Abstract
Purpose
Genetic and preclinical studies have implicated fibroblast growth factor receptor (FGFR) signaling in the pathogenesis of adenoid cystic carcinoma (ACC). Dovitinib, a suppressor FGFR activity, may be active in ACC.
Methods
In a two-stage phase II study, 35 patients with progressive ACC were treated with dovitinib 500mg orally for 5 of 7 days continuously. The primary endpoints were objective response rate (ORR) and change in tumor growth rate (TGR). Progression-free survival (PFS), overall survival (OS), metabolic response, biomarker and QOL were secondary endpoints.
Results
Of thirty-four evaluable patients, two (6%) had a partial response and 22 (65%) had stable disease >4 months. Median PFS was 8.2 months and OS was 20.6 months. The slope of the overall TGR fell from 1.95 to 0.63 on-treatment (p<0.001). Toxicity was moderate; 63% of patients developed grade 3–4 toxicity, 94% required dose modifications, and 21% stopped treatment early. An early metabolic response based on 18FDG-PET scans was seen in 3/15 patients but did not correlate with RECIST response. MYB gene translocation was observed and significantly correlated with over-expression of MYB but did not correlate with FGFR1 phosphorylation or clinical response to dovitinib.
Conclusion
Dovitinib produced few objective responses in patients with ACC but did suppress the TGR with a PFS that compares favorably to those reported with other targeted agents. Future studies of more potent and selective FGFR inhibitors in biomarker-selected patients will be required to determine if FGFR signaling is a valid therapeutic target in ACC.
Keywords: dovitinib, TKI258, adenoid cystic carcinoma, MYB, FGFR, tumor growth rate
INTRODUCTION
Adenoid cystic carcinomas (ACC) are rare malignancies that afflict 1,200 individuals per year in the United States. The most common primary sites are the salivary gland and other regions in the head and neck, but they may also arise in the skin, breast, and uterus (1). Localized ACCs are usually treated with surgery and/or radiotherapy but local and distant recurrences are frequent due to the tumor’s propensity for perineural infiltration and to metastasize to the lung. Although the median survival for patients with metastatic ACC is about 4 years the disease often becomes more aggressive later in the course such that the median survival for patients whose disease had progressed within the previous 6 months is in the range of 18–20 months (1–4). Unfortunately, there is no effective systemic treatment for advanced ACC as standard cytotoxic chemotherapy agents produce tumor response rates in the range of 5–15% without a clear impact on patient survival (5–7). Targeted agents including cetuximab, erlotinib, gefitinib, vorinostat, and others have also shown limited activity against ACCs (3;4;8;9). One potential target, the tyrosine kinase receptor KIT, is overexpressed in the majority of these tumors but treatment of ACC patients with the KIT inhibitor, imatinib, produced no or little clinical benefit (10–12). Similarly, the multi-kinase inhibitors sunitinib and sorafenib, whose targets include VEGFR and PDGFR, rarely cause objective responses although they may confer a modest improvement in progression-free survival (PFS) (3;4).
Genetic analyses have shown that ACCs frequently acquire chromosomal translocations such as t(6;9)(q22–23;p23–24) that result in the over-expression of the oncogenic transcription factors MYB or MYBL1(13–19). The relevance of MYB to the pathogenesis of ACCs is supported by the observation that inhibition of its transcriptional regulatory functions by the BET domain inhibitor JQ1 suppresses the growth of lower grade ACCs propagated as primary xenografts (15). An alternative is to target downstream effectors of MYB which may include the fibroblast growth factor receptors (FGFR). Among the potential consequences of MYB over-expression appears to be up-regulation of FGFR2 and its ligand FGF2, perhaps related to MYB DNA binding sites that are located near the corresponding genes (15;17;20). This, perhaps in conjunction elevated FGFR1 expression, could result in autocrine receptor signaling and promote tumor growth (21). This scenario is consistent with proteomic studies of primary ACC xenografts which detected spontaneous phosphorylation of peptides derived from the carboxyterminus of FGFR1 (22). In addition, 4–12% of ACCs harbor mutations that are predicted to activate or enhance FGFR signaling independently of MYB overexpression (16;19). A role for FGFR signaling in the pathogenesis of ACC is further supported by the observation that inhibitors of the receptor kinase can suppress the growth of primary ACC xenografts (23).
Dovitinib (TKI258) is a multi-tyrosine kinase inhibitor that not only inhibits the VEGFR, PDGFR, c-Kit, CSF-1R, RET, TrkA, and FLT3 receptor kinases, but also FGFRs 1–3 (IC50 of 10 nmol/L) (24;25). This contrasts with sorafenib and sunitinib that have similar activity against the VEGFRs and other shared targets but have only weak activity against the FGFRs (IC50 = 580 and 880 nM, respectively). Given that MYB gene alterations are found in the majority of ACCs and may drive autocrine activation of FGFRs, we postulated that the ability of dovitinib to inhibit signaling by these receptors would result in a high tumor response rate in patients with advanced and progressive tumors. To test this hypothesis, we conducted a phase II study of dovitinib in patients whose tumors had progressed in the last six months. Objective tumor response was set as the primary end-point but we also measured alterations in the tumor growth rate as determined by change point analysis in order to detect more subtle anti-tumor drug effects that may be clinically relevant. Other secondary endpoints included estimation of the PFS, OS and clinical benefit rate, evaluation of the adverse events, and descriptions of the early metabolic response rate, changes in quality of life, and correlation between biomarkers and clinical outcome.
