooled Alt-Pre, Pre PBS, Pre Scop) ANOVA revealed no main effect [F(1,29) = .27, p = .61] or interaction [F(2, 29) = 0.08, p = .93] involving this factor. A single outlier was removed from each of the three experimental groups and final group sizes were as follows: Pooled Alt-Pre n = 13, Pre Scop n = 12, and Pre PBS n = 10. A three group one-way ANOVA (Pooled Alt-Pre, Pre Scop, and Pre PBS) revealed a significant effect of condition [F(1,32) = 18.27, p < .001] such that Pre PBS was significantly elevated above both Pre Scop and Pooled Alt-Pre (p’s < .001) however there was no difference between Pre Scop and Pooled Alt-Pre (p = .54). These results suggest that mPFC muscarinic activity is necessary for training-day processes during the CPFE.
Figure 5.
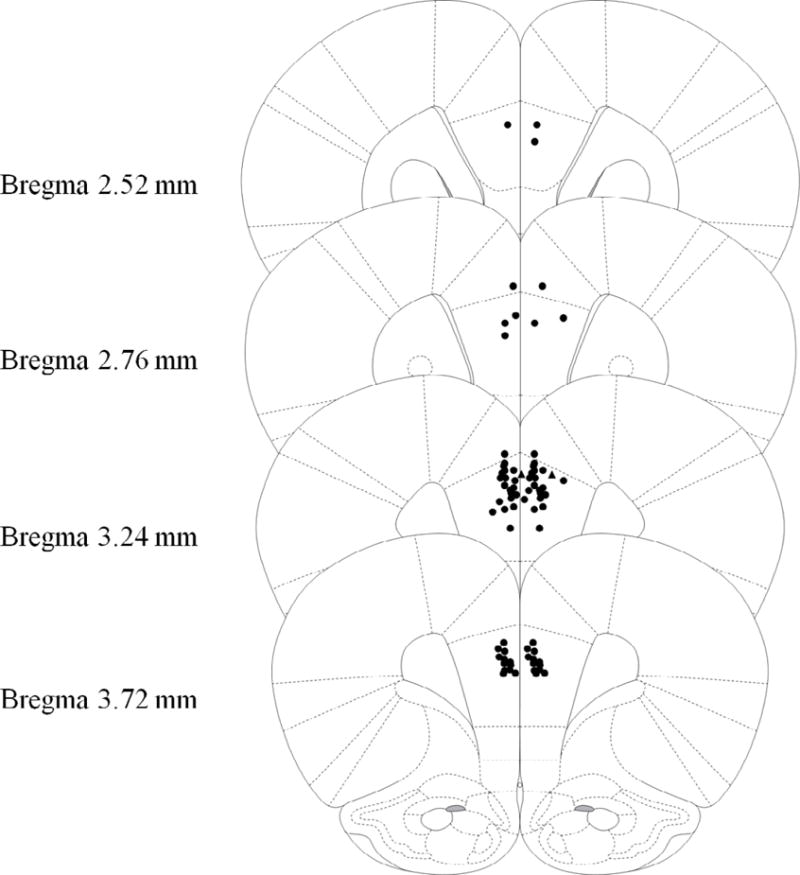
Schematic representation of injection cannula tip placement in the mPFC for Experiment 3. Animals included in final analyses are represented by filled black circle while animals excluded as mPFC placement misses are filled black triangle. Extremely anterior (Bregma 4.68mm or more) or posterior (Bregma 2.28 or less) were automatically excluded and are not represented in the following figure. Coronal brain images are adapted from the rat brain atlas of Paxinos and Watson (2007).
Experiment 4
Intra-mPFC Scopolamine does not affect fear memory retrieval or performance of the CPFE when administered prior to testing (Figure 8)
Figure 8.
