Abstract
Drug combinations that include a psychostimulant such as methylphenidate (Ritalin) and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine are indicated in several medical conditions. Co-exposure to these drugs also occurs with “cognitive enhancer” use by individuals treated with SSRIs. Methylphenidate, a dopamine reuptake inhibitor, by itself produces some addiction-related gene regulation in the striatum. We have demonstrated that co-administration of SSRIs potentiates these methylphenidate-induced molecular effects, thus producing a more “cocaine-like” profile. There is evidence that the 5-HT1B serotonin receptor subtype mediates some of the cocaine-induced gene regulation. We thus investigated whether the 5-HT1B receptor also modifies methylphenidate-induced gene regulation, by assessing effects of a selective 5-HT1B receptor agonist (CP94253) on immediate-early gene markers (Zif268, c-Fos, Homer1a) in adolescent male rats. Gene expression was measured by in situ hybridization histochemistry. Our results show that CP94253 (3, 10 mg/kg) produced a dose-dependent potentiation of methylphenidate (5 mg/kg)-induced expression of Zif268 and c-Fos. This potentiation was widespread in the striatum and was maximal in lateral (sensorimotor) sectors, thus mimicking the effects seen after cocaine alone, or co-administration of fluoxetine. However, in contrast to fluoxetine, this 5-HT1B agonist did not influence methylphenidate-induced expression of Homer1a. CP94253 also potentiated methylphenidate-induced locomotor activity. These findings indicate that stimulation of the 5-HT1B receptor can enhance methylphenidate (dopamine)-induced gene regulation. This receptor may thus participate in the potentiation induced by fluoxetine (serotonin) and may serve as a pharmacological target to attenuate methylphenidate+SSRI-induced “cocaine-like” effects.
Keywords: dopamine, serotonin, gene expression, psychostimulant, serotonin reuptake inhibitor, SSRI antidepressant, striatum
Introduction
There is increasing use of psychotropic medications (e.g., psychostimulants, antidepressants) in children and adolescents. For example, psychostimulants such as the dopamine (and norepinephrine) reuptake inhibitor methylphenidate (MP) are useful in the treatment of attention-deficit/hyperactivity disorder (ADHD) (Castle et al., 2007; Kollins, 2008), which is diagnosed in up to 12% of school-age children in the United States (Kollins, 2008; Collins and Cleary, 2016). According to recent reports, up to 3.5 million US children age 4–17 years were taking psychostimulant medications in 2011 (Swanson et al., 2011; Visser et al., 2014). Perhaps more troubling is the increasing abuse of medical psychostimulants such as MP as “cognitive enhancers” or party drugs, as such use tends to entail more high-level drug exposure (Steiner and Van Waes, 2013). Such non-medical use is well-documented (Kollins, 2008; Wilens et al., 2008; Benson et al., 2015). For example, a 2008 US survey found that about 8.5% of the population age 12 and older admitted to a history of non-medical use of prescription psychostimulants, and it was estimated that as many as 11 million prescriptions out of 38 million may have been diverted for non-medical use (Swanson et al., 2011).
Exposure to psychotropic agents in children and adolescents is of great concern because preclinical studies show that such drugs have the potential to interfere with normal brain development and facilitate long-term neurobehavioral maladaptations, including addiction and other neuropsychiatric disorders (for reviews, see Carlezon and Konradi, 2004; Andersen, 2005; Marco et al., 2011; Steiner et al., 2014).
Long-term neuronal and behavioral changes induced by such drugs are typically mediated by altered gene regulation. Research shows that MP, for example, can produce changes in the expression of many genes, similar to the effects of illicit psychostimulants such as cocaine (Yano and Steiner, 2007; Steiner and Van Waes, 2013), although other genes appear less affected (Yano and Steiner, 2007). Cocaine primarily blocks the reuptake of dopamine and serotonin, while MP blocks the dopamine transporter, but not the serotonin transporter (e.g., Kuczenski and Segal, 1997; see Yano and Steiner, 2007). Dopamine action is critical for the molecular impact of cocaine (e.g., Bhat and Baraban, 1993), but an important contribution of serotonin to the behavioral (Muller and Huston, 2006) and molecular effects of cocaine (Bhat and Baraban, 1993; Lucas et al., 1997; Horner et al., 2005) is also well established. The lack of serotonin effects for MP may therefore explain its more moderate effects on gene regulation (Yano and Steiner, 2007).
This notion is supported by our findings from a series of studies showing that serotonin action potentiates MP-induced gene regulation. More specifically, combining MP with a selective serotonin reuptake inhibitor (SSRI; SERT) antidepressant, such as fluoxetine (FLX) or citalopram, in doses that by themselves have no effect on gene regulation, potentiates MP-induced gene regulation in the striatum (for review, see Van Waes and Steiner, 2015). This potentiating effect was demonstrated for acute induction of immediate-early genes (IEGs) such as Zif268, c-Fos, and Homer1a by MP (Steiner et al., 2010; Van Waes et al., 2010), as well as for expression of neuropeptides (Van Waes et al., 2015) and blunting (repression; Renthal and Nestler, 2008) of IEG induction (Beverley et al., 2014; Van Waes et al., 2014), after repeated MP+FLX treatment.
These gene regulation effects are strikingly similar to those induced by illicit psychostimulants such as cocaine and amphetamine (Steiner and Van Waes, 2013), and are considered integral part of the molecular basis of addiction (Berke and Hyman, 2000; Nestler, 2001; Nestler, 2012). Zif268 and c-Fos encode transcription factors that regulate the activity of other genes (Knapska and Kaczmarek, 2004), and both are implicated in long-term neurobehavioral changes induced by psychostimulants. For example, Zif268 is critical for place preference conditioning by cocaine (Valjent et al., 2006) and for reconsolidation of cocaine memories (Lee et al., 2005; Théberge et al., 2010), among other effects (Valjent et al., 2006). Homer proteins are scaffolding proteins that help cluster glutamate receptors at the synapse and link these to internal calcium stores. Homer1 (Homer1a) also plays a role in receptor trafficking and other mechanisms of synaptic plasticity (Xiao et al., 2000; Thomas, 2002; Diering et al., 2017), and has long been implicated in drug-induced abnormal neuroplasticity related to addiction (for review, see Szumlinski et al., 2008).