MATERIALS AND METHODS
Patients
Eligible patients were 18 years of age or older with a diagnosis of ACC confirmed by expert review (Drs. C. Moskaluk and H. Frierson). All patients had unresectable and/or metastatic measurable disease with evidence of progression within the previous six months based on imaging and RECIST 2.0 criteria. All patients were required to submit remote pre-treatment cross-sectional films (minimum of 3 months prior to baseline film) in order to calculate the pre-treatment tumor growth rate (TGR). Up to 5 target lesions were chosen from scans 3–6 months prior to enrollment and assessed by a radiologist. There was no limit on the number of prior therapies but patients must not have had chemotherapy, radiotherapy, surgery, or investigational treatments in the previous 4 weeks. Other eligibility criteria: ECOG performance status of 0 to 2, life expectancy of at least 16 weeks, neutrophil count ≥1,500/μL, platelet counts ≥100,000/μL, hemoglobin >9 g/dL, total bilirubin ≤1.5× upper limit of normal (ULN), AST and/or ALT ≤3× ULN, creatinine ≤1.5× ULN or creatinine clearance ≥30 mL/min, absence of brain metastases, no other active malignancy in the last 3 years (adequately treated cervical carcinoma-in-situ and non-melanoma skin cancers excepted), no serious medical conditions, left ventricular ejection fraction >45%, and absence of uncontrolled hypertension, malabsorption, cirrhosis, warfarin therapy, pregnancy, and active breastfeeding. Referring hospitals were asked to submit tissue blocks for biomarker analysis. This study was approved by the University of Virginia Institutional Review Board for Health Sciences Research and patients signed informed consent before study entry.
Study design and treatment
This was a modified two-step phase II clinical trial in which patients were treated with dovitinib (supplied by Novartis Pharmaceuticals Corporation) at a starting dose of 500 mg taken orally for 5 days on and 2 days off; cycle length was 28 days. Treatment was continued until disease progression, unacceptable toxicity, patient refusal, or at the physician’s discretion. Dose reductions and delays were allowed as per protocol. Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. For intolerable grade 2, or for grade 3 or 4 toxicity, dovitinib was withheld until the toxicity returned to grade 1 or baseline, and the dose was reduced to 400 mg at the same schedule. If the toxicity recurred, the drug was stopped again until the toxicity returned to grade 1 or baseline, and the dose was reduced to 300 mg. For asymptomatic grade 3 hypertension, hyperlipidemia, or cytopenias, dovitinib was withheld until the toxicity resolved to grade 1, and then resumed at the same dose or decreased one dose level at physician discretion. If the grade 3 or 4 toxicity recurred, the drug was withheld until recovery to grade 1, and the dose was again reduced. For a third recurrence of any grade 3 or 4 toxicity, the drug was discontinued. Patients were also taken off treatment if the interruption for toxicity lasted longer than 21 days without recovery to grade 1 or baseline or if they developed a grade 4 toxicity that was judged to be life threatening.
Statistical Analyses
The primary end-points were objective tumor response rate (ORR; complete and partial responses) and drug-induced changes in the tumor growth rate (TGR) using the change point method (26). Tumor response was determined by RECIST 1.1 criteria from computed tomography or magnetic resonance images obtained at 2, 4, and 8 months then every 4 cycles until progression. The modified two-stage trial design incorporated the alternative hypothesis that dovitinib would produce an ORR of at least 18% as compared to 1%, with approximate power of 90% and a 1-sided type I error rate of 5%. The first stage allowed the study to be terminated for drug inactivity if no responses were seen in the first 16 patients. If at least one objective response was observed, 5 additional patients would be enrolled. If ≥ 2 of the 21 patients experienced an objective response, a subsequent protocol amendment recommended the recruitment of 14 additional evaluable patients to more precisely evaluate the change in the TGR. The two stage design was chosen to allow an early “go or no go” decision to minimize patient exposure to dovitinib.
For change point analysis, piecewise linear models of tumor growth were fit to describe a patient’s tumor growth profile while on treatment compared to their pre-treatment profile (using up to five index lesions from pre-baseline films). A preliminary analysis of data from 5 untreated ACC patients allowed us to estimate the variance and power to detect reductions in slopes using change point analysis. In each model we allowed for one change point, one slope measured from -6 months to time 0 (TG0) compared to the slope (TG1) for the period from study entry to a minimum of 4 months post the start of treatment (time 0 to time 4 months). The effect of treatment on tumor growth was assessed by measuring the change in slope between the pre- and post-treatment periods. Using the estimated variance of change in tumor growth from studies of untreated patients (data not shown) we designed this study to detect a change in the TGR. With a total of 35 patients, there would be 80% power to detect an absolute reduction of 0.62 in the slope of the line that represents the tumor growth rate.
Secondary objectives were to estimate PFS, the clinical benefit rate (CR + PR + stable disease >4mo), OS, and to determine the adverse event profile. Exploratory analyses included evaluation of early metabolic tumor response rate (day 12), changes in quality of life (QOL), and biomarker studies. Early metabolic responses were defined as 25% reduction in the maximal standard uptake (SUV) of 18F-fluorodeoxyglucose (18FDG) on PET scans done on day 12 as compared to pre-treatment values (27). QOL outcomes were monitored by the FACT-G questionnaires at each clinic visit. The biomarker studies looked for potential correlations between MYB:NFIB chromosomal translocation and the expression of c-MYB, phosphorylated FGFR1, and clinical end-points (supplemental data).