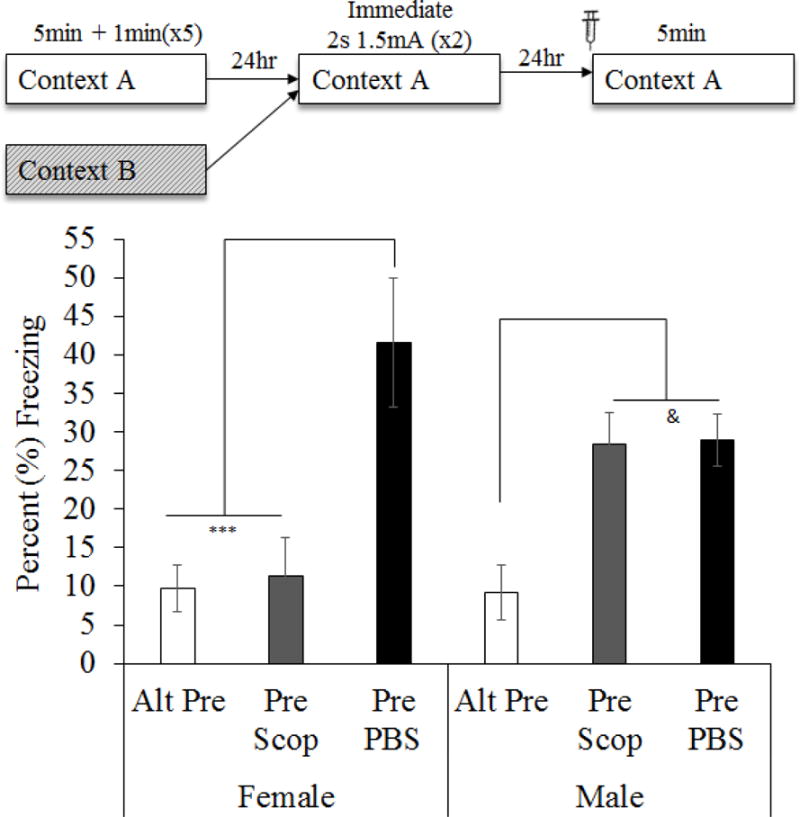
Mean (±SEM) percent time freezing during a 5min test of conditioned fear. Animals were given either PBS or scopolamine prior to the testing phase of the CPFE. 2 (Sex: Female, Male) × 3 (Condition: Alt-Pre, Pre Scop, Pre PBS) factorial ANOVA. Scopolamine significantly impaired CPFE performance in males. Asterisks indicate significance relative to Pre PBS group. Ampersand indicates significance relative to Alt-Pre. & = p < .05; *** = p < .001
Methods and Results
The current experiment infused scopolamine into the mPFC prior to fear memory testing to examine effects on retrieval or expression of the CPFE. Forty three rats from 21 litters were grouped by drug (PBS v Scop) and preexposure condition (Pre v Alt-Pre). Six animals were removed due to misplaced cannula (Figure 7; Alt-Pre PBS n = 2, Pre PBS n = 1, Pre Scop n = 3). Alt-Pre groups were unaffected by drug [F(1,10) = .004, p = .95] so a Pooled Alt-Pre group was used. Although 2 (Sex: Male, Female) × 3 (Condition: Pooled Alt-Pre, Pre PBS, Pre Scop) ANOVA revealed no main effect of Sex [F(1,34) = .12, p = .73], there was an effect of Condition [F(1,34) = 14.60, p < .001] and a Sex × Condition interaction [F(2, 34) = 5.40, p < .01]. The interaction was driven by a significant difference between female and male animals in the Pre Scop group (p = .01) so there were no subsequent analyses that pooled across this variable. One outlier was removed from each of the six groups. Group sizes were as follows: Female Pooled Alt-Pre n = 5, Female Pre PBS n = 5, Female-Pre Scop n = 6, Male Pooled Alt-Pre n = 7, Male Pre PBS n= 8, Male-Pre Scop n = 9. Newman-Keuls post hoc tests revealed that in males, there was no significant difference observed between Pre Scop and Pre PBS (p = .94) and a significant CPFE was evident regardless of testing day drug (ps < .05 relative to Pooled Alt-Pre). However, in females the significant CPFE observed between Pre PBS and Pooled Alt-Pre (p < .001) was not evident between Pre Scop and Pooled Alt-Pre (p = .81). This evidence that mPFC scopolamine influences expression of the CPFE in females but not males should be regarded with caution as it may reflect sampling error. Analysis of effect sizes indicates that twice as much of the variance results from the drug condition (ηp2 = .462) than the sex × drug condition interaction (ηp2 = .241) and nearly none of the variance is due to sex alone (ηp2 = .003). We have also not seen similar sex differences following systemic or intra-hippocampal scopolamine administration (Robinson-Drummer et al., 2016). On the other hand, there is some evidence that the mPFC may contribute to sex differences observed in eyeblink conditioning following stress (Maeng & Shors, 2013; Maeng, Waddell, & Shors, 2010; Wood & Shors, 1998). Whether the present findings represent this type of outcome or are merely sampling error is a question that requires further study.
