Abstract
Previous studies in our laboratory identified that 3-deazaneplanocin A (DZNep), a carbocyclic adenosine analog and histone methyl transferase inhibitor, suppresses TGFβ-induced epithelial-to-mesenchymal (EMT) characteristics. In addition, DZNep epigenetically reprograms miRNAs (miRs) to regulate endogenous TGFβ1 levels via miR-663/4787 mediated RNA interference (Mol Cancer Res. 2016 Sep 13. pii: molcanres.0083.2016) (1). While DZNep also attenuates exogenous TGFβ-induced EMT response, the mechanism of this inhibition was unclear. Here, DZNep induced miR-202-5p to target both TGFβ receptors, TGFBR1 and TGFBR2, for RNA interference and thereby contribute to the suppression of exogenous TGFβ-induced EMT in pancreatic cancer cells. Lentiviral overexpression of miR-202 significantly reduced the protein levels of both TGFβ receptors and suppressed TGFβ signaling and EMT phenotypic characteristics of cultured parenchymal pancreatic cancer cells. Consistently, transfection of anti-miRs against miR-202-5p resulted in increased TGFBR1 and TGFBR2 protein expressions and induced EMT characteristics in these cells. In stellate pancreatic cells, miR-202 overexpression slowed growth as well as reduced stromal extracellular membrane matrix (ECM) protein expression. In orthotopic pancreatic cancer mouse models, both immunodeficient and immunocompetent, miR-202 reduced tumor burden and metastasis. Together, these findings demonstrate an alternative mechanism of DZNep in suppressing TGFβ signaling at the receptor level and uncover the EMT suppressing role of miR-202 in pancreatic cancer.
Implications
These findings support the possibility of combining small molecule- (e.g., DZNep analogs) or large molecule- (e.g., miRs) based epigenetic modifiers with conventional nucleoside analogs (e.g., gemcitabine, capecitabine) to improve the anti-metastatic potential of current pancreatic cancer therapy.
Keywords: DZNep, miR-202-5p, TGFBR1, TGFBR2, EMT, Pancreatic Cancer
INTRODUCTION
One of the key reasons for the aggressiveness of pancreatic tumors is attributed to a tumor cell remodeling process called epithelial-to-mesenchymal transition (EMT) (2–4). Mechanistically, EMT is characterized by the loss of cell-cell contacts and cell polarity and gain of mesenchymal phenotypes including increased motility, invasiveness, and chemoresistance (2–4). Transforming Growth Factor-beta 1 (TGF-β1) is a multifunctional cytokine secreted into the tumor microenvironment which primarily promotes the EMT process (5, 6). Although TGF-β1 is a growth inhibitory cytokine to normal cells, parenchymal tumor cells exposed to TGF-β do not exhibit growth inhibition, but instead favors increased tumor cell dissemination, colonization, and clonal expansion with acquisition of drug refractiveness (5–9). In addition, TGF-β induces significant stromal reorganization to provide a structural framework necessary for supporting tumor malignancy (10, 11). Thus, a therapeutic strategy to curtail the TGF-β-triggered EMT process is expected to improve pancreatic cancer therapy, although several challenges exist for translational advancements in this direction.
TGF-β signaling is initiated by the binding of the TGF-β ligand to its receptor (TGFBR2) (12). The receptor-ligand complex recruits TGFBR1 to phosphorylate downstream signaling players including receptor-associated Smad2 and Smad3 (R-Smads) (12). The R-Smads further complex with a coSmad, Smad4, to translocate to the nucleus which leads to the activation of critical transcription factors governing EMT. These include the Snail family of zinc-finger transcription factors (Snail1, Snail2, and Snail3), Smad-interacting proteins (ZEB2), and basic helix-loop-helix factors (Twist1 and Twist2) (13). In addition to the canonical players involved in the Smad-dependent pathway, TGF-β also works in concert with several non-canonical players, including those in the PI3K/AKT, MEK/ERK, and β-catenin/WNT pathways, to co-regulate the EMT process (13). Several mutations, frameshift deletions, and monoallelic losses of genes involved in the TGF-β signaling pathway are reported to contribute to aberrant functional consequences of TGF-β signaling in cancers (14).
In addition to genetic alterations, epigenetic alterations including DNA and histone methylations are now increasingly being recognized to play critical roles in the interconversions of epithelial-mesenchymal plasticity. In particular, EZH2 histone methyl transferase (a part of the Polycomb Repressive Complex 2) that mediates trimethylation of lysine 27 in histone H3 was recently reported to epigenetically silence genes essential in maintaining an epithelial phenotype (15, 16). In addition, overexpression of EZH2 protein or mutations in the EZH2 gene is also linked to the acquisition of EMT characteristics and cancer progression in tumor settings (17). These findings support the notion that targeting of EZH2 could be of potential therapeutic value for treating EMT-active tumors such as pancreatic tumors. As a result, synthetic EZH2 inhibitors are currently being evaluated in preclinical and clinical trials for various solid tumors (18), although their applicability in pancreatic cancer treatment remains less understood.
An earlier study in our laboratory demonstrated 3-deazaneplanocin A (DZNep), a carbocyclic analog of adenosine that depletes cellular levels of EZH2 to presensitize pancreatic cancer cells to nucleoside analog drugs (19). Recently, we reported DZNep to inhibit TGF-β induced EMT characteristics of pancreatic cancer cells (1). The latter study also revealed DZNep to induce distinct epigenetic reprogramming of miRNAs, a class of small non-coding RNAs that downmodulate target gene expression, to attenuate endogenous synthesis of TGF-β1 in parenchymal pancreatic cancer cells (1). Particularly, two DZNep-reprogrammed and PDAC-downregulated miRNAs, miR-663a and miR-4787-5p, were identified to directly target TGF-β1 for RNA interference (1). As a result, DZNep not only inhibited the synthesis of TGF-β1 but also its secretion into the extracellular environment and its associated autocrine and paracrine responses. Intriguingly, in addition to attenuation of endogenous TGF-β, we observed DZNep to inhibit exogenous TGF-β stimulated EMT response in pancreatic cancer cells (1). However, the mechanism(s) regarding this process was not known and suggested the presence of other modes of action.
In this study, we addressed whether any of the DZNep induced miRNAs were capable of attenuating exogenous TGF-β1 stimulated EMT responses in pancreatic cancer cells. We report the DZNep-induced miR-202-5p to target both TGFB receptors, TGFBR1 and TGFBR2, for RNA interference to attenuate the exogenous TGF-β1-induced EMT response at the receptor level. MiR-202 also inhibited cell migration and invasion as well as metastasis in orthotopic pancreatic cancer mouse models. Together, these data uncovered a novel mechanism of DZNep action in suppressing the EMT phenotype and further support the pharmacotherapeutic evaluation of miR-202 for the intervention of pancreatic cancer progression.