RESULTS
Between February 2012 and December 2013, 65 patients were screened and 38 patients were consented (Table 1). Thirty-four patients were evaluable for response and 35 for toxicity. Two patients signed consent but were never treated (financial burden for one and a pneumonia for another). The median number of completed cycles was 8 (range 0–20). Twenty-two patients came off study due to progressive tumor, 8 because of toxicity, three due to withdrawals, one due to disease-related adverse event and one due to death.
Table 1.
Characteristics of 35 patients with Adenoid Cystic Carcinoma
| Characteristic | Median (range) |
|---|---|
|
| |
| Age, years | 56 (28–75) |
|
| |
| No. of patients (%) | |
|
| |
| Gender | |
| Female | 18 (51) |
| Male | 17 (49) |
|
| |
| Ethnicity | |
| Hispanic or Latino | 3 ( 9) |
| Non-Hispanic | 32 (91) |
|
| |
| Race | |
| Asian | 1 ( 3) |
| Black or African American | 2 ( 6) |
| More than one race | 1 ( 3) |
| White | 30 (86) |
| Other | 1 ( 3) |
|
| |
| ECOG Performance Status | |
| 0 | 12 (34) |
| 1 | 21 (60) |
| 2 | 2 ( 6) |
|
| |
| Primary Site | |
| Parotid gland | 10 (29) |
| Minor salivary gland | 12 (34) |
| Other oro-naso-pharyngeal site | 8 (23) |
| Trachea | 3 ( 9) |
| Bartholin’s gland | 1 ( 3) |
| Lacrimal | 1 ( 3) |
|
| |
| Number of prior systemic regimens | |
| 0 | 18 (51) |
| 1 | 9 (26) |
| 2 | 4 (11) |
| 3 | 1 ( 3) |
| 4 or more | 3 ( 9) |
|
| |
| Metastatic sites (multiple possible) | |
| Lung | 32 (91) |
| Liver | 15 (43) |
| Bone | 16 (46) |
| Kidney or spleen | 4 (11) |
| Soft tissue | 11 (31) |
Clinical responses and stable disease were observed in treated patients
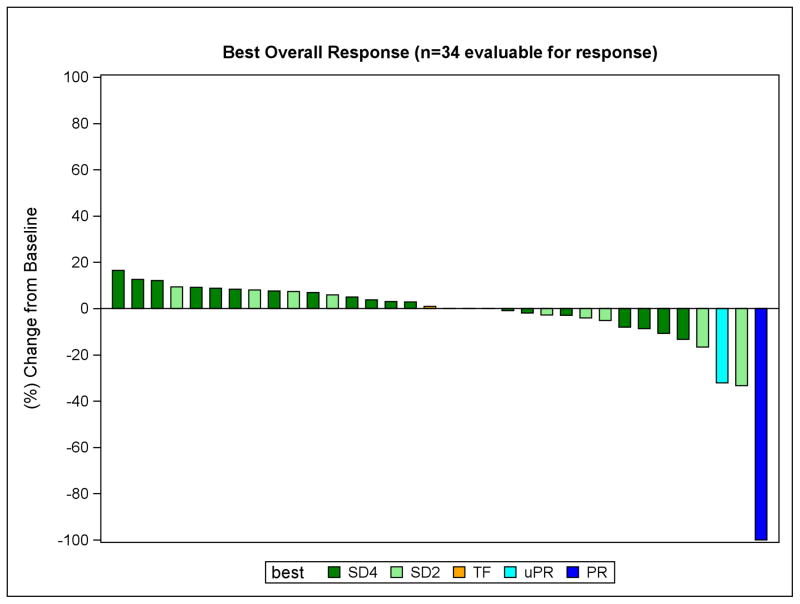
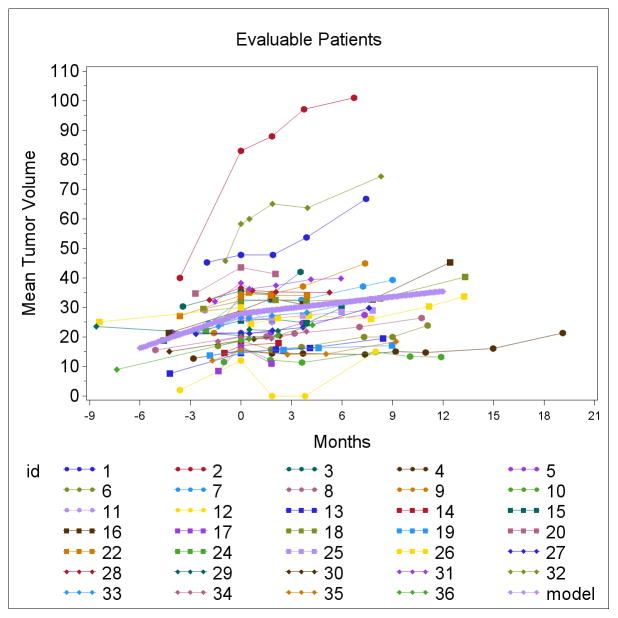
Partial tumor responses were observed in two patients, including one with a complete response of the measurable tracheal lesion without changes in non-measurable bone metastases (Table 2). The other experienced a 32% decrease in RECIST measurements at 2 months, primarily due to marked regression of the largest lung metastasis and also a dramatic decrease in cancer pain. However, although this response was apparent on the follow-up scan 4 months later, the appearance of a new ground glass area made this an unconfirmed response by standard RECIST criteria (unclear whether pneumonia vs tumor per radiologist). Given the extent of the initial response and the relatively long interval between scans, we chose to list this case as a response but denote it as unconfirmed. Overall 14 patients (41%) experienced any degree of tumor regression at any point (Figure 1). In addition, dovitinib significantly suppressed the TGR (figure 2). Prior to treatment, the slope of the line representing TGR was 1.95 which dropped to 0.63 after four cycles of dovitinib (p<0.001). In an unplanned analysis, we noted that beyond 4 months the slope increased to 1.06 but remained significantly lower than the pre-treatment level (p<0.001). This suggested the emergence of partial drug-resistance with an on-going drug effect at least up