Figure 7.
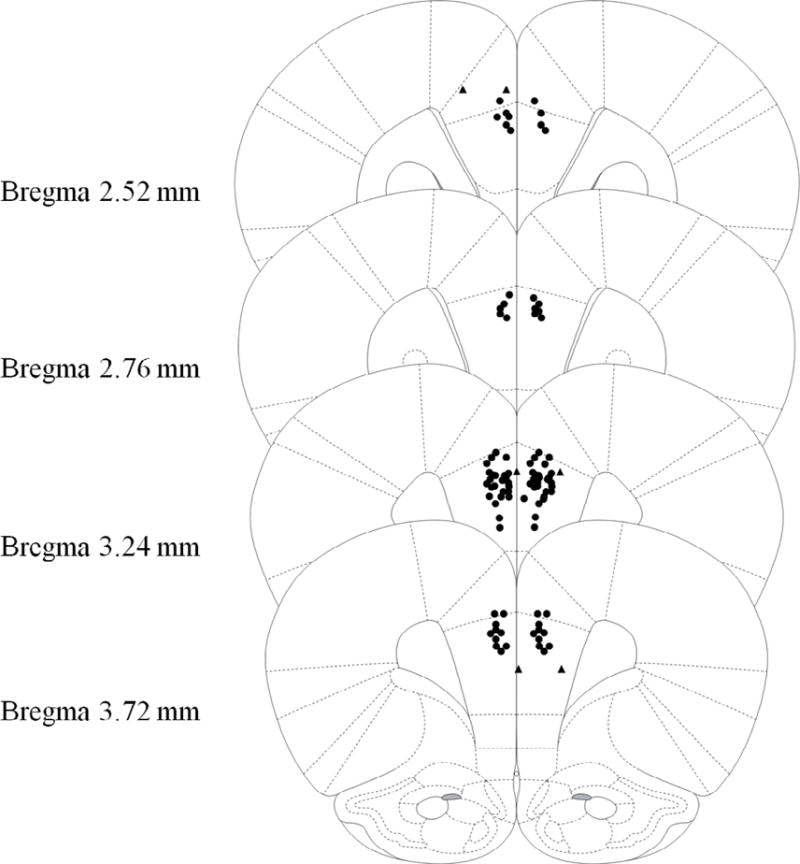
Schematic representation of injection cannula tip placement in the mPFC for Experiment 4. Animals included in final analyses are represented by filled black circle while animals excluded as mPFC placement misses are filled black triangle. Extremely anterior (Bregma 4.68mm or more) or posterior (Bregma 2.28 or less) were automatically excluded and are not represented in the following figure. Coronal brain images are adapted from the rat brain atlas of Paxinos and Watson (2007).
Discussion
The current experiments demonstrate a necessary role of muscarinic cholinergic receptor function in the medial prefrontal cortex in the CPFE. This system contributes to learning-related activity on the preexposure (Experiment 2) and training (Experiment 3) days but not to memory retrieval or CPFE performance on the testing day (Experiment 4), at least in males. The effects observed in Experiments 2 and 3 are unlikely to be state-dependent as the animals were able to express fear during testing while on the drug. Furthermore, a state-dependent account of the current results predicts that there would be no effect of the scopolamine when the drug is given during all three phases of the CPFE, a prediction that was not supported by the outcome of Experiment 1.