The mechanisms underlying this serotonin potentiation of MP (dopamine)-induced gene regulation are not clear. This is partly due to the complexity in the interactions between serotonin and dopamine transmission as these are mediated by several serotonin receptor subtypes in multiple brain regions (Muller and Huston, 2006; Cunningham and Anastasio, 2014; De Deurwaerdère and Di Giovanni, 2017). For example, a number of serotonin receptor subtypes are expressed in striatal neurons themselves (Barnes and Sharp, 1999; De Deurwaerdère and Di Giovanni, 2017), and these could thus directly modulate the molecular effects of dopamine input in these neurons.
One of the most highly expressed serotonin receptors in the striatum is the 5-HT1B receptor (Barnes and Sharp, 1999; De Deurwaerdère and Di Giovanni, 2017). A number of studies have demonstrated a role for this receptor in the regulation of behavioral effects (e.g., Parsons et al., 1998; Neumaier et al., 2002; Pentkowski et al., 2012) and gene regulation induced by cocaine (Lucas et al., 1997; Castanon et al., 2000). Moreover, a facilitatory role for 5-HT1B in behavioral activation by MP has also been shown (Borycz et al., 2008). We previously investigated whether MP and/or FLX treatment regulated the expression of 5-HT1B in the striatum (Van Waes et al., 2015). Indeed, our results demonstrated that repeated treatment with MP also increases the expression of 5-HT1B (but not 5-HT2C) in striatal neurons and that this effect is also potentiated by co-treatment with the SSRI FLX (Van Waes et al., 2015). Moreover, the normal expression of 5-HT1B is highest in the lateral striatum (Van Waes et al., 2015), which roughly matches the distribution of the FLX-induced potentiation of gene regulation seen in our studies (see above).
In the present study, we investigated whether activation of 5-HT1B regulates MP-induced gene expression by assessing the effects of the selective 5-HT1B agonist CP94253 (Borycz et al., 2008; Pentkowski et al., 2008) on the induction of our IEG markers Zif268, c-Fos and Homer1a. These effects were contrasted with those previously observed with FLX. Our results show that the 5-HT1B agonist CP94253 potentiates MP-induced expression of Zif268 and c-Fos, but not Homer1a, thus matching in part the effects induced by FLX co-treatment.
Materials and methods
Animals
Male Sprague–Dawley rats (five weeks old at the time of the drug treatment; Harlan, Madison, WI, USA) were housed 2–3 per cage under standard laboratory conditions (12:12h light/dark cycle, lights on at 07:00h; with food and water available ad libitum). Experiments were performed between 13:00 and 17:00h. Prior to the drug treatment, the rats were allowed one week of acclimation during which time they were repeatedly handled. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
Drug treatment
Rats (n=5–9 per group) received an injection of vehicle (V; i.p.), or the selective 5-HT1B receptor agonist CP94253 [3 (CP3) or 10 mg/kg (CP10) in 0.02% ascorbic acid, 1 ml/kg; Tocris Bioscience, Minneapolis, MN, USA] (Borycz et al., 2008; Pentkowski et al., 2008), followed 15 min later by an injection of V or methylphenidate HCl (5 mg/kg, MP; Sigma, St. Louis, MO, USA). MP plus fluoxetine HCl (5 mg/kg each, MP+FLX; Sigma) were administered in the same injection (Steiner et al., 2010; Van Waes et al., 2014). After the last injection, the rat was placed in an open-field apparatus (43 × 43 cm), and locomotion (ambulatory counts) was measured for 40 min with an activity monitoring system (Truscan, Coulbourn Instruments, Allentown, PA, USA).
The MP and FLX doses were based on our previous studies. The MP dose of 5 mg/kg produces robust changes in striatal gene regulation, and these effects are potentiated by adding FLX (5 mg/kg) (e.g., Steiner et al., 2010; Van Waes et al., 2014). This MP dose is at the upper limit or above of doses used clinically and is relevant for MP abuse (see Steiner and Van Waes, 2013). The FLX dose is in the clinically relevant range (Dulawa et al., 2004; Rantamäki et al., 2007).
Tissue preparation and in situ hybridization histochemistry
After the behavioral test, the rats were killed with CO2, and the brain was rapidly removed, frozen in isopentane cooled on dry ice and then stored at −30 °C until cryostat sectioning. Coronal sections (12 μm) were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL, USA), dried on a slide warmer and stored at −30 °C. In situ hybridization histochemistry was performed as described before (Willuhn et al., 2003; Van Waes et al., 2015). Oligonucleotide probes (48-mers; Invitrogen, Rockville, MD, USA) were labeled with [33P]-dATP. The probes had the following sequence: Zif268 (Egr1), complementary to bases 352–399, GenBank accession number M18416; c-Fos, bases 207–254, X06769; Homer1a, bases 1493–1540, AB003726. Hybridization and washing procedures were as reported (Willuhn et al., 2003; Van Waes et al., 2015). The sections were apposed to X-ray film (BioMax MR-2, Kodak) for 5–9 days.
Analysis of autoradiograms
Gene expression in the striatum was assessed in sections from 3 rostrocaudal levels [rostral, approximately at +1.6 mm relative to bregma (Paxinos and Watson, 1998); middle, +0.4 (Fig. 1); caudal, −0.8] in a total of 23 sectors. These sectors reflect functional domains and are mostly defined by their predominant cortical inputs (see Willuhn et al., 2003; Yano and Steiner, 2005a). Eighteen of these sectors represent the caudate-putamen, and 5 the nucleus accumbens (Fig. 2).