until the time treatment was stopped, the consequence of the frequent dose reductions of dovitinib, and/or the influence of drug-independent events. Given the low rate of clinical responses, no robust association of TGR with outcome (OR, PFS or OS) was observed in an exploratory analysis. It is noted that the two partial responders had relatively steep TGR’s prior to treatment but so did some non-responders. It is noteworthy that half of the patients (49%) on this trial had prior systemic therapies (table 1) for ACC (possibly owing to the lack of effective therapies for ACC.) Of the participants, 37% (13 of 35) had a prior systemic therapy in the 6 months prior to enrollment.
Table 2.
Summary of Responses and reasons for removal from study.
| Best response | n=34 (%; 95%CI ) | Reason off study |
|---|---|---|
| PR/UcPR | 2 (5.9; 0.7–19.7) | Progression (1) AEs (1) |
| Stable 2 month scan | 9 (26.5; 12.9–44.4) | Early death (1) Progression (3) AEs (4) Withdrawal (1) |
| Stable ≥4 months | 22 (64.7; 46.5–80.3) | Progression (18) AEs (2) withdrawal (2) |
| Failure/death | 1 (2.9; 0.1–15.3) | AE |
| PR/uPR+SD4 | 24 (70.6; 52.5–84.9) |
Figure 1.
Response to treatment. Maximum percentage change from baseline in target lesions. SD4 indicates stable disease at 4 month assessment. SD2 indicates stable disease at 2 month assessment. TF indicates a patient with stable disease who died while on study. uPR indicates the unconfirmed partial response. PR indicates the confirmed partial response.
Figure 2.
Change point analysis plot. This figure demonstrates the assessment of tumor growth kinetics based on pre-study and on-study measurements of the sum of the longest diameters of target lesions. The measurements at month zero are the on-study scans. Up to 3 pre-study scans were evaluated up to 9 months prior to study enrollment. The tumor diameter sums are shown for 34 patients. The thick blue line is the summary change point analysis line as modeled using piecewise linear models of tumor growth allowing for one inflection point for the pre and post treatment periods. The component of the summary line for the slope of tumor growth rate pretreatment was 1.95. The slope of that blue summary line post treatment was 0.63 (p<0.001).
Repeated measures model results:
| Label | Estimate | Std Error | t-value | Pr>|t| |
|---|---|---|---|---|
| Pre slope | 1.95 | 0.29 | 6.71 | <0.001 |
| Post slope | 0.63 | 0.18 | 3.41 | <0.001 |
| Post – Pre | −1.32 | 0.35 | −3.77 | <0.001 |
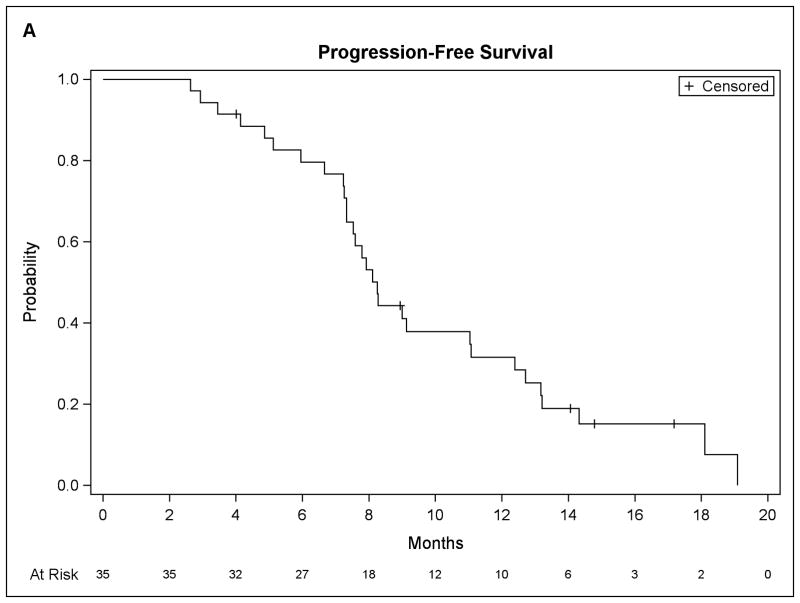
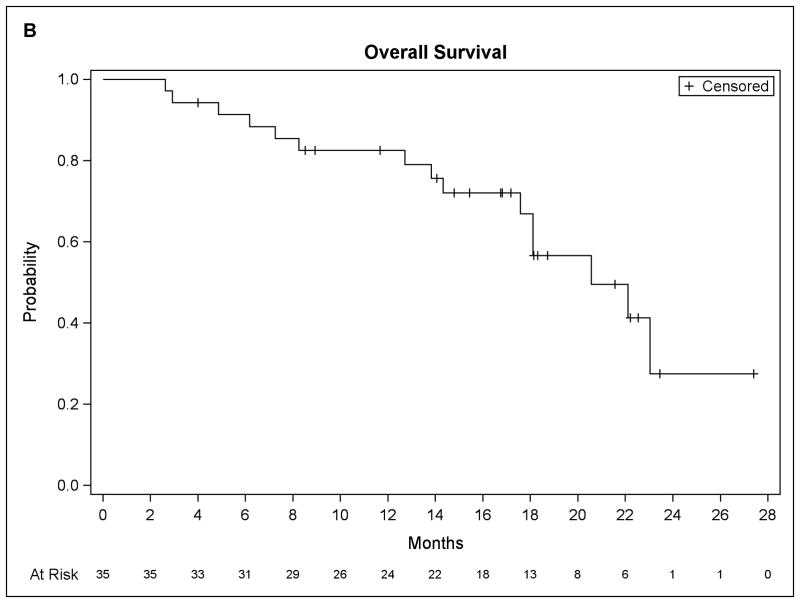
At last follow-up, fifteen patients have died and twenty patients remain alive. The median PFS for the entire group (figure 3) was 8.2 months (90% CI, 7.3 to 11.0) with a median OS of 20.6 months (90% CI, 17.6 to unmet). Of 34 evaluable patients, all but one had stable or responsive disease at 2 months, whereas the clinical benefit rate (CR+PR+ stable disease ≥ 4 months) was 70.6%.
Figure 3.
Patient survival data. (A) Progression-free survival. (B) Overall survival.