The results of Experiments 2 and 3 support and extend previous reports of a role of the mPFC in the acquisition of contextual fear conditioning. Acquisition of background contextual conditioning (i.e. when the context is not the sole predictor of the US) is significantly impaired by disrupting neural activity or plasticity in the mPFC prior to conditioning (Gilmartin & Helmstetter, 2010; Gilmartin, Kwapis, & Helmstetter, 2013). However, the co-occurrence of context learning and context-shock association during sCFC in these previous studies makes it difficult to parse out which process is affected by disruption of mPFC function. The current results extend previous knowledge by suggesting a necessary role for cholinergic mPFC function for acquisition of both the context representation and the context-shock association. Intra-hippocampal scopolamine significantly impairs CPFE performance when infused post-training while post-preexposure infusions have no effect suggesting a consolidation deficit on the training day and an encoding deficit on the preexposure day (Chang & Liang, 2012). Other results from our lab (Heroux, Robinson-Drummer, Sanders, Rosen and Stanton, submitted) suggest that the current training day effects may also reflect a consolidation (or reconsolidation) effect. Pre-training mPFC inactivation spares post-shock freezing, suggesting inactivation does not prevent rats from retrieving the context representation and momentarily associating it with a foot shock. Furthermore, if scopolamine targets consolidation processes, post-conditioning infusions should have the same effect on CPFE performance as the current experiments. Although this has been reported following intra-hippocampal scopolamine (Chang & Liang, 2012), additional experimentation is necessary to dissociate the effect of mPFC scopolamine on acquisition versus consolidation of contextual fear conditioning.
The lack of a deficit following pre-testing scopolamine in Experiment 4 was surprising as the mPFC (specifically the prelimbic region) is thought to be the primary region responsible for fear expression (Giustino & Maren, 2015). Whereas the current results suggest no contribution of the mPFC cholinergic system to testing-day processes, previous results do demonstrate a need for a functional mPFC either for memory retrieval or expression of contextual fear (Corcoran & Quirk, 2007; Heroux et al., submitted). In their review, Giustino and Maren (2015) suggest that the mPFC is differentially recruited for fear memory acquisition or expression depending on the task requirements. However they do not speculate on the mechanism by which this task-dependent recruitment is achieved. It is likely that this recruitment is dependent on circuit-level interactions of the mPFC with other regions necessary for contextual conditioning. Prefrontal inhibition during contextual fear conditioning disrupts entorhinal-hippocampal activity and these circuit-level changes are associated with reduced fear memory during testing 24hr later (Bero et al., 2014). Additionally, in a model of mPFC regulation of attention, the ability for the mPFC to switch cue-processing modes depends on both tonic and transient cholinergic function from the basal forebrain (BF; Hasselmo & Sarter, 2011). Tonic BF cholinergic activity regulates cue-evoked glutamatergic input from thalamic nuclei that in turn may modulate transient cholinergic changes from projections that enhance cue detection. Taken together, testing day exposure to the training context may not trigger (contextual) cue-related mAChr-type mPFC activity, either directly or through afferent projections from other regions. Whether these types of circuit- and transmitter-level neuromodulations are acting to control mPFC involvement in the CPFE across the different phases is an interesting question to be explored in future studies.
Several neurotransmitter systems have been identified across the contextual fear circuit as being crucial for conditioning however their role varies by region and phase of conditioning. In the hippocampus, both muscimol (GABAA agonist) and scopolamine (muscarinic cholinergic antagonist) impair the CPFE when administered prior to any single phase of the CPFE protocol (Matus-Amat et al., 2004; Robinson-Drummer et al., 2016). However, intra-hippocampal NMDA-type glutamate antagonists only impair the CPFE when they are administered prior to preexposure (Matus-Amat et al., 2007; Schiffino et al., 2011). In the amygdala, APV (NMDA-type glutamate antagonist) significantly impairs CPFE performance when administered prior to immediate shock training but not prior to preexposure or testing (Matus-Amat et al., 2007). (Matus-Amat et al., 2007). In these regions, as well as the mPFC, afferent connections from other regions and the distribution of neurotransmitter receptors on pre- and post-synaptic neuronal projections likely give rise to the specialized function of that region during the particular CPFE phases. Specifically how these neurotransmitter systems, both singularly and in concert, contribute to specific prefrontal mnemonic functions (e.g. context learning vs. context-shock association) during the CPFE is a fruitful direction for future research.