MATERIALS AND METHODS
Materials and Reagents, Cell Culture, Western Blotting, Real-time PCR Analysis, MiRNA Overexpression, Knockdown of miRNAs, Scratch Wound Assay, MTT Growth Assays and RT2 Profiler PCR Array
These procedures were carried out as described previously (1, 20). A breast cancer cell line, MCF-7 was received from the American Type Culture Collection (ATCC) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS. The murine PDAC cell line, PAN 02 was procured from National Cancer Institute DCTDC Tumor Repository (Frederick, MD) and were grown in RPMI 1640 media supplemented with 10% FBS and 2 mM L-Glutamine. The details of KPC cell line established from a genetically engineered mouse model (GEMM) of pancreatic cancer, PDX-1-CRE, LSL-KRasG12D, LSL-Trp53−/− are described earlier (21, 22) and was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (without sodium pyruvate) supplemented with 10% FBS. Primary human pancreatic stellate cells (PSCs) (#3830) were purchased from ScienCell Research Laboratories (Carlsbad, California).
Growth conditions of PSCs
PSCs were grown in stellate cell medium (ScienCell Research Laboratories, #5301) supplemented with FBS, Stellate Cell Growth Supplement, and penicillin/streptomycin solution (provided with the media) in poly-lysine coated flasks and were used in the study within 10 passages. Procedures including miRNA-202 overexpression in PSCs and subsequent analysis including Western Blotting, MTT Growth assays, and Real-time PCR Analysis were carried out in a similar fashion as for other pancreatic cancer cell lines. Collection of PSCs-conditioned media was carried out as per procedure described elsewhere (23).
Antibodies
The rabbit polyclonal TGFBR1 (sc-398), TGFBR2 (sc-400), collagen 1alpha1 (sc-8784-R), and fibronectin (sc-9068) antibodies, the mouse monoclonal Smad3 (sc-101154) antibody, and the goat polyclonal collagen 3alpha1 (sc-8781) antibody were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). The rabbit polyclonal p-Smad2 (#3101), rabbit monoclonal Smad2 (#3122), and vimentin (#5741) antibodies and the mouse monoclonal cytokeratin 8/18 (#4546) antibody were purchased from Cell Signaling Technology (Danvers, MA). The mouse E-cadherin antibody was kind a gift from Dr. Parmender Mehta (University of Nebraska Medical Center, Nebraska). The mouse monoclonal N-cadherin antibody was purchased from BD Biosciences (San Jose, CA). The mouse monoclonal α-smooth muscle action (SMA) (A2547) and β-actin (A1978) antibody were purchased from Sigma-Aldrich (St. Louis, MO).
Primers and Constructs
The Taqman primer probes for EZH2 (Hs01016789_m1), TGFBR1 (Hs00610320_m1), TGFBR2 (Hs00234253), CTGF (Hs01026927_g1), GUSB (Hs00939627_m1), miR-202-5p (002362), pri-miR-663a (Hs03304850_pri), miR-4787-5p (464332_mat), and RNU6B (001093) were purchased from Applied Biosystems (Foster City, CA). Pre-miR-202 lentiviral constructs (HmiR0166-MR03) for overexpressing miR-202-5p and the miRNA scrambled control clone (CmiR0001-MR03) was purchased from GeneCopoeia, Inc (Rockville, MD). The GIPZ lentiviral EZH2 shRNAs (RHS4531-EG2146) were purchased from Dharmacon GE (Lafayette, CO). For knockdown studies, miR-202-5p inhibitors (Assay ID: MH12682) were purchased from Applied Biosystems (Foster City, CA).
Luciferase In Vitro Reporter Assay
The luciferase reporter assay was carried out as described elsewhere (20) with some modifications. Overall, 5X103 cells (empty vector or miR-202-5p overexpressing) were seeded in a 96-well plate. Within 24 h, cells were transfected with 0.1 μg of TGFBR1 (ID: HmiT064498-MT01, GeneCopoeia) or TGFBR2 (ID: HmiT018053-MT01, GeneCopoeia) 3′UTR dual luciferase vector and treated with DZNep (10 μM), antimiR-202-5p (50 nM), or solvent. Within 24 to 48 h, cells were lysed and subjected to the Dual-Luciferase assay (Promega) as per the manufacturer’s instructions.
Orthotopic Pancreatic Tumor Models
Animal experiments were performed as per the protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Georgia and The Ohio State University. Orthotopic pancreatic cancer xenograft mice models were generated as described elsewhere (1). Briefly, cells grown to 90% confluency in culture dishes were trypsinized, centrifuged, and resuspended at a concentration of 1×105 cells/100 μl in sterile HBSS containing 1% v/v serum-free Matrigel. Eight week old female athymic nude mice (homozygous; 002019, Jackson Laboratories) were injected with cells orthotopically in the tail region of the pancreas. The cell suspension was allowed to solidify, and mice were recovered and monitored for changes in their weight twice a week. Eight weeks after cell injection, tumors were excised and assessed for weight and size for end-point assays. Tumor volume was measured with a digital caliper and calculated by the formula: tumor volume = π/6 (L × S × S), where L = longest diameter and S = shortest diameter of the tumors. In addition, the liver was monitored for metastatic tumor lesions, and tissue sections at the normal-tumor interface were collected for histological examination of metastatic foci. For establishing syngeneic orthotopic model of pancreatic cancer, 1×106 KPC cells were injected in 8–10 week old female C57BL/6 mice as described above. Three weeks after cell injection, tumors were excised and assessed for weight. For another orthotopic model of pancreatic cancer in immunocompetent mice, 0.5×106 PAN 02 cells were injected in 8–10 week old female C57BL/6 mice as described above. Nine weeks after cell injections, tumors were weighed and tumor progression was monitored and metastasis and hemorrhagic ascites were assessed.
Statistical Analysis
Data analysis was conducted using GraphPad Prism 6 (Graphpad Software, Inc.). The statistical significance of the data was determined using the Student’s t-test between two groups or using one-way (one variable) or two-way (two independent variables) analysis of variance (ANOVA) with multiple comparisons test for multiple experimental groups. All p-values <0.05 were considered significant unless otherwise stated. P-values <0.05, <0.01, and <0.005 are indicated with *, **, and *** respectively unless otherwise stated.