In an exploratory study, fifteen patients underwent PET imaging with 18FDG before and 12 days after starting treatment to determine if FGFR inhibition alters tumor glucose metabolism and if metabolic tumor response correlates with subsequent clinical outcome (27–29). Three patients (20%) had metabolic responses with no discernable changes in tumor size. Overall, the tumor SUV decreased in eight patients and increased in six. However, there was no apparent association between metabolic response and other study end-points (supplemental Table 4).
QOL as assessed by FACT-G questionnaires are summarized in Supplemental Figure 2. In modeling overall QOL, there was a significant association between overall QOL and time to treatment failure, where higher scores of QOL were associated with a smaller instantaneous hazard of end of treatment (p-value 0.073). Time is the only significant covariate in the model, where there is an average decrease in QOL of 1.2 points for each additional month in the study (p-value 0.014). The subset of patients who experienced pain relief while on dovitinib generally had the least decline in FACT-G scores but there was no correlation with objective tumor response or PFS. Notably, overall QOL scores trend lower over time suggesting either morbidity from disease progression or cumulative toxicity from treatment or a combination.
MYB gene rearrangements and exome sequencing results
Tumor samples from a subset of patients were available for biomarker and genetic testing. Tissue samples were frequently insufficient or had been depleted by prior pathologic studies during clinical analyses of this rare tumor type. Serial biopsies were not pursued for safety considerations; in many patients this would require lung biopsies. Of the 15 tested tumors, ten had MYB gene rearrangements as determined by the break-apart FISH assay. Despite the limited sample sizes, we found a significant associations between MYB translocation and higher MYB expression (p=0.021) as determined by immunohistochemistry; however, there was no apparent association with FGFR1 phosphorylation (p=0.99) or clinical endpoints. Nine tumor samples yielded DNA suitable for exome sequence analysis of the Foundation One gene panel. This revealed gene mutations previously reported to be altered in ACCs but no alterations in the FGF or FGFR genes (supplemental table 4).
Toxicity
Dovitinib was generally tolerable although dose reductions were required in all but two patients and eight stopped treatment due to toxicity, most often due to fatigue and anorexia. Grade 1 and 2 toxicities that were seen in more than 50% of patients included fatigue, nausea, diarrhea, anorexia, and acneiform rash (Supplemental table 3). Hyperphosphatemia was not observed. Grade 3 or higher toxicity was observed in 63% of the patients, including 9 with hypertriglyceridemia (Table 3). One patient with locally recurrent stage III ACC of the parotid developed central necrosis of tumor after two months of therapy but died from pneumonia while on study.
Table 3.