The CPFE emerges between 2 and 3 weeks postnatally and does not develop further behaviorally between adolescence and adulthood (Jablonski et al., 2012; Robinson-Drummer & Stanton, 2015; Schiffino et al., 2011). However, the current report does not include a comparison with adults so it is possible that there is a differential involvement of the mPFC in fear conditioning in the CPFE between adolescence and adulthood. The mPFC likely performs a similar role in acquisition of contextual fear conditioning but may play a different role during long-term memory between adolescence and adulthood. Adults and adolescent rats show similar learning-related changes in mPFC gene expression during the CPFE (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014) and the mPFC is involved in context conditioning in adults rats (Zelikowsky et al., 2013; Zelikowsky, Hersman, Chawla, Barnes, & Fanselow, 2014). However, long-term retention of context learning changes between adolescence and adulthood. Adult rats can retain memory of a context for at least 3 weeks (Robinson-Drummer & Stanton, 2015; Rudy & Wright-Hardesty, 2005) however adolescent rats can only remember contexts for two weeks (Robinson-Drummer & Stanton, 2015). It is possible that the remaining maturation in the prefrontal cortex observed between adolescence and adulthood (Coyle & Yamamura, 1976; Ferguson & Gao, 2014; Lee, Nicklaus, Manning, & Wolfe, 1990; Van Eden & Uylings, 1985) contributes not to the initial acquisition of contextual learning but to the long-term consolidation of previously acquired memory (Frankland & Bontempi, 2005; Wiltgen & Tanaka, 2013). This is an interesting and understudied area in ontogeny of memory that should be explored in future reports.
The significant impairment in CPFE performance observed following cholinergic antagonism in the mPFC may be relevant to behavioral impairments in fetal alcohol spectrum disorder (FASD). Cholinergic dysfunction has been implicated in learning-related impairmentsin FASD in humans and rodent models (Fryer et al., 2007; Jacobson et al., 2008; Lebel, Roussotte, & Sowell, 2011; Lewis et al., 2015; Malisza et al., 2005; Mattson, Crocker, & Nguyen, 2011; Murawski, Moore, Thomas, & Riley, 2015; Rasmussen, 2005). Converging evidence from these studies implicate, in addition to the hippocampus, significant impairments in prefrontal structure and function. The CPFE is disrupted by neonatal alcohol exposure (Hamilton et al., 2011; Jablonski & Stanton, 2014; Murawski & Stanton, 2010, 2011) and this impairment is rescued by enhancement of cholinergic function (Dokovna et al., 2013). Furthermore, alcohol-induced behavioral and mnemonic impairments as well as molecular changes in the hippocampus and prefrontal cortex can be rescued with cholinergic supplementation (Monk, Leslie, & Thomas, 2012; Otero, Thomas, Saski, Xia, & Kelly, 2012; Schneider & Thomas, 2016; Thomas, Biane, O’Bryan, O’Neill, & Dominguez, 2007; Thomas, Idrus, Monk, & Dominguez, 2010; Thomas & Tran, 2012; Wagner & Hunt, 2006). Taken together, these studies and the results of the current report support a potential role of prefrontal cholinergic dysfunction in learning and memory impairment in FASD that should be more directly explored in future experiments.