RESULTS
DZNep inhibits TGF-β receptor expressions and induces miR-202-5p expression in pancreatic cancer cells
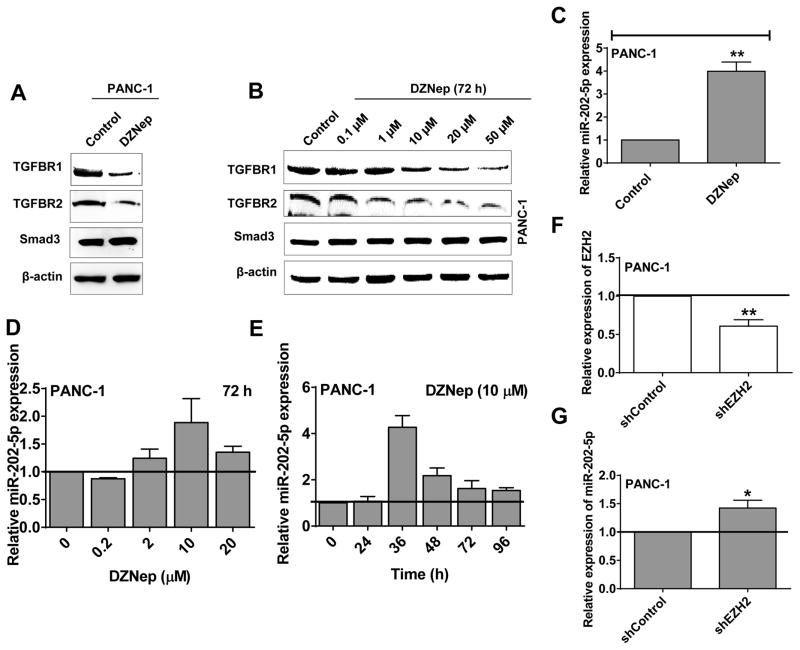
DZNep’s resistance to exogenous TGF-β1-induced EMT characteristics could be due to the functional inhibition of TGF-β receptors or intracellular Smad proteins. To test this possibility, we assayed for the changes in the expression levels of TGF-β receptor I (TGFBR1), TGF-β receptor II (TGFBR2), Smad2, and Smad3 in control and DZNep treated cells. As shown by Western blotting analysis in Fig. 1A, DZNep distinctly reduced the expression of both TGFBR1 and TGFBR2 in PANC-1 without altering the expression of Smads (Smad3 in Fig. 1A; Smad2 shown in previous study (1)). Similar results were also obtained in another pancreatic cancer cell line (BxPC-3) and a breast cancer cell line (MCF-7) (both of which are previously shown to undergo EMT on TGF-β treatment) as well as in other pancreatic cancer cell lines (Capan-1, L3.6pl, and MIA PaCa-2) and murine PDAC cell lines (KPC and PAN 02) (Fig. S1A). DZNep inhibited TGFBR1 and TGFBR2 protein expressions in a dose-dependent manner within a concentration range of 1–50 μM (72 h treatment) (Fig. 1B). These results identify DZNep’s inhibition of TGFBR1 and TGFBR2 protein expression and possibly also DZNep’s action at the receptor level to inhibit exogenous TGF-β stimulated EMT response.
Figure 1. DZNep decreases TGFBR1 and TGFBR2 levels and induces miR-202-5p expression in pancreatic cancer.
A. DZNep decreased TGFBR1 and TGFBR2 but not Smad3 protein levels in pancreatic cancer cells (PANC-1). Whole cell lysates (50 μg) of DZNep (10 μM; 72 h)-treated cells subjected to Western blotting for TGFBR1, TGFBR2, and Smad3 protein levels. β-actin used as a loading control. B. DZNep decreased TGFBR1 and TGFBR2 but not Smad3 protein levels in a dose-dependent fashion in pancreatic cancer. C. DZNep (10 μM; 36 h) increased miR-202-5p expression in pancreatic cancer cells. Total RNA from cells subjected to qRT-PCR analysis to determine the expression levels of miR-202-5p with a taqman probe. U6B was used as an endogenous control. D and E. DZNep increased miR-202-5p expression in a dose- (D) and time- (E) dependent manner in PANC-1. F. Transcript levels (Average) of EZH2 in EZH2 knockdown stables made by using short-hairpin RNAs (shRNAs) (n=5) in PANC-1 was analyzed with qRT-PCR and compared against control cells. GUSB was used as an internal control. G. Knockdown of EZH2 by shRNAs induces miR-202-5p expression in PANC-1 as analyzed by qRT-PCR using taqman probe with U6B as an internal control. Bars, SD or SEM. n=2–5.
Since our previous studies identified DZNep to significantly reprogram miRNA expression in human PDAC (1), we postulated that one or more of the DZNep induced miRNA(s) could be involved in the regulation of TGF-β receptor expressions. Bioinformatic analysis predicted DZNep-induced miRNAs, miR-4646-5p and miR-361-5p, to directly target TGFBR1 while miR-1273g-3p, miR-19a-3p, and miR-3182 were predicted to directly target TGFBR2 for RNA interference (1). Furthermore, DZNep-induced miR-320a/c/d and miR-202-5p were predicted to target both TGFBR1 and TGFBR2. As DZNep maximally induced miR-202-5p (~7 folds; microarray data) as compared with miR-320 members (~2 folds; microarray data) and since two independent algorithms (miRDB and microRNA.org) predicted TGFBR1 and TGFBR2 as direct targets for miR-202-5p (in contrast to only TargetScan for miR-320 members), we further directed our research on miR-202-5p. Real-time PCR analysis of DZNep-treated (10 μM; 36 h) cells validated that DZNep induced the expression levels of miR-202-5p by at least 4–5-fold in pancreatic cancer cells in which it is otherwise generally poorly expressed (Fig. 1C). Similarly, DZNep induced miR-202-5p levels in BxPC-3 and MCF-7 (TGF-β-EMT responsive cell lines) as well as in KPC cells (Fig. S1B). Furthermore, DZNep induced miR-202-5p levels in PANC-1 in a dose- and time-dependent fashion with maximal induction at 10 μM for 36–48 h of treatment (Fig. 1D–E). Addtionally, EZH2 shRNAs (Fig. 1F) increased miR-202-5p levels suggesting that DZNep’s actions are at least partially mediated via EZH2 inhibition (Fig. 1G).