Grade 3/4 Toxicities Believed to be Possibly, Probably, or Definite Related to Dovitinib
| Toxicity (Grade 3 or 4) | Number |
|---|---|
| Anemia | 1 |
| Constipation | 1 |
| Diarrhea | 1 |
| Mucositis | 1 |
| Nausea/vomiting | 1 |
| Stomach pain | 2 |
| Fatigue | 1 |
| Urinary Tract Infection | 1 |
| Transaminitis | 1 |
| GGT elevation | 4 |
| Dehydration | 1 |
| Neutropenia | 1 |
| Hypertriglyceridemia | 9 |
| Anxiety | 1 |
| Thromboembolic event | 1 |
| Hypertension | 1 |
| Pain | 2 |
| Acneiform rash | 1 |
DISCUSSION
This study revealed that dovitinib has demonstrable but limited anti-tumor activity in patients with recently progressed ACC. Although the ORR (6%) was not sufficient to reject the null hypothesis, dovitinib significantly suppressed the overall TGR as determined by change point analysis. Consistent with this, about 40% of the patients had some reduction in tumor volume and 65% had stable disease for greater than 4 months. We also observed that three of 15 evaluable patients had early metabolic responses as determined by 18FDG PET imaging. Although a high proportion (43%) of our patients had liver involvement, an adverse prognostic factor, the median PFS (8.2 months) and OS (21 months) compared favorably with those reported for ACC patients treated with other kinase inhibitors. For instance, median PFS for patients treated with cetuximab, lapatinib, imatinib or regorafenib in phase II studies is in the range of 2.5 – 6 months whereas the values for those given sunitinib or sorafenib were 7.2 and 11.3 months, respectively (3;4;8;12;16). The prolonged PFS in the sorafenib study may reflect the inclusion of patients without evidence of recent tumor progression; also despite the reported PFS, the OS (19.6 months) was similar to that seen in our dovitinib-treated patients (4). The anti-tumor effects of dovitinib were achieved at the expense of moderate toxicity as dose reductions were required in almost all patients and one in five stopped treatment due to adverse effects. That dovitinib has limited activity against ACC is supported by a recently reported trial in which only one of 32 treated ACC patients responded to this drug although 69% did exhibit minor responses (30).
The results of our trial leave open the question of whether or not FGFR is an appropriate therapeutic target in ACC. We had postulated that FGFR signaling acted downstream of the frequently acquired MYB gene mutations and inhibition of this signaling would produce at least a moderate objective tumor response rate. This was not observed; however, treatment was associated with a reduction in TGR and a favorable PFS. Because dovitinib is a multi-kinase inhibitor, we cannot determine if the observed anti-tumor effects were due to inhibition of signaling by the FGFR or other target receptors, or a combinatorial effect. In this regard, sunitinib and sorafenib which lack activity against FGFRs nonetheless confer PFS or OS similar to that seen in our dovitinib-treated patients. Thus, the similarities in clinical outcome seen with these three kinase inhibitors may stem from suppression of shared targets, such as the VEGFRs.
One possible explanation for the low ORR of ACCs to dovitinib is failure of the drug to adequately suppress oncogenic FGFR signaling in tumor tissues. Although dovitinib has been shown to increases the blood levels of FGF23, a biomarker of systemic FGFR inhibition, the drug produces little or no hyperphosphatemia which is a marker of more robust receptor inhibition (32–34). That dovitinib may not produce sufficient FGFR suppression in vivo is supported by phase II studies of patients with bladder cancer who were treated with dovitinib or the more potent and specific FGFR inhibitor BGJ398. Dovitinib produced only one response in 54 treated patients and none in 12 patients whose tumors harbored FGFR3 gene mutations (35). In contrast, BGJ398 caused hyperphosphatemia in up to one-half of treated patients and an ORR of 36% in those whose tumors contained FGFR gene alterations (32). Thus, it is possible that the more potent and specific FGFR inhibitors such as BGJ398 may be more effective than dovitinib in the treatment of ACC.
Another explanation for the few objective responses in our study is that the FGFRs are not major drivers of tumorigenesis in the majority of ACCs, irrespective of MYB gene status. In the ancillary studies, we found that 10 of the 15 tumors that were studied contained detectable MYB gene rearrangements and this correlated with higher MYB expression as determined by IHC. However, in this limited sampling there was no apparent association between MYB mutation or expression and the level of FGFR1 phosphorylation (p=0.99) or clinical outcome. This suggests that MYB translocation is not tightly associated with FGFR1 activation and therefore may not be a reliable marker of sensitivity to FGFR inhibitors. However, our observations do not exclude the possibility that the response rate to dovitinib would be much higher in the small subset of ACC patients with tumors that contain activating mutations in the FGFR or FGF genes (16;19).
This study did confirm that change point analysis of TGR of ACCs is feasible and capable of detecting statistically significant reductions in the TGR following within 4 months of initiating drug treatment. However, additional studies will be needed to determine the degree of TGR suppression required to confer a true clinical benefit. Once established, the specified reduction in TGR could be used as an early end-point in trials of ACC designed to identify agents likely to be clinically effective. In this regard, reductions in the TGR (measured by a distinct method) were linked to improved OS in patients with renal cancer treated with kinase inhibitors and with favorable PFS in patients entered into a series of phase I trials (36–38).
In summary, we found that dovitinib has limited but demonstrable anti-tumor activity against ACCs. However, it is not clear if these limitations are related to inability of the drug to adequately suppress oncogenic FGFR signaling or the fact that FGFRs are not valid therapeutic targets in the majority of tumors. These issues can be clarified by additional pre-clinical studies to confirm that MYB gene translocation is associated with autocrine FGFR signaling. If so, this would justify a follow-up phase II trial of a potent FGFR-specific inhibitor along with companion genetic and pharmacodynamic studies to verify that most tumor contain MYB mutations and that FGFR signaling is effective suppressed. In addition, a trial of FGFR inhibitors for the treatment of the 4–12% of ACCs with FGFR or FGF gene mutations is also appropriate. However, because of their rarity, these tumors would probably be best studied in the context of a “basket trial” which enrolls patients with different types of tumors that contain similar FGFR gene alterations.