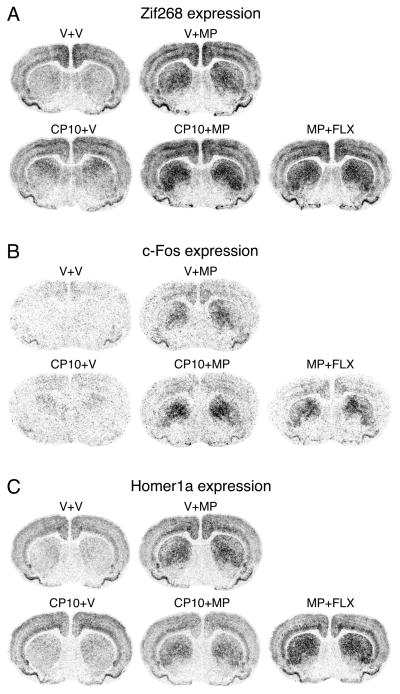
Figure 1.
Differential effects of 5-HT1B receptor stimulation on IEG expression induced by methylphenidate (MP) in the striatum. Illustrations of film autoradiograms depict Zif268 (A), c-Fos (B) and Homer1a expression (C) in coronal sections from the middle striatum in rats that received an injection of vehicle (V) or the 5-HT1B receptor agonist CP94253 (10 mg/kg; CP10), followed 15 min later by an injection of V, MP (5 mg/kg), or MP plus fluoxetine (5 mg/kg each; MP+FLX) (groups V+V, V+MP, CP10+V, CP10+MP or MP+FLX), and were killed 40 min later. The maximal hybridization signal is in black. The 5-HT1B receptor agonist potentiated MP-induced expression of Zif268 and c-Fos, but not Homer1a (MP+CP10). In contrast, FLX potentiates MP-induced expression of all three IEGs (MP+FLX).
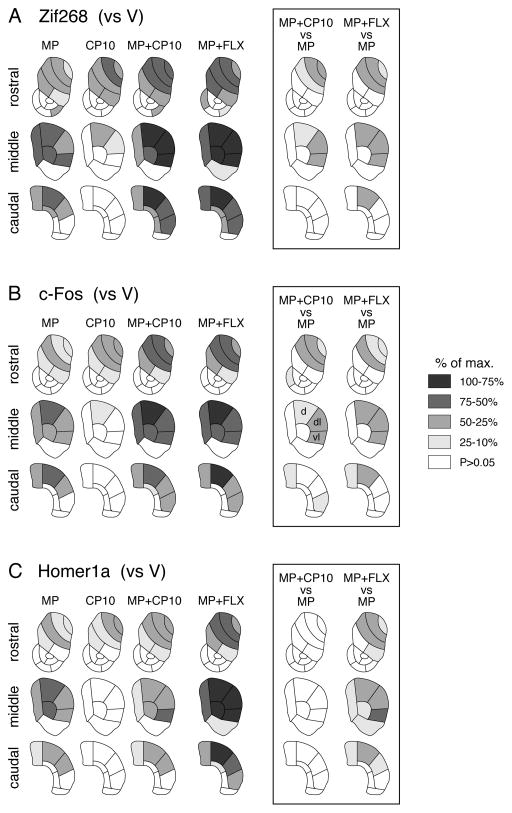
Figure 2.
Topography of 5-HT1B agonist effects on basal and methylphenidate (MP)-induced gene expression. Maps depict the distribution of gene induction (i.e., the difference vs. vehicle controls, V) for Zif268 (A), c-Fos (B) and Homer1a (C) across the 23 sectors in the rostral, middle and caudal striatum in rats treated with MP (5 mg/kg), the 5-HT1B receptor agonist CP94253 (10 mg/kg; CP10), MP+CP10, or MP plus fluoxetine (5 mg/kg; MP+FLX). The differences between MP+CP10 and MP, and between MP+FLX and MP groups are also shown (box). The data are normalized relative to the maximal induction observed (% of max.) for each gene. Sectors with a statistically significant induction or difference (P<0.05) are shaded as indicated. Sectors without significant difference are in white. The 5-HT1B agonist by itself (CP10) produced some increase in expression for all three genes. This effect was maximal in the rostral dorsal striatum and dissipated towards caudal. CP94253 potentiated MP-induced gene expression (MP+CP10 vs. MP), which was most robust in the lateral sectors of the middle striatum. However, this effect was only seen for Zif268 and c-Fos, but not for Homer1a. In contrast, FLX, in a dose that by itself produces no gene regulation (see text), potentiated induction of all three IEGs (MP+FLX vs. MP). This effect was also maximal in the lateral striatum on the middle level. d, dorsal; dl, dorsolateral; vl, ventrolateral.
Hybridization signals on film autoradiograms were measured by densitometry (NIH Image; Wayne Rasband, NIMH, Bethesda, MD, USA), as described (Van Waes et al., 2015). Mean densities were corrected for background by subtracting mean density values measured over white matter (corpus callosum). Values from corresponding regions in the two hemispheres were then averaged. The illustrations of film autoradiograms displayed in Figure 1 are computer-generated images, and are contrast-enhanced where necessary.
Statistics
Treatment effects were determined by one-factor ANOVA. Newman-Keuls post hoc tests were used to describe differences between individual groups (Statistica, StatSoft, Tulsa, OK, USA). The distribution of changes in gene induction throughout the striatum was illustrated by maps (Fig. 2). For these maps, the difference in gene expression (vs. V or as indicated) in a given sector was expressed as the percentage of the maximal difference observed for either gene (% max.). The regional distributions of gene regulation effects across the 23 striatal sectors were compared by Pearson correlations.
Results
Effects of 5-HT1B receptor agonist alone: Increased gene expression in the rostral dorsal striatum for all three IEGs
Acute administration of the 5-HT1B receptor agonist CP94253 (10 mg/kg; CP10 group) alone was sufficient to induce modest IEG expression in the striatum (Figs. 1, 2, 3). This response had a distinctive regional distribution (Figs. 1, 2). Gene induction followed a rostrocaudal gradient, with the highest levels in the dorsal and dorsolateral striatal sectors on the rostral level and minor (dorsal) or no effects on the middle and caudal levels. This effect was found for all three IEGs [Zif268, c-Fos, Homer1a: rostral: significantly increased (vs. V group) in 5, 5, 4 of the 10 sectors, respectively; middle: 2, 1, 0 of 6 sectors; caudal: 0, 0, 0 of 7 sectors; Fig. 2] and was indeed highly correlated between the three IEGs across the 23 striatal sectors (Zif268 x c-Fos, r=0.9781, P<0.001; Zif268 x Homer1a, r=0.9274, P<0.001; c-Fos x Homer1a, r=0.9069, P<0.001).