miR-202-5p directly targets TGFBR1 and TGFBR2 for RNA interference
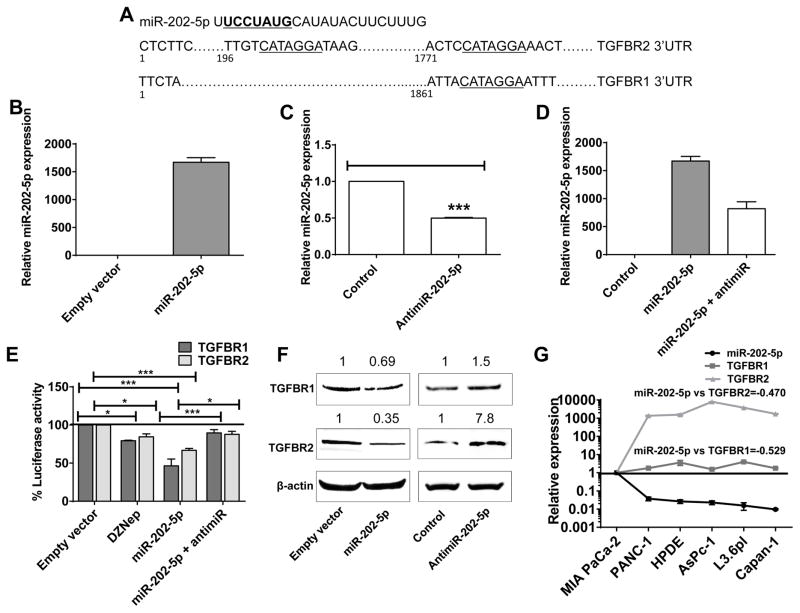
Sequence analysis showed two complementary binding regions to the miR-202-5p seed sequence within the 3′UTRs of TGFBR2 and a single binding site within the 3′UTR of TGFBR1 (Fig. 2A). To experimentally test whether miR-202-5p binds to the 3′UTRs of TGFBR1 and/or TGFBR2 for targeting to RNA interference, we transiently transfected PANC-1 cells stably expressing miR-202-5p (Fig. 2B–D) with a dual luciferase reporter construct in which the TGFBR1 3′UTR or TGFBR2 3′UTR were cloned downstream of a firefly luciferase cDNA (see Methods). PANC-1 cells were chosen for this analysis because they endogenously expressed abundant levels of both TGFBR1 and TGFBR2 and harbored no receptor mutations or loss of SMAD expression. In addition, our earlier studies identified DZNep to significantly reprogram miRNA expression in PANC-1 (1). As shown in Fig. 2E, both miR-202-5p overexpression and DZNep treatment significantly reduced luciferase activities demonstrating a functional role of miR-202-5p on DZNep inhibition of TGFBR1 and TGFBR2 expressions. Supportively, the reductions in luciferase activities by DZNep and miR-202-5p were effectively counteracted by a miR-202-5p antagomiric construct (50 nM for 48 h treatment) (Fig. 2E). Western blotting analysis using cell lysates of miR-202-5p overexpressing PANC-1 showed a reduction of both TGFBR1 and TGFBR2 protein expressions, similar to that observed with DZNep (Fig. 2F). Consistently, depletion of endogenous miR-202-5p in PANC-1 cells by transfection of antimiR-202-5p constructs (50 nM for 48 h treatment) increased the protein expression of both receptors when compared with that in control vector transfected cells (Fig. 2F). These results were independently verified in MIA PaCa-2 wherein miR-202-5p expression was the most abundant strand (Fig. S2–3). However, the changes were observed only for TGFBR1 protein levels and not TGFBR2 (due to lower expression of TGFBR2) with miR-202-5p alterations in MIA PaCa-2. Finally, we investigated if there was any correlation between miR-202-5p and its targets, TGFBR1 and TGFBR2, in pancreatic cancer. For this, we compared the expression levels of miR-202-5p with that of TGFBR1 and TGFBR2 (Fig. S2) in a panel of pancreatic cancer cells to determine the Pearson’s correlation coefficient. As shown in Fig. 2G, miR-202-5p expression levels negatively correlated with both TGFBR1 and TGBR2 with Pearson r = −0.529 and −0.470, respectively. Overall, these results support that DZNep-induced miR-202-5p directly targets TGFBR1 and TGFBR2 for RNA interference in pancreatic cancer.
Figure 2. MiR-202-5p directly targets TGFBR1 and TGFBR2 for RNA interference.
A. Schematic representation of predicted miR-202-5p binding sites in the TGFBR1 and TGFBR2 3′-UTRs. B–D. Stable overexpression (B and D) or knockdown (C and D) of miR-202-5p in a pancreatic cancer cell line, PANC-1. Expression levels of miR-202-5p in PANC-1 stably overexpressing miR-202-5p (B and D) or transfected with miR-202-5p antagomiR (50 nM; 48 h) analyzed by qRT-PCR with a taqman probe. RNU6B used as internal control. E. MiR-202-5p directly binds to TGFBR1 and TGFBR2 3′UTRs. Cells treated with DZNep (10 μM; 24–48 h) or stably overexpressing miR-202-5p with or without miR-202-5p antagomiR (antimiR-202-5p; 48 h) were transfected with the TGFBR1 or TGFBR2 3′-UTR luciferase reporter and percent activities recorded. F. MiR-202-5p overexpression decreased TGFBR1 and TGFBR2 while antimiR-202-5p increased TGFBR1 and TGFBR2 protein levels. Whole cell lysates (50 μg) of cells stably overexpressing miR-202-5p or transfected with antimiR-202-5p (50 nM; 48 h) subjected to Western blotting to determine TGFBR1 and TGFBR2 protein levels with β-actin as a loading control. G. MiR-202-5p is inversely correlated with TGFBR1 (r=−0.53) and with TGFBR2 (r=−0.47) in pancreatic cancer cell lines. Total RNA from PDAC cells subjected to qRT-PCR analysis to determine the expression levels of miR-202-5p, TGFBR1, and TGFBR2 with respective taqman probes. U6B and GUSB were used as internal controls for miR-202-5p and TGFBR1/TGFBR2, respectively. Relative expressions of miR-202-5p and TGFBR1/TGFBR2 were plotted and Pearson’s correlation coefficients were calculated. Bars, SEM or SD. n=2–5.