Based on the accumulated literature from our lab and others that use the CPFE, both the mPFC and dHPC play significant and varied roles during all three CPFE phases. This developing knowledge of contextual fear conditioning challenges previous models of system consolidation that suggest separate roles for the dHPC and mPFC in recent and remote memories, respectively (Frankland & Bontempi, 2005; Quinn, Ma, Tinsley, Koch, & Fanselow, 2008). The current report shows that the role of mPFC is not confined to remote memory. Rather, it is likely a site of early acquisition and consolidation of fear memories as well as a participant in the long term retrieval and expression of that memory (Giustino & Maren, 2015; Heroux et al., submitted). Additionally, mPFC activity (as measured by relative gene expression) during the CPFE not only changes during context exposure but increases in a learning-related way following immediate shock training (Asok et al., 2013; Schreiber et al., 2014). It seems that as new contextual conditioning parameters are explored many of the canonical models of mPFC contributions to contextual fear memory will need to be revised to include a role of the mPFC in the early acquisition, encoding and consolidation of memories.
Taken together, our lab has demonstrated that the mPFC contributes to the early stages of contextual fear conditioning during the CPFE (Heroux et al., submitted) and that the cholinergic system in the mPFC contributes to acquisition or consolidation of context- and context-shock learning but not retrieval or expression of this learning during the test phase of the CPFE. These findings encourage further, more nuanced exploration of mPFC involvement in the early stages of contextual fear conditioning and neurobehavioral deficits following neonatal alcohol exposure.
Highlights.
Rats were giving intra-mPFC scopolamine prior to each day of conditioning during the CPFE
CPFE disrupted in animals given scopolamine prior to preexposure or training
Testing day performance may not require muscarinic-type cholinergic function in mPFC
mPFC cholinergic function contributes to context learning and context shock association in CPFE
Acknowledgments
Supported by NIH grant R01 HD075066-01A1. We thank Lauren Miller, Hollie Sanders and Katelyn Buban for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64(3):191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology. 1999;21(6):731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiol Learn Mem. 2013;106:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Meng J, Cho S, Shen AH, Canter RG, Ericsson M, Tsai LH. Early remodeling of the neocortex upon episodic memory encoding. Proc Natl Acad Sci U S A. 2014;111(32):11852–11857. doi: 10.1073/pnas.1408378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito GN, Davis BJ, Stopp LC, Stanton ME. Memory and the septo-hippocampal cholinergic system in the rat. Psychopharmacology (Berl) 1983;81(4):315–320. doi: 10.1007/BF00427569. [DOI] [PubMed] [Google Scholar]
- Broersen LM, Heinsbroek RPW, de Bruin JPC, Uylings HBM, Olivier B. The role of the medial prefrontal cortex of rats in short-term memory functioning: further support for involvement of cholinergic, rather than dopaminergic mechanisms. Brain Research. 1995;674(2):221–229. doi: 10.1016/0006-8993(95)00025-l. [DOI] [PubMed] [Google Scholar]
- Brown KL, Kennard JA, Sherer DJ, Comalli DM, Woodruff-Pak DS. The context preexposure facilitation effect in mice: a dose-response analysis of pretraining scopolamine administration. Behav Brain Res. 2011;225(1):290–296. doi: 10.1016/j.bbr.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T, Asok A, Stanton ME, Rosen JB. Variants of contextual fear conditioning induce differential patterns of Egr-1 activity within the young adult prefrontal cortex. Behav Brain Res. 2016;302:122–130. doi: 10.1016/j.bbr.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SD, Liang KC. Roles of hippocampal GABA(A) and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: a double dissociation study. Neurobiol Learn Mem. 2012;98(1):17–24. doi: 10.1016/j.nlm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004;20(4):1081–1088. doi: 10.1111/j.1460-9568.2004.03548.x. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn Mem. 2004;11(1):78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27(4):840–844. doi: 10.1523/jneurosci.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Yamamura HI. Neurochemical aspects of the ontogenesis of cholinergic neurons in the rat brain. Brain Res. 1976;118(3):429–440. doi: 10.1016/0006-8993(76)90310-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behav Brain Res. 2013;248:114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18(3):264–270. doi: 10.3758/bf03205285. [DOI] [Google Scholar]