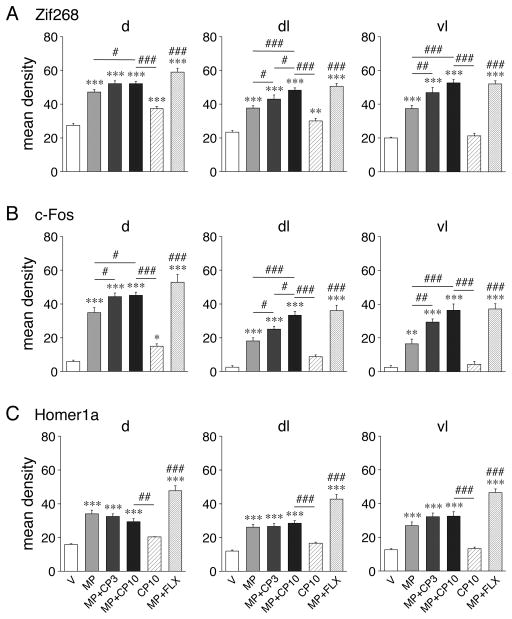
Figure 3.
5-HT1B agonist-induced potentiation of methylphenidate (MP)-induced IEG expression in the middle striatum. Mean density values (mean ± SEM) for Zif268 (A), c-Fos (B) and Homer1a expression (C) in rats that received vehicle (V), MP (5 mg/kg), MP plus the 5-HT1B receptor agonist CP94253 (3 or 10 mg/kg; MP+CP3, MP+CP10), CP10 alone, or MP plus fluoxetine (5 mg/kg; MP+FLX) are given for the dorsal (d), dorsolateral (dl), and ventrolateral (vl) sectors on the middle level. CP94253 potentiated MP-induced expression of Zif268 and c-Fos, but not Homer1a, in a dose-dependent manner. This effect was most robust in the lateral sectors. FLX potentiated MP-induced expression of all three IEGs. *P<0.05, **P<0.01, ***P<0.001 vs. V; #P<0.05, ##P<0.01, ###P<0.001 vs. MP (potentiation) and as indicated.
5-HT1B receptor stimulation potentiates methylphenidate-induced expression of Zif268 and c-Fos, but not Homer1a
MP (5 mg/kg) alone induced IEG expression with a different striatal distribution (Figs. 1, 2, 3). This effect was present on rostral, middle and caudal levels and was maximal in the dorsal and central striatum on the middle and caudal levels (Figs. 1, 2) (Zif268, c-Fos, Homer1a: rostral: 6, 5, 4 of 10 sectors, respectively; middle: 5, 5, 5 of 6; caudal: 4, 3, 3 of 7; Fig. 2). Again, this distribution was similar for all three IEGs (Zif268 x c-Fos, r=0.9478, P<0.001; Zif268 x Homer1a, r=0.9589, P<0.001; c-Fos x Homer1a, r=0.9582, P<0.001).
The 5-HT1B agonist CP94253 given together with MP (MP+CP) produced more pronounced induction of Zif268 and c-Fos than MP alone (potentiation), on all three striatal levels (Fig. 1A and B, 2A and B, 3A and B). This effect was dose-dependent; MP+CP10 produced a more robust increase than MP+CP3 in the lateral striatum (Fig. 3A, B). Overall, the striatal sectors affected by MP+CP10 (Zif268, c-Fos: rostral: 6, 5 of 10 sectors; middle: 5, 5 of 6; caudal: 6, 5 of 7; Fig. 2) were similar to those with MP alone. However, the maps (Fig. 2) show that adding CP10 to MP produced a shift in the peak induction towards the lateral striatum compared to MP alone, for both Zif268 and c-Fos. This potentiation was most robust on the middle level and displayed a medial-lateral gradient, with greatest increases in the dorsolateral and ventrolateral sectors on the middle level (Fig. 3). The statistical comparison of the effects in MP+CP10 vs. MP groups (Fig. 2, box) confirmed that MP+CP10 animals displayed significantly stronger Zif268 and c-Fos responses in the rostral (3 and 3 sectors), middle (3, 3), and caudal striatum (0, 2), with the most pronounced differences occurring in the middle, lateral striatum. Considering that part of the signal in the rostral striatum likely reflects an effect by CP10 alone (see above), the potentiation was most robust in the middle, lateral striatum.
In contrast to Zif268 and c-Fos, Homer1a induction by MP was not affected by adding CP94253 (Figs. 1C, 2C, 3C). In MP+CP10-treated animals, no significantly greater Homer1a induction than in MP-treated animals was seen in any of the striatal sectors (MP+CP10 vs. MP, potentiation; Fig. 2C, box). This difference was also reflected by weaker or no correlations between Homer1a vs. Zif268 and c-Fos (MP+CP10 minus MP; Zif268 x c-Fos, r=0.8320, P<0.001; Zif268 x Homer1a, r=0.6670, P<0.001; c-Fos x Homer1a, r=0.4821, P<0.05).
For a further comparison of these responses, we also assessed the effects of a MP+FLX combination (Figs. 1, 2, 3). As previously reported (Steiner et al., 2010; Van Waes et al., 2010), FLX (5 mg/kg), which by itself has no effect on these genes (Van Waes et al., 2010; Van Waes et al., 2014; Van Waes et al., 2015), potentiated MP-induced gene expression for all three IEGs. MP+FLX-treated animals showed increased expression (vs. V controls) on rostral, middle and caudal levels (Zif268, c-Fos, Homer1a: rostral: 7, 5, 5 of 10 sectors; middle: 6, 5, 6 of 6; caudal: 6, 4, 5 of 7; Fig. 2). A significantly greater response in MP+FLX- than in MP-treated animals (potentiation) was seen for all three IEGs (Zif268, c-Fos, Homer1a: rostral: 3, 3, 4 of 10 sectors; middle: 3, 3, 6 of 6; caudal: 1, 2, 3 of 7; Fig. 2). Consequently, these differences between MP+FLX- and MP-treated animals were highly correlated between all three IEGs (Zif268 x c-Fos, r=0.9498, P<0.001; Zif268 x Homer1a, r=0.9179, P<0.001; c-Fos x Homer1a, r=0.9498, P<0.001). Thus, in contrast to CP94253, FLX also potentiates MP effects on Homer1a expression.