miR-202 resists TGF-β1-induced EMT in pancreatic cancer
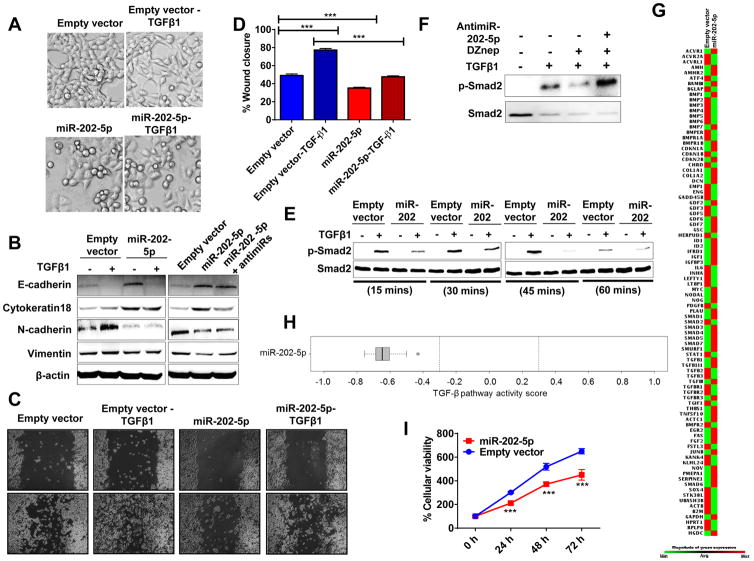
Since both TGFB receptors are indispensable for TGF-β1-mediated actions (e.g., EMT) and miR-202-5p expression inhibited TGFB receptor expressions, we directly tested whether miR-202-5p can counteract TGF-β1-mediated EMT in pancreatic cancer (as seen with DZNep) (1). To do so, we treated PANC-1 transfected with empty vector or stably overexpressing miR-202-5p with TGF-β1 and analyzed for possible changes in cellular morphology, EMT markers, and cell migration. As expected, recombinant-derived human TGF-β1 (10 ng/mL; 48 h) induced distinct EMT-like morphological changes as judged from scattering of cells, more spindle-shaped cells, elongated cellular processes, and reduced cell-to-cell contacts in empty vector-transfected cells (Fig. 3A). However, such morphological changes were significantly inhibited in miR-202-5p overexpressing cells (Fig. 3A) similar to that observed with DZNep although the magnitude of inhibition of miR-202 was lesser than DZNep (1). Additionally, cells stably overexpressing miR-202-5p appeared more epithelial-like (Fig. 3A) as compared with control cells, suggesting that miR-202-5p is resisting endogenous TGF-β1-induced changes as well. This is expected when miR-202 targets nascent TGFBR1 and TGFBR2 transcripts for RNA interference. Western blotting analysis of control transfected cells treated with TGF-β1 (10 ng/mL; 48 h) showed reduced expression of epithelial markers (e.g., E-cadherin) and increased expression of mesenchymal markers (e.g., N-cadherin and vimentin) (Fig. 3B, left panel). However, such changes were absent or reduced, especially for changes in mesenchymal markers N-cadherin and vimentin, in miR-202-5p overexpressing cells treated with TGF-β1 (Fig. 3B, left panel). Consistent with morphological observations of miR-202-5p overexpressing cells appearing more epithelial-like (Fig. 3A, left, bottom panel), these cells showed increased expression of the epithelial markers E-cadherin and cytokeratin 18 and reduced expression of the mesenchymal markers N-cadherin and vimentin (Fig. 3B). Additionally, such changes were reduced or absent when miR-202-5p overexpressing cells were transfected with anti-miR-202-5p (50 nM; 48 h) (Fig. 3B; right panel) confirming miR-202-5p to play a role in EMT resistance.
Figure 3. MiR-202-5p resists TGF-β1 signaling pathway, counteracts EMT features, and slows cellular growth in pancreatic cancer cells.
A. MiR-202-5p resisted TGF-β1-induced morphological EMT features in PANC-1. Phase contrast images of live cells after TGF-β1 treatment (10 ng/mL; 48 h). Original magnification, X10. B. MiR-202-5p overexpression resisted TGF-β1-induced changes while antimiR-202-5p antagonized miR-202-5p-induced changes in epithelial and mesenchymal markers. Whole cell lysates (50 μg) of empty vector-transfected cells or miR-202-5p-transfected cells (with or without antimiR-202-5p) untreated or treated with TGF-β1 (10 ng/mL; 48 h) were subjected to Western blotting for EMT markers with β-actin as a loading control. C and D. MiR-202-5p decreased cell migration. Representative images of cells subjected to a scratch wound assay (C) and quantification of wound closure measurements (D). Original magnification X4. E. MiR-202-5p reduced TGF-β1-induced p-Smad2 levels in PANC-1. Whole cells lysates of TGF-β1 (10 ng/mL; 15–60 minutes) treated cells subjected to Western blotting to detect total and p-Smad2. F. DZNep-induced reduction in p-Smad2 level is rescued with antimiR-202-5p in PANC-1. Whole cell lysates of TGF-β1-treated (10 ng/mL; 60 mins) PANC-1 with or without DZNep pre-treatment (10 μM; 36 h) (in presence or absence of antimiR-202-5p; 50 nM) were subjected to Western blotting to detect total and p-Smad2 & Smad2 levels. G–H. MiR-202-5p overexpression altered TGF-β signaling genes and attenuated TGF-β1 signaling pathway. Total RNA from cells subjected to a RT2-PCR profiler array. Heat maps showing transcript level changes of TGF-β-responsive genes (G) and overall TGF-β pathway activity scores depicted (H). I. MiR-202-5p reduced cellular proliferation as determined by MTT. Points, mean of duplicate or triplicate; bars, SD or SEM. n=2 or 3.
Subsequent to biochemical characterization of EMT markers, we examined the role of miR-202-5p in modulating a key phenotypic EMT characteristic, i.e., cell migration. MiR-202-5p reduced cell migration in a wound-healing assay both in the presence and absence of TGF-β1 treatment (Fig. 3C–D). Further, to test whether miR-202 can significantly influence TGF-β-induced activation of TGF signaling in pancreatic cancer cells, we treated control or miR-202 overexpressing PANC-1 with TGF-β1 and observed for changes in the phosphorylation of Smad2. Western blotting analysis of control transfected cells treated with TGF-β1 (10 ng/mL; 15–60 minutes) showed increased phosphorylated levels of Smad2 with no significant changes in total Smad2 levels, while in miR-202-5p overexpressing cells, such increments in p-Smad2 levels were either absent or reduced (Fig. 3E). More importantly, antimiR-202-5p treatment (50 nM; 36 h) rescued DZNep’s effects on p-Smad2 levels in TGF-β1-treated PANC-1 suggesting that DZNep’s effects are at least partially mediated by miR-202-5p in pancreatic cancer. Since antimiR-202-5p was observed to significantly antagonize both miR-202-5p as well as DZNep’s actions, we assessed the effect of antimiR-202-5p on miR-663a and miR-4787-5p (DZNep-induced miRNAs previously shown to target TGF-β1 ligand) in PANC-1 (1). As shown in Fig. S4, antimiR-202-5p was found to be specific to its target (miR-202) and did not alter the levels of TGF-β1-targeting miR-663a and miR-4787-5p in PANC-1. To extend the understanding of the effect of miR-202-5p on the players involved in the TGF-β signaling axis, we profiled several genes involved in TGF-β signaling using a nested PCR array (1). Analysis of Ct values of all 84 genes profiled in the RT-PCR array identified a strong positive pathway score of +0.865 (p<0.001) in PANC-1 treated with TGFβ indicating the activation of TGF-β pathway as shown in our previous studies (1). In contrast, a negative TGF-β pathway score of (~−0.6) (p<0.005) was determined in PANC-1 with miR-202-5p overexpression, thereby confirming the repressive effect of miR-202-5p on the TGF-β signaling axis (Fig. 3G–H). The extent of inhibition was much higher than that observed with either miR-663a (−0.4; (p<0.01)) or miR-4787 (−0.2; miR-4787-5p (p=n.s.)) which act on the TGF-β1 ligand, suggesting a stronger influence of DZNep on TGF-β signaling axis through acting on the receptors (1). In addition, MTT assays showed that miR-202-5p reduced the cellular proliferation rate in pancreatic cancer cells (Fig. 3I).