- Ferguson BR, Gao WJ. Development of thalamocortical connections between the mediodorsal thalamus and the prefrontal cortex and its implication in cognition. Front Hum Neurosci. 2014;8:1027. doi: 10.3389/fnhum.2014.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31(8):1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11(4):371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17(6):289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20(6):290–294. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Murawski NJ, St Cyr SA, Jablonski SA, Schiffino FL, Stanton ME, Klintsova AY. Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats. Brain Res. 2011;1412:88–101. doi: 10.1016/j.brainres.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27(3):654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux NA, Robinson-Drummer PA, Rosen JB, Stanton ME. NMDA receptor antagonism disrupts acquisition and retention of the context preexposure facilitation effect in adolescent rats. Behav Brain Res. 2016;301:168–177. doi: 10.1016/j.bbr.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Richardson R. Pharmacological dissociation of trace and long-delay fear conditioning in young rats. Neurobiol Learn Mem. 2007;87(1):86–92. doi: 10.1016/j.nlm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev Psychobiol. 2012;54(7):714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Stanton ME. Neonatal alcohol impairs the context preexposure facilitation effect in juvenile rats: dose-response and post-training consolidation effects. Alcohol. 2014;48(1):35–42. doi: 10.1016/j.alcohol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Watson DJ, Stanton ME. Role of medial prefrontal NMDA receptors in spatial delayed alternation in 19-, 26-, and 33-day-old rats. Dev Psychobiol. 2010;52(6):583–591. doi: 10.1002/dev.20465. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21(2):102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Nicklaus KJ, Manning DR, Wolfe BB. Ontogeny of cortical muscarinic receptor subtypes and muscarinic receptor-mediated responses in rat. J Pharmacol Exp Ther. 1990;252(2):482–490. [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39(4):724–732. doi: 10.1111/acer.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Shors TJ. The stressed female brain: neuronal activity in the prelimbic but not infralimbic region of the medial prefrontal cortex suppresses learning after acute stress. Front Neural Circuits. 2013;7:198. doi: 10.3389/fncir.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J Neurosci. 2010;30(48):16188–16196. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58(6):1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153(1):63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22(8):1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Moore EM, Thomas JD, Riley EP. Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies. Alcohol Res. 2015;37(1):97–108. doi: 10.35946/arcr.v37.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. Behav Brain Res. 2010;212(2):133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcohol Clin Exp Res. 2011;35(6):1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Cholinergic deafferentation of prefrontal cortex increases sensitivity to cross-modal distractors during a sustained attention task. J Neurosci. 2008;28(10):2642–2650. doi: 10.1523/JNEUROSCI.5112-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero NK, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res. 2012;36(10):1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem. 2008;15(5):368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. Executive Functioning and Working Memory in Fetal Alcohol Spectrum Disorder. Alcoholism: Clinical & Experimental Research. 2005;29(8):1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Dokovna LB, Heroux NA, Stanton ME. Cholinergic mechanisms of the context preexposure facilitation effect in adolescent rats. Behav Neurosci. 2016;130(2):196–205. doi: 10.1037/bne0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Stanton ME. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol Behav. 2015;148:22–28. doi: 10.1016/j.physbeh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learn Mem. 2004;11(1):102–107. doi: 10.1101/lm.64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Wright-Hardesty K. The temporal dynamics of retention of a context memory: something is missing. Learn Mem. 2005;12(2):172–177. doi: 10.1101/lm.84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD. Adolescent Choline Supplementation Attenuates Working Memory Deficits in Rats Exposed to Alcohol During the Third Trimester Equivalent. Alcohol Clin Exp Res. 2016;40(4):897–905. doi: 10.1111/acer.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber WB, Asok A, Jablonski SA, Rosen JB, Stanton ME. Egr-1 mRNA expression patterns in the prefrontal cortex, hippocampus, and amygdala during variants of contextual fear conditioning in adolescent rats. Brain Res. 2014;1576:63–72. doi: 10.1016/j.brainres.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121(1):120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88(10):827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22(3):619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241(3):253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120(2):482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Stanton ME. Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem. 2009;92(1):89–98. doi: 10.1016/j.nlm.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Tanaka KZ. Systems consolidation and the content of memory. Neurobiol Learn Mem. 2013;106:365–371. doi: 10.1016/j.nlm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95(7):4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci U S A. 2013;110(24):9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34(25):8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