Comparison of 5-HT1B agonist effects with distribution of 5-HT1B receptor expression in the striatum
We next used correlation analysis to compare the effects of these drugs on IEG expression with the distribution of 5-HT1B receptor mRNA in the striatum, as reported before (Van Waes et al., 2015). The distribution of the effects of CP94253 (10 mg/kg) alone was not or poorly related to that of 5-HT1B mRNA expression (values taken from Van Waes et al., 2015) across the 23 striatal sectors (Zif268 x 5-HT1B, r=0.3350, P>0.05; c-Fos x 5-HT1B, r=0.3098, P>0.05; Homer1a x 5-HT1B, r=0.4231, P<0.05). On the other hand, the regional distribution of the CP10-induced potentiation of MP-induced IEG expression (i.e., MP+CP10 minus MP values) better matched the receptor mRNA distribution, for Zif268 (Zif268 x 5-HT1B, r=0.6319, P<0.002) and c-Fos (c-Fos x 5-HT1B, r=0.4392, P<0.05), but not Homer1a (no potentiation; Homer1a x 5-HT1B, r=0.3476, P>0.05).
5-HT1B receptor stimulation potentiates methylphenidate-induced locomotor activity
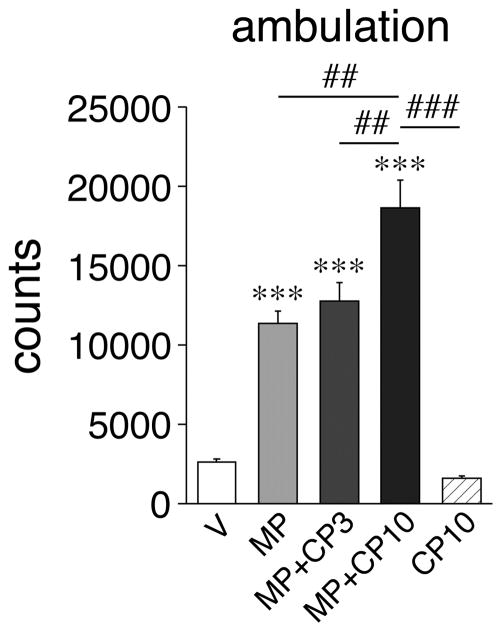
MP treatment alone significantly increased ambulation in the open-field test, while CP94253 (10 mg/kg) alone had no effect, as compared with V controls (Fig. 4). The MP+CP10 combination produced a significantly greater increase in ambulation than MP alone (potentiation) (Fig. 4), consistent with findings by others (Borycz et al., 2008). Overall, this behavioral profile matched the profile of IEG induction in the middle lateral striatum (see Fig. 3).
Figure 4.
Drug effects on open-field behavior. Ambulation counts (mean ± SEM) during 40 min after the last drug injection are shown for animals that received vehicle (V), methylphenidate (5 mg/kg; MP), MP plus the 5-HT1B receptor agonist CP94253 (3 or 10 mg/kg; MP+CP3, MP+CP10), or CP10 alone. CP94253 potentiated MP-induced ambulation in a dose-dependent manner. ***P<0.001 vs. V; ##P<0.01, ###P<0.001 vs. MP (potentiation) and as indicated.
Discussion
The present study investigated potential mechanisms mediating the potentiation of MP-induced gene regulation in the striatum by SSRIs, such as FLX, resulting in a “cocaine-like” profile of gene expression. Our main findings demonstrate that acute stimulation of the 5-HT1B receptor by the selective 5-HT1B agonist CP94253 potentiates the induction of the IEGs Zif268 and c-Fos by MP, thus mimicking previously observed FLX effects. The regional distribution of this potentiation matched that of the FLX-induced potentiation of gene regulation as well as the distribution of 5-HT1B receptor expression in the striatum. However, in contrast to FLX, CP94253 had no effect on MP-induced expression of the IEG Homer1a. These findings are consistent with a role for 5-HT1B in FLX-induced gene regulation, but indicate that other serotonin receptor subtypes may also be involved in these FLX effects.
Interactions between dopamine and serotonin receptors in gene regulation in the striatum: Effects of the 5-HT1B agonist CP94253
Psychostimulant-induced gene regulation in the striatum is the result of interactions between several neurotransmitter systems (Steiner and Van Waes, 2013). For example, numerous studies have demonstrated that the molecular effects of cocaine are principally a result of dopamine receptor stimulation (for review, see Steiner and Van Waes, 2013), but this impact of cocaine is also influenced by serotonin (e.g., Bhat and Baraban, 1993). Further studies have identified the 5-HT1B serotonin receptor subtype as a facilitator of the molecular effects of cocaine (Lucas et al., 1997; Castanon et al., 2000).
The gene regulation effects of the dopamine reuptake inhibitor MP are also mediated by dopamine receptors (Yano et al., 2006; Alburges et al., 2011). However, our previous studies showed that these effects are more limited compared to those of cocaine (Yano and Steiner, 2007). Indeed, we then demonstrated that increasing the serotonin tone, by combining MP with FLX, potentiates MP-induced gene regulation (Van Waes and Steiner, 2015). Given the known facilitatory role for the 5-HT1B receptor in cocaine-induced gene regulation (Lucas et al., 1997; Castanon et al., 2000), the present study assessed the impact of 5-HT1B stimulation on MP-induced IEG expression.