Supplementary Material
Translational Relevance.
FGFR signaling in adenoid cystic carcinoma (ACC) is believed to be upregulated by recurring MYB:NFIB translocations seen in the majority of ACC tumor samples or uncommon mutations in components of the FGFR signaling pathway. Dovitinib, a multikinase inhibitor that suppresses FGFR 1–3 signaling, reduces growth of some ACC xenografts. This manuscript describes an open-label, phase II study of the efficacy of dovitinib treatment for patients with recently progressive tumors. Clinical response rate was low but disease stabilization was common. Dovitinib also induced a significant decrease in tumor growth rate as measured by change point analysis. Analysis of a subset of tumors showed that MYB:NFIB translocations correlated significantly with increased MYB expression. FGFR1 phosphorylation was frequent but did not correlate with MYB expression or with clinical end-points. Although dovitinib has some activity against ACC, additional studies of FGFR-specific inhibitors in biomarker-selected patients along with companion pharmacodynamic studies are needed to determine if FGFR signaling is a valid therapeutic target in ACC.
Acknowledgments
Author financial support/Grant support: This study (NCT01524692) was funded by Novartis Pharmaceuticals. Some biomarker studies were supported by the UVA Cancer Center, Charlottesville, VA (P Dillon and C Moskaluk). Statistical analysis was by the Biostatistics Shared Resource, University of Virginia Cancer Center, University of Virginia (P30 CA044579).
We thank all of the patients and their families for their participation. We also thank the University of Virginia Cancer Center and the Adenoid Cystic Carcinoma Research Foundation.
Abbreviations list
- ACC
adenoid cystic carcinoma
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CTCAE
common terminology criteria for adverse events
- CR
complete response
- FDG
fluorodeoxyglucose
- FGFR
fibroblast growth factor receptor
- FISH
fluorescence in-situ hybridization
- ORR
overall response rate
- OS
overall survival
- PDGFR
platelet derived growth factor receptor
- PET
positron emission tomography
- PFS
progression free survival
- PF
partial response
- QOL
quality of life
- SUV
standardized uptake value
- TGR
tumor growth rate
- ULN
upper limit of normal
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Clinicaltrials.gov Registry Number: NCT01524692
Reference List
- 1.Sessions RHL, Forastiere A. Tumors of the salivary glands and paragangliomas. In: Devita VT, Hellman S, Rosenberg SA, editors. Principles and Practice of Oncology. Philadelphia: Lippincott-Raven Publishers; 2000. [Google Scholar]
- 2.Argiris A, Ghebremichael M, Burtness B, Axelrod RS, Deconti RC, Forastiere AA. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: a trial of the Eastern Cooperative Oncology Group (E1303) Cancer. 2011;117(15):3374–3382. doi: 10.1002/cncr.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23(6):1562–1570. doi: 10.1093/annonc/mdr522. [DOI] [PubMed] [Google Scholar]
- 4.Thomson DJ, Silva P, Denton K, Bonington S, Mak SK, Swindell R, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37(2):182–187. doi: 10.1002/hed.23577. [DOI] [PubMed] [Google Scholar]
- 5.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid Cystic Carcinoma: A Review of Recent Advances, Molecular Targets and Clinical Trials. Head Neck. 2014 doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Verweij J, De Mulder PH, Cognetti F, Clavel M, Rodenhuis S, et al. Epirubicin in patients with advanced or recurrent adenoid cystic carcinoma of the head and neck: a phase II study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1993;4(9):785–788. doi: 10.1093/oxfordjournals.annonc.a058665. [DOI] [PubMed] [Google Scholar]
- 7.Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van HC, et al. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33(6):905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- 8.Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25(25):3978–3984. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves P, Kummar S, SL, HA A phase II study of sueroylanilide hydroxamic acid (SAHA) in subjects with locally advanced, recurrent, or metastatic adenoid cystic carcinoma (ACC) J Clin Oncol. 2013 Jun 12;31 Ref Type: Abstract. [Google Scholar]
- 10.Ghosal N, Mais K, Shenjere P, Julyan P, Hastings D, Ward T, et al. Phase II study of cisplatin and imatinib in advanced salivary adenoid cystic carcinoma. Br J Oral Maxillofac Surg. 2011;49(7):510–515. doi: 10.1016/j.bjoms.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Holst VA, Marshall CE, Moskaluk CA, Frierson HF., Jr KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol. 1999;12(10):956–960. [PubMed] [Google Scholar]