MiR-202-5p overexpression in PSCs slows growth, suppresses stromal-associated ECM proteins, and reduces viability of pancreatic cancer cells grown in PSC-conditioned media
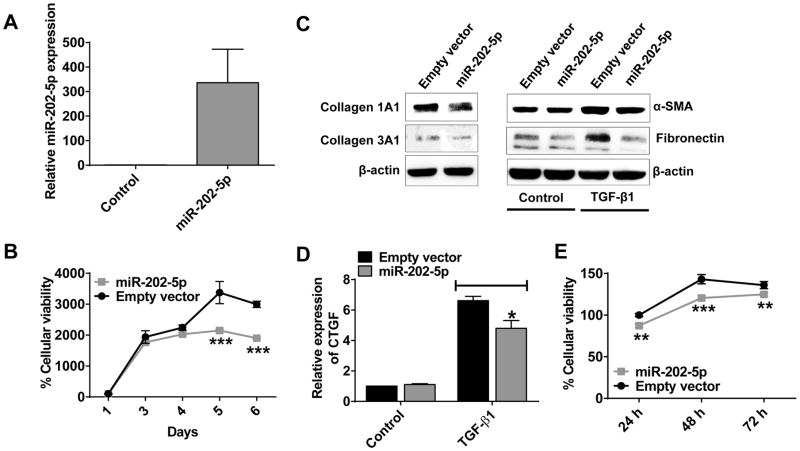
Pancreatic stellate cells (PSCs) are the main cell types that form the fibrotic stroma in PDAC (24). TGF-β signaling plays an important role in the activation of PSCs that leads to an elevated production and accumulation of ECM proteins and thereby fibrotic stromal deposition (11, 24). As such, the activated PSCs act as supporting cells for PDAC progression and metastasis. Since miR-202-5p suppressed TGF-β signaling in pancreatic cancer cells, we next wanted to investigate if the pathophysiological role of miR-202-5p was similar in pancreatic stellate cells. We overexpressed miR-202-5p in PSCs (Fig. 4A) using a lentiviral gene transfer method (20) and evaluated the effects of miR-202-5p on various phenotypes in PSCs. To begin, we evaluated the effects of miR-202-5p on the growth of PSCs especially because we found miR-202-5p to suppress growth of pancreatic cancer cells (Fig. 3I). As shown by the MTT cell proliferation assay, miR-202-5p significantly slowed the growth of PSCs as compared with control cells (Fig. 4B). We next wanted to investigate whether miR-202-5p suppressed TGF-β signaling in PSCs and thereby activated PSC-mediated production of ECM proteins. Hence, we checked the levels of three ECM proteins (Collagen 1alpha1, collagen 3alpha1, and fibronectin), a PSC activation marker (α-Smooth Muscle Actin), and a downstream mediator of TGF-β1-induced fibrotic effect (CTGF; Connective Tissue Growth Factor). Indeed, overexpression of miR-202-5p reduced ECM protein levels in PSCs as compared with control cells (Fig. 4C, left Panel). In addition, TGF-β1 induced levels of α-SMA and fibronectin indicating that the activated state of PSCs was effectively counteracted with miR-202-5p overexpression in PSCs (Fig. 4C, right Panel). This was also evident from the reduction in the TGF-β1-induced expression of CTGF by miR-202-5p in PSCs (Fig. 4D). Finally, to check the paracrine effects of miR-202-5p overexpressing PSCs on the growth of the pancreatic cancer cells, we grew PANC-1 cells in conditioned media of miR-202-5p overexpressing or control activated PSCs and measured cell viability. As shown in Fig. 4E, PANC-1 cells in conditioned media of miR-202-5p overexpressing and activated PSCs showed a reduced growth as compared with control. Taken together, these results show that miR-202-5p suppresses the TGF-β signaling in PSCs and thereby the autocrine and paracrine effects of PSCs and pancreatic cancer cells.
Figure 4. MiR-202-5p slows PSCs growth, reduces stromal ECM protein levels, and suppresses viability of pancreatic cancer cells grown in conditioned media of PSCs.
A. Overexpression of miR-202-5p in PSCs as analyzed by qRT-PCR with a taqman probe. RNU6B used as internal control. B. MiR-202-5p reduced PSCs cellular proliferation compared with control as determined by MTT assay. 1X103 PSCs with miR-202-5p overexpression or control seeded in a 96-well plate subjected to MTT assay at indicated time points. C and D. MiR-202-5p reduced ECM protein levels as well as suppressed TGF-β1-induced activation of PSCs. PSCs overexpressing miR-202-5p or control with or without TGF-β1 treatment (10 ng/mL; 48 h) lysed and subjected to Western blotting (C) or qRT-PCR analysis (D) to check the levels of ECM proteins (Collagens, and Fibronectin) or activation of PSCs (α-SMA, CTGF). β-actin used as a loading control for Western blotting while GUSB used as an internal control for qRT-PCR. E. Conditioned media of PSCs with miR-202-5p overexpression reduced pancreatic cancer cells viability. Cellular viability of pancreatic cancer cells (PANC-1) grown in conditioned media of PSCs with miR-202-5p overexpression or control was measured with MTT cellular proliferation assay over 72 h. Points, mean of duplicate or triplicate; bars, SD. n=2 or 3.