Consistent with a facilitatory role for 5-HT1B, our findings show that the 5-HT1B agonist CP94253 combined with MP enhanced, in a dose-dependent manner, the expression of Zif268 and c-Fos over the expression induced by MP alone, thus mimicking FLX effects in inducing a “cocaine-like” pattern. CP94253 by itself also produced some (modest) IEG induction in some striatal areas, most notably in the rostral dorsal striatum. The increase by the combined treatment may thus reflect an additive effect in those areas. In contrast, in the middle and caudal striatum, where CP94253 alone had minimal or no effects, this increase represents a potentiation of MP-induced IEG expression. This potentiation was maximal in the lateral (sensorimotor) striatum on the middle level, which also mimicked the regional distribution of the FLX-induced potentiation of MP-induced IEG regulation (Van Waes et al., 2010; Van Waes et al., 2014).
The IEG response to CP94253 alone in the rostral striatum was seen for all three IEGs, including Homer1a. This finding notably contrasted with the CP94253-induced potentiation in the middle and caudal striatum that was only found for Zif268 and c-Fos, but not Homer1a. Moreover, unlike the potentiation, this effect by CP94253 alone did not match the distribution of striatal 5-HT1B expression. This dissociation indicates differential underlying mechanisms. Given that striatal gene regulation is also dependent on glutamate receptor stimulation/cortical input (Steiner and Van Waes, 2013), it is possible that this response in the rostral striatum reflected enhanced cortical (or other) input as a consequence of 5-HT1B receptor stimulation outside of the striatum.
On the other hand, the CP94253-induced potentiation of MP-induced IEG expression in the middle and caudal striatum paralleled the distribution of 5-HT1B mRNA expression, consistent with a role for 5-HT1B receptors expressed by striatal neurons in this potentiation.
Potential mechanisms mediating the potentiation of IEG induction by 5-HT1B stimulation
In our studies, systemic drug treatments were employed, which precludes conclusions regarding the specific site of action of the 5-HT1B agonist. For example, we cannot exclude potential metabolic or pharmacokinetic interactions between the 5-HT1B agonist and MP, which might have resulted in increased MP levels in the brain (Zhu et al., 2010). However, elevated MP plasma levels would be expected to enhance striatal gene expression throughout the striatum, as we have shown for higher MP doses (Yano and Steiner, 2005b), but this is not the case for MP+CP94253 (or MP+FLX) combinations. Instead, our present and previous findings show that the FLX- or CP94253-induced potentiation of gene regulation by MP preferentially occurs in the lateral striatum, where levels of 5-HT1B mRNA are highest (Van Waes et al., 2015). These matching regional patterns (correlations) for the Zif268/c-Fos potentiation and 5-HT1B receptors (mRNA) in the striatum suggest that local receptors may play a role.
Several mechanisms involving such 5-HT1B receptors are conceivable. The 5-HT1B receptor is predominantly located presynaptically on neuronal terminals and inhibits transmitter release (Boschert et al., 1994; De Deurwaerdère and Di Giovanni, 2017). Given that the distribution of the potentiation of gene regulation in striatal projection neurons roughly matches the striatal 5-HT1B mRNA distribution, 5-HT1B receptors expressed by striatal projection neurons (heteroreceptors) are likely involved, rather than 5-HT1B receptors on serotonin terminals (autoreceptors; Barnes and Sharp, 1999; De Deurwaerdère and Di Giovanni, 2017) or other striatal inputs (De Deurwaerdère and Di Giovanni, 2017). 5-HT1B receptors expressed by striatal projection neurons are located in their terminal regions, for example, in the substantia nigra, but also on the extensive local axon collaterals in the striatum (Wilson and Groves, 1980).
One potential mechanism for 5-HT1B action, as previously proposed by Hen and colleagues (Lucas et al., 1997; Castanon et al., 2000), involves disinhibition of dopamine input to the striatum, mediated by serotonin action on 5-HT1B receptors in the substantia nigra. In this scenario, the relevant 5-HT1B receptors would be expressed by striatonigral (direct pathway) neurons and be situated on their terminals in the substantia nigra to inhibit GABA release from these neurons (Ding et al., 2015; see Castanon et al., 2000, for discussion). Reduced GABA release would be expected to increase (disinhibit) dopamine input to striatal neurons (Castanon et al., 2000). Enhanced dopamine input would be consistent with increased dopamine-mediated gene regulation after MP plus SSRI co-treatment. Alternatively, local inhibition of GABA release by serotonin acting on 5-HT1B receptors located on terminals of local striatal axon collaterals of striatonigral neurons (Wilson and Groves, 1980), or other GABA neurons, and consequent disinhibition of striatal neurons is another potential mechanism by which 5-HT1B receptors could enhance gene regulation by dopamine input. Future studies with local experimental manipulations will have to discern between these mechanistic possibilities.
Differential effects of 5-HT1B stimulation on Zif268, c-Fos vs. Homer1a expression in the striatum
As mentioned above, the 5-HT1B agonist CP94253 (10 mg/kg) potentiated the MP-induced expression of Zif268 and c-Fos, but not Homer1a. This differential response contrasts with the effects of FLX. FLX potentiates MP-induced regulation of Homer1a to a similar degree as regulation of Zif268 and c-Fos. This was demonstrated for acute IEG induction by MP+FLX (present results) as well as for blunting (repression) of IEG induction after repeated MP+FLX treatment (Beverley et al., 2014; Van Waes et al., 2014).
Differential regulation for Homer1a vs. Zif268/c-Fos was also seen in some of our earlier studies, and serotonin appeared to be a factor as well. Thus, repeated cocaine treatment (i.e., dopamine+serotonin activation) resulted in similar robust blunting for Homer1a and Zif268 induction (Unal et al., 2009). In contrast, repeated methylphenidate (dopamine) treatment produced robust Zif268 blunting, but had minimal or no effects for Homer1a (Cotterly et al., 2007). However, as discussed above, adding FLX (serotonin) to MP produced similar blunting for both Homer1a and Zif268 (Beverley et al., 2014; Van Waes et al., 2014). These results highlight the critical role for serotonin in Homer1a regulation.