- 12.Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23(3):585–590. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- 13.Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent Fusions in MYB and MYBL1 Define a Common, Transcription Factor-Driven Oncogenic Pathway in Salivary Gland Adenoid Cystic Carcinoma. Cancer Discov. 2016;6(2):176–187. doi: 10.1158/2159-8290.CD-15-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brill LB, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 15.Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48(3):265–272. doi: 10.1038/ng.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho AH, Sherman EJ, Baxi SS, Haque S, Ni A, Antonescu CR, et al. Phase II study of regorafenib in progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. 2016 Jun 20;34(18_suppl) doi: 10.1200/JCO.18.01859. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106(44):18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persson M, Andren Y, Moskaluk CA, Frierson HF, Jr, Cooke SL, Futreal PA, et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer. 2012;51(8):805–817. doi: 10.1002/gcc.21965. [DOI] [PubMed] [Google Scholar]
- 19.Stephens PJ, Davies HR, Mitani Y, Van LP, Shlien A, Tarpey PS, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123(7):2965–2968. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miglarese MR, Halaban R, Gibson NW. Regulation of fibroblast growth factor 2 expression in melanoma cells by the c-MYB proto-oncoprotein. Cell Growth Differ. 1997;8(11):1199–1210. [PubMed] [Google Scholar]
- 21.Myoken Y, Myoken Y, Okamoto T, Sato JD, Kan M, McKeehan WL, et al. Immunohistochemical study of overexpression of fibroblast growth factor-1 (FGF-1), FGF-2, and FGF receptor-1 in human malignant salivary gland tumours. J Pathol. 1996;178(4):429–436. doi: 10.1002/(SICI)1096-9896(199604)178:4<429::AID-PATH495>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Moskaluk CA, Baras AS, Mancuso SA, Fan H, Davidson RJ, Dirks DC, et al. Development and characterization of xenograft model systems for adenoid cystic carcinoma. Lab Invest. 2011;91(10):1480–1490. doi: 10.1038/labinvest.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frierson HF, Jr, Moskaluk CA. Mutation signature of adenoid cystic carcinoma: evidence for transcriptional and epigenetic reprogramming. J Clin Invest. 2013;123(7):2783–2785. doi: 10.1172/JCI69070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood. 2007;110(10):3729–3734. doi: 10.1182/blood-2007-02-074286. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Lopes de MD, Vora J, Harris A, Ye H, Nordahl L, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11(10):3633–3641. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- 26.Milella M. Optimizing clinical benefit with targeted treatment in mRCC: “Tumor growth rate” as an alternative clinical endpoint. Crit Rev Oncol Hematol. 2016;102:73–81. doi: 10.1016/j.critrevonc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Dos Anjos RF, Dos Anjos DA, Vieira DL, Leite AF, Figueiredo PT, de Melo NS. Effectiveness of FDG-PET/CT for evaluating early response to induction chemotherapy in head and neck squamous cell carcinoma: A systematic review. Medicine (Baltimore) 2016;95(32):e4450. doi: 10.1097/MD.0000000000004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas JM, Matsen ME, Mundinger TO, Morton GJ, Stefanovski D, Bergman RN, et al. Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Mol Metab. 2015;4(8):561–568. doi: 10.1016/j.molmet.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Keam B, Kim SB, Shin SH, Cho BC, Lee KW, Kim MK, et al. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer. 2015;121(15):2612–2617. doi: 10.1002/cncr.29401. [DOI] [PubMed] [Google Scholar]
- 31.Keam B, Kim SB, Shin SH, Cho BC, Lee KW, Kim MK, et al. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer. 2015 doi: 10.1002/cncr.29401. [DOI] [PubMed] [Google Scholar]
- 32.Pal SK, Rosenberg JE, Keam B, Wolf J, Berger R, Dittrich C, et al. Efficacy of BGJ398, a fibroblast growth factor receptor (FGFR) 1–3 inhibitor, in patients (pts) with previously treated advanced/metastatic urothelial carcinoma (mUC) with FGFR3 alterations. J Clin Oncol. 2016 Jun 20;34(18 suppl) doi: 10.1158/2159-8290.CD-18-0229. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escudier B, Grunwald V, Ravaud A, Ou YC, Castellano D, Lin CC, et al. Phase II results of Dovitinib (TKI258) in patients with metastatic renal cell cancer. Clin Cancer Res. 2014;20(11):3012–3022. doi: 10.1158/1078-0432.CCR-13-3006. [DOI] [PubMed] [Google Scholar]
- 34.Yanochko GM, Vitsky A, Heyen JR, Hirakawa B, Lam JL, May J, et al. Pan-FGFR inhibition leads to blockade of FGF23 signaling, soft tissue mineralization, and cardiovascular dysfunction. Toxicol Sci. 2013;135(2):451–464. doi: 10.1093/toxsci/kft161. [DOI] [PubMed] [Google Scholar]
- 35.Milkowski M, Dittrich C, Martinez ID, Jagdev S, Millard FE, Sweeney C. Final results of a multicenter, open-label phase II trial of dovitinib (TKI258) in patients with advanced urothelial carcinoma with either mutated or nonmutated FGFR3. J Clin Oncol. 2013 Jun 18;31(6 suppl) Ref Type: Abstract. [Google Scholar]
- 36.Ferte C, Koscielny S, Albiges L, Rocher L, Soria JC, Iacovelli R, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65(4):713–720. doi: 10.1016/j.eururo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Ferte C, Fernandez M, Hollebecque A, Koscielny S, Levy A, Massard C, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20(1):246–252. doi: 10.1158/1078-0432.CCR-13-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein WD, Wilkerson J, Kim ST, Huang X, Motzer RJ, Fojo AT, et al. Analyzing the pivotal trial that compared sunitinib and IFN-alpha in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res. 2012;18(8):2374–2381. doi: 10.1158/1078-0432.CCR-11-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.