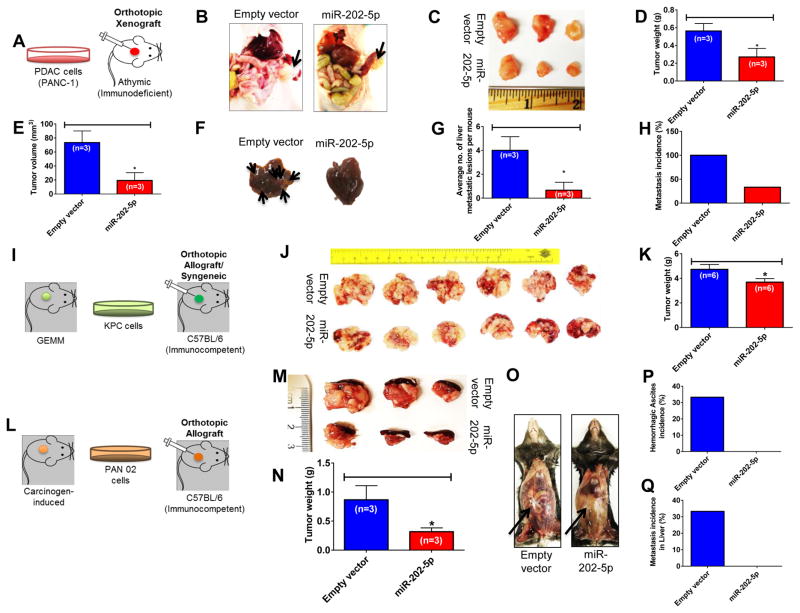
Exogenous Overexpression of MiR-202 suppresses the growth and metastasis of pancreatic cancer xenografts in immunocompromised and immunocompetent mouse models
To study whether miR-202-5p can direct in vivo suppression of the TGF-β signaling-induced increase in metastasis, we investigated whether the in vitro effects of miR-202-5p could be recapitulated in athymic nude mice orthotopically implanted with PANC-1 cells stably overexpressing miR-202-5p or empty vector-transfected cells (Fig. 5A). As shown in Fig. 5B–C, mice transplanted with miR-202-5p overexpressing cells at the end of 8 weeks show reduced tumor growth as compared with mice transplanted with empty vector-transfected cells. Consistently, average tumor weights (n=3) (Fig. 5D) and average tumor volumes (n=3) (Fig. 5E) were significantly reduced in mice with miR-202-5p overexpressing xenografts as compared with control. Moreover, gross pathological examination of the liver, one of the main sites for pancreatic tumor metastasis, showed the average number of metastatic lesions per mouse to be reduced in the miR-202-5p group of mice as compared with control group (Fig. 5F and 5G). Furthermore, 100% of mice (3 out of 3) in the control group showed liver metastases compared with a reduction to ~33% (1 out of 3) in the miR-202-5p group (Fig. 5H). Overall, there were no significant changes in mice body weights indicating that miR-202-5p was well tolerated by the mice (data not shown). These results support that miR-202-5p can suppress metastasis in vivo in an orthotopic model of pancreatic tumor xenografts.
Figure 5. MiR-202-5p suppresses pancreatic cancer growth and metastasis in vivo.
A–H. MiR-202-5p decreased primary tumor burden and metastasis in an orthotopic pancreatic xenograft model A. Establishment of an orthotopic pancreatic xenograft model in an athymic nude (immunodeficient) mice. Representative photographs of tumor xenografts (B) and excised tumor specimens (C) after 8 weeks of cell injections. D & E. MiR-202-5p decreased primary tumor growth in an orthotopic pancreatic xenograft model. Average tumor weights (D) and volumes (E) in mice injected with empty vector- or miR-202-5p-transfected cells. F. Representative images of tumor metastases to the liver with arrows indicating metastatic foci. Tumor characteristics were confirmed by histopathology. G & H. MiR-202-5p reduced overall metastasis in liver. G. The total number of metastatic nodules in liver per mouse was calculated and plotted. The bar indicates mean number of lesions in liver per mouse within a group. H. Metastatic incidences in liver. I–K. MiR-202-5p decreased primary tumor burden in a syngeneic orthotopic model of pancreatic cancer. I. Establishment of a syngeneic orthotopic model of pancreatic cancer in an immunocompetent mice. Representative photographs of tumors (along with spleen) (J) and average tumor weights (K) after 21 days of KPC (Empty vector or miR-202-5p overexpressing) cell injections in C57BL/6 mice. L–Q. MiR-202-5p decreased primary tumor burden and metastasis in another orthotopic mouse model of pancreatic cancer. L. Establishment of an orthotopic mouse model of pancreatic cancer in an immunocompetent mice. M & N. MiR-202-5p decreased primary tumor burden in an orthotopic mouse model of pancreatic cancer. Representative photographs of excised tumor specimens (along with spleen) (M) and average tumor weights (N) after 9 weeks of PAN 02 (Empty vector or miR-202-5p overexpressing) cell injections in C57BL/6 mice. O–Q. MiR-202-5p suppressed aggressiveness and reduced overall metastasis of pancreatic cancer. O. Representative photographs of mice bearing PAN 02 tumors demonstrating reduced aggressiveness in miR-202 group as evidenced by the absence of hemorrhagic ascites and peritoneal invasion (indicated by arrow) as compared with control. P. Incidence of hemorrhagic ascites. Q. Metastatic incidences in liver. Bars, SEM. n=3 or 6.
In order to evaluate if miR-202-5p exerted similar effects in a mouse model with an active immune system, we established a syngeneic mouse model of pancreatic cancer by orthotopically implanting KPC cells (from genetically engineered mouse model of pancreatic cancer) in the pancreas of C57BL/6 mice (Fig. 5I). Consistent with the human pancreatic xenograft model, miR-202-5p overexpression reduced tumor burden in the KPC syngeneic model as well (Fig. 5J–K). However, the KPC syngeneic mice developed extensive peritoneal carcinomatosis and dissemination along with intense hemorrhagic ascites and gastric obstruction (data not shown). Hence, these mice were sacrificed as early as on day 21 post cell-implantation, at which none had developed liver metastasis. As a result, we were not able to evaluate the effect of miR-202-5p on liver metastasis in syngeneic model of pancreatic cancer. Hence, we carried out a similar study in another immunocompetent mouse model of pancreatic cancer which was developed in a similar fashion using PAN 02 cells (from carcinogen-induced mouse model of pancreatic cancer) (Fig 5L). Indeed, miR-202-5p not only reduced primary tumor burden (Fig. 5M–N) but also reduced the aggressiveness of PAN 02 pancreatic tumors as evidenced by the absence of hemorrhagic ascites as well as liver metastasis (Fig. 5O–Q). Overall, these results show that miR-202-5p can reduce tumor aggressiveness in mouse models with an active immune system as well.