In the present study, we did not assess whether a higher dose of the 5-HT1B receptor agonist CP94253 might potentiate induction of Homer1a to determine whether stimulation of 5-HT1B can be sufficient also for Homer1a. In either case, this observed dissociation for Zif268 and c-Fos vs. Homer1a demonstrates differential regulatory mechanisms for these genes.
The mechanisms underlying these effects remain unclear. Transcription dynamics may play a role. In the striatum, robust psychostimulant-induced Zif268 and c-Fos signals are detected within 15 min, and peak around 30–60 min after drug administration (i.e., cocaine, MP; Steiner and Van Waes, 2013). In contrast, the time course for the Homer 1a signal is delayed (Yano and Steiner, 2005a; Steiner and Van Waes, 2013). Homer 1 is a larger gene (~100 kb, 10 exons), and the Homer 1a-specific signal (intron 5) only appears around 30 min after transcription initiation (Bottai et al., 2002). For MP, this signal peaks around 60 min after drug administration or later (Yano and Steiner, 2005a; Steiner and Van Waes, 2013). At 40 min (present study), this signal was thus on the ascending limb. While these differential dynamics may indicate a less robust Homer 1a signal at 40 min, this signal was significantly above threshold following treatment with the 5-HT1B receptor agonist CP94253 (10 mg/kg) alone (in the rostral striatum) and for MP alone (all 3 striatal levels), yet the 5-HT1B agonist did not potentiate the MP-induced signal. These differential dynamics can thus hardly account for the lack of potentiation for Homer 1a by CP94253.
Given that FLX (serotonin) did potentiate the Homer 1a signal in the present study, while the 5-HT1B agonist was insufficient, the most parsimonious explanation is that stimulation of additional 5-HT receptor subtypes is necessary for the Homer 1a potentiation, but not Zif268/c-Fos potentiation. Several other serotonin receptor subtypes are present in the striatum, and some of these also facilitate psychostimulant-induced gene regulation (e.g., 5-HT2A, Szucs et al., 2005; 5-HT3, Genova and Hyman, 1998). In addition, it is conceivable that serotonin receptor subtypes outside of the striatum are involved, by facilitating cortico-/thalamostriatal input which drives psychostimulant-induced gene regulation in the striatum (Steiner and Van Waes, 2013). Future studies will have to clarify whether other serotonin receptors, alone or in combination with 5-HT1B, contribute to facilitate MP-induced gene regulation.
Clinical relevance and conclusions
Our findings show that combining SSRIs with MP potentiates gene regulation in the striatum, which mimics gene regulation by cocaine in several aspects (Steiner and Van Waes, 2013). Such combination treatments are indicated for various conditions. These include ADHD/depression comorbidity (Safer et al., 2003; Bhatara et al., 2004; Kollins, 2008), which occurs with up to 40% prevalence in pediatric ADHD (Waxmonsky, 2003; Spencer, 2006), as well as other conditions (e.g., Lavretsky et al., 2003; Nelson, 2007; Ishii et al., 2008). Dual exposure to these medications also occurs as a result of “cognitive enhancer” use (Kollins, 2008) by patients on SSRIs.
Given that the affected genes are implicated in addiction (Berke and Hyman, 2000; Nestler, 2001; Nestler, 2012), the question arises whether this MP+SSRI combination treatment may increase the risk for subsequent substance use disorder. Preclinical studies suggest that this may be the case. For example, MP+FLX treatment in juvenile rats enhanced the rewarding effects of cocaine later in life, as determined in the cocaine place preference conditioning model (Warren et al., 2011). Moreover, such a combination treatment has been shown to facilitate the subsequent acquisition of cocaine self-administration in rats (Marinelli et al., 2015). Both effects are suggestive of an increased cocaine abuse/addiction risk after MP+SSRI exposure. Identifying serotonin receptors that participate in this SSRI-mediated potentiation of MP-induced gene regulation and associated behavioral changes may provide pharmacological targets to attenuate this risk.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Institute on Drug Abuse grants DA031916 and DA011261.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alburges ME, Hoonakker AJ, Horner KA, et al. Methylphenidate alters basal ganglia neurotensin systems through dopaminergic mechanisms: A comparison with cocaine treatment. J Neurochem. 2011;117:470–478. doi: 10.1111/j.1471-4159.2011.07215.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18:50–76. doi: 10.1007/s10567-014-0177-z. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Beverley JA, Piekarski C, Van Waes V, et al. Potentiated gene regulation by methylphenidate plus fluoxetine treatment: Long-term gene blunting (Zif268, Homer1a) and behavioral correlates. Basal Ganglia. 2014;4:109–116. doi: 10.1016/j.baga.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- Bhatara V, Feil M, Hoagwood K, et al. National trends in concomitant psychotropic medication with stimulants in pediatric visits: practice versus knowledge. J Atten Disord. 2004;7:217–226. doi: 10.1177/108705470400700404. [DOI] [PubMed] [Google Scholar]
- Borycz J, Zapata A, Quiroz C, et al. 5-HT(1B) receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008;33:619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, et al. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bottai D, Guzowski JF, Schwarz MK, et al. Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. J Neurosci. 2002;22:167–175. doi: 10.1523/JNEUROSCI.22-01-00167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WAJ, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, et al. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol Biochem Behav. 2000;67:559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Castle L, Aubert RE, Verbrugge RR, et al. Trends in medication treatment for ADHD. J Atten Disord. 2007;10:335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- Collins KP, Cleary SD. Racial and ethnic disparities in parent-reported diagnosis of ADHD: National Survey of Children’s Health (2003, 2007, and 2011) J Clin Psychiatry. 2016;77:52–59. doi: 10.4088/JCP.14m09364. [DOI] [PubMed] [Google Scholar]
- Cotterly L, Beverley JA, Yano M, et al. Dysregulation of gene induction in corticostriatal circuits after repeated methylphenidate treatment in adolescent rats: Differential effects on zif 268 and homer 1a. Eur J Neurosci. 2007;25:3617–3628. doi: 10.1111/j.1460-9568.2007.05570.x. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76:460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, Di Giovanni G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog Neurobiol. 2017;151:175–236. doi: 10.1016/j.pneurobio.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Diering GH, Nirujogi RS, Roth RH, et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017;355:511–515. doi: 10.1126/science.aai8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Robust presynaptic serotonin 5-HT(1B) receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J Neurophysiol. 2015;113:3397–3409. doi: 10.1152/jn.00831.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, et al. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Genova LM, Hyman SE. 5-HT3 receptor activation is required for induction of striatal c-Fos and phosphorylation of ATF-1 by amphetamine. Synapse. 1998;30:71–78. doi: 10.1002/(SICI)1098-2396(199809)30:1<71::AID-SYN9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Horner KA, Adams DH, Hanson GR, et al. Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Ishii M, Tatsuzawa Y, Yoshino A, et al. Serotonin syndrome induced by augmentation of SSRI with methylphenidate. Psychiatry Clin Neurosci. 2008;62:246. doi: 10.1111/j.1440-1819.2008.01767.x. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord. 2008;12:115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kim MD, Kumar A, et al. Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J Clin Psychiatry. 2003;64:1410–1414. doi: 10.4088/jcp.v64n1202. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, et al. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Segu L, Hen R. 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol Pharmacol. 1997;51:755–763. doi: 10.1124/mol.51.5.755. [DOI] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Ruocco LA, et al. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci Biobehav Rev. 2011;35:1722–1739. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Beverley JA, Lamoureux L, et al. Fluoxetine potentiates methylphenidate-induced behavioral stereotypies and subsequent cocaine self-administration in rats. Soc Neurosci Abstr. 2015;44:51, 21. doi: 10.1016/j.addicn.2023.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol Sci. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nelson JC. Augmentation strategies in the treatment of major depressive disorder. Recent findings and current status of augmentation strategies. CNS Spectr. 2007;12(Suppl 22):6–9. doi: 10.1017/s1092852900016011. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci. 2012;10:136–143. doi: 10.9758/cpn.2012.10.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, et al. Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Pentkowski NS, Cheung TH, Toy WA, et al. Protracted withdrawal from cocaine self-administration flips the switch on 5-HT(1B) receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry. 2012;72:396–404. doi: 10.1016/j.biopsych.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegaliński E, Gołda A, Filip M. Effects of serotonin (5-HT)(1B) receptor ligands on cocaine-seeking behavior in rats. Pharmacol Rep. 2008;60:798–810. [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, DosReis S. Concomitant psychotropic medication for youths. Am J Psychiatry. 2003;160:438–449. doi: 10.1176/appi.ajp.160.3.438. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. ADHD and comorbidity in childhood. J Clin Psychiatry. 2006;67(Suppl 8):27–31. [PubMed] [Google Scholar]
- Steiner H, Van Waes V. Addiction-related gene regulation: Risks of exposure to cognitive enhancers vs. other psychostimulants. Prog Neurobiol. 2013;100:60–80. doi: 10.1016/j.pneurobio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Van Waes V, Marinelli M. Fluoxetine potentiates methylphenidate-induced gene regulation in addiction-related brain regions: Concerns for use of cognitive enhancers? Biol Psychiatry. 2010;67:592–594. doi: 10.1016/j.biopsych.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Warren BL, Van Waes V, et al. Life-long consequences of juvenile exposure to psychotropic drugs on brain and behavior. Prog Brain Res. 2014;211:13–30. doi: 10.1016/B978-0-444-63425-2.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Wigal TL, Volkow ND. Contrast of medical and nonmedical use of stimulant drugs, basis for the distinction, and risk of addiction: comment on Smith and Farah (2011) Psychol Bull. 2011;137:742–748. doi: 10.1037/a0024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs RP, Frankel PS, McMahon LR, et al. Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behav Neurosci. 2005;119:1173–1183. doi: 10.1037/0735-7044.119.5.1173. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge FR, Milton AL, Belin D, et al. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Unal CT, Beverley JA, Willuhn I, et al. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: Effects on zif 268 and homer 1a. Eur J Neurosci. 2009;29:1615–1626. doi: 10.1111/j.1460-9568.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbillé AG, et al. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Beverley J, Marinelli M, et al. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)-induced gene regulation in the adolescent striatum. Eur J Neurosci. 2010;32:435–447. doi: 10.1111/j.1460-9568.2010.07294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Ehrlich S, Beverley JA, et al. Fluoxetine potentiation of methylphenidate-induced gene regulation in striatal output pathways: Potential role for 5-HT1B receptor. Neuropharmacology. 2015;89:77–86. doi: 10.1016/j.neuropharm.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Steiner H. Fluoxetine and other SSRI antidepressants potentiate addiction-related gene regulation by psychostimulant medications. In: Pinna G, editor. Fluoxetine: Pharmacology, Mechanisms of Action and Potential Side Effects. Hauppauge, NY: Nova Science Publishers; 2015. p in press. [Google Scholar]
- Van Waes V, Vandrevala M, Beverley J, et al. Selective serotonin re-uptake inhibitors potentiate gene blunting induced by repeated methylphenidate treatment: Zif268 versus Homer1a. Addict Biol. 2014;19:986–995. doi: 10.1111/adb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014;53:34–46. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, et al. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxmonsky J. Assessment and treatment of attention deficit hyperactivity disorder in children with comorbid psychiatric illness. Curr Opin Pediatr. 2003;15:476–482. doi: 10.1097/00008480-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular injection of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yano M, Beverley JA, Steiner H. Inhibition of methylphenidate-induced gene expression in the striatum by local blockade of D1 dopamine receptors: Interhemispheric effects. Neuroscience. 2006;140:699–709. doi: 10.1016/j.neuroscience.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005a;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Topography of methylphenidate (Ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005b;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Appel DI, Peterson YK, et al. Identification of selected therapeutic agents as inhibitors of carboxylesterase 1: potential sources of metabolic drug interactions. Toxicology. 2010;270:59–65. doi: 10.1016/j.tox.2010.01.009. [DOI] [PubMed] [Google Scholar]