DISCUSSION
Previous studies in our laboratory showed DZNep to suppress TGF-β1-induced EMT characteristics in PDAC (1, 19). We identified epigenetic reprogramming of miRNAs, especially miR-663a and miR-4787-5p that directly targeted TGF-β1 for RNA interference, as a mechanism of DZNep inhibition of endogenous TGF-β1. However, the post-transcriptional mechanism of DZNep-mediated inhibition of endogenous TGF-β1 did not provide explanations for DZNep resisting exogenous TGF-β1-induced EMT effects. The current study contributes to the better understanding of the pharmacological mechanisms of DZNep by revealing the miR-202-5p effect on TGF-β receptors. To our knowledge, this is the first study that demonstrates the direct effects of a histone methylation inhibitor (DZNep) in reducing both TGFBR1 and TGFBR2 protein expression across a panel of PDAC cells. The mechanism of DZNep inhibition of TGFBR1 and TGFBR2 was attributed, at least partially, to miR-202-5p which directly targeted both TGFBR1 and TGFBR2 3′UTRs for RNA interference. MiR-202 overexpression reduced the protein levels of both TGFBR1 and TGFBR2, and consequently, resisted TGF-β1-induced EMT-like characteristics in PDAC. Thus, these findings uncover a novel mechanism of DZNep-mediated inhibition of TGF-β1-induced EMT in PDAC.
We suspected DZNep to affect TGF-β1 signaling either by targeting the TGF-β1 receptors or players downstream of TGF-β1 in the pathway. By screening endogenous expression profiles in basal states, we observed expressional differences in the endogenous levels of TGFB receptors (TGFBR1 and TGFBR2) and Smads (Smad2, Smad3, and Smad 4) (data not shown) in cell lines representing various subtypes of PDAC. While this partially explains the inherent differences in TGF-β signaling noted between various PDAC cell lines, DZNep’s effect was consistent in reducing the protein levels of TGFBR1 and TGFBR2 across multiple PDAC cell lines. However, DZNep effects were independent of Smad protein alterations. The strong reduction in TGF-β expression and lack of alteration of Smad proteins is likely to be beneficial from a therapeutic perspective considering the tumor suppressor effects of certain Smads in pancreatic cancer.
Next, DZNep’s ability to induce several miRNAs that could target TGFB receptors also suggested a post-transcriptional mechanism of TGFBR1 and TGFBR2 inhibition. It is likely that several DZNep-induced miRNAs could target TGFBR1 and/or TGFBR2 and possibly mediate DZNep-induced EMT effects in PDAC. Particularly, we focused on miR-202-5p in this study since it was predicted to bind to the 3′UTRs of both TGFBR1 and TGFBR2 for RNA interference and it was one of the miRNAs maximally induced by DZNep in PANC-1 (~4–5-fold). While miR-202-3p has been previously reported to be a tumor suppressor miRNA for various types of cancer (e.g., hepatocellular carcinoma, osteosarcoma, and colorectal cancer) (25–27), its role in any other types of cancer including pancreatic cancer is not investigated. Previous studies identified both miR-202-3p and miR-202-5p as evolutionarily conserved gonadal miRNAs regulated by a sex-determining transcription factor, SOX-9, with a potential role in gonadal differentiation (28). However, the current study for the first time presents miR-202-5p as potential growth suppressing miRNA candidate that targets both TGFBR1 and TGFBR2 in PDAC. Additionally, miR-202-5p suppresses the TGF-β signaling axis, reduces the expression of several genes associated with TGF-β signaling, and counteracts TGF-β1-induced EMT-like characteristics of PDAC. In this study, we overexpressed miR-202 with a lentiviral construct of pre-miR-202 that resulted in overexpression of both miR-202-5p and miR-202-3p strands. Interestingly, in addition to miR-202-5p, bioinformatics analysis also predicted miR-202-3p to target TGFBR1 3′UTR. Although we confirmed that the observed phenotypic effects of miR-202 were specific to miR-202-5p (using antagomiRs specific for miR-202-5p and not -3p), it is not unlikely that contributions may have also occurred from miR-202-3p. In addition to miR-202-5p, DZNep induced the expression of other miRNAs including miR-4646-5p, miR-361-5p, miR-3182, miR-320a, miR-320c, miR-320d, miR-1273g-3p, and miR-19a-3p (1) which were also predicted to target TGFBR1 and/or TGFBR2. However, understanding the possible functional contributions of these miRNAs in mediating DZNep-induced inhibitions of TGFBR1 and TGFBR2, and thereby resistance to TGF-β-induced EMT, warrant further studies.
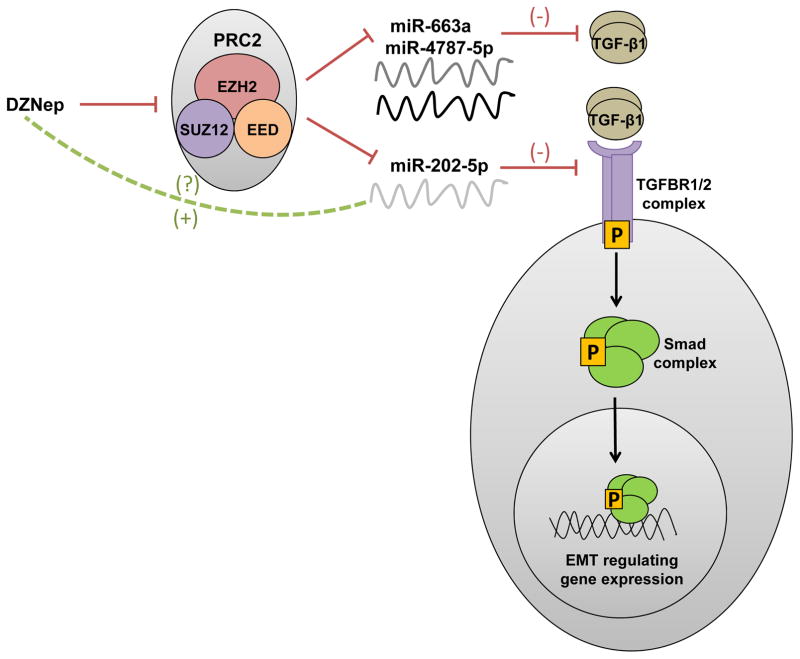
While we have earlier demonstrated DZNep to reprogram miR-663a and miR-4787-5p to inhibit TGF-β1 (1), in this study, we found DZNep to induce miR-202-5p to inhibit TGFBR1 and TGFBR2 for RNA interference in PDAC (Fig. 6). Since silencing EZH2 with shRNAs moderately increased the levels of these miRNAs in pancreatic cancer cells, it is likely that the DZNep’s action of miRNAs induction is partially via EZH2 inhibition. Taken together, these findings explain the role and mechanistic aspects of synthetic histone methylation reversal agents in inhibiting TGF-β signaling and TGF-β-induced EMT in PDAC. Indeed, as we complete our studies, another independent group very recently showed DZNep’s effect in inhibiting TGF-β induced activation of renal fibroblasts and deposition of extracellular matrix protein (ECM) in an obstructed kidney by preventing Smad7 (an inhibitor of TGF-β signaling) degradation and reducing TGFBR1 expression (29). Hence, it is also likely that DZNep can also suppress TGF-β-induced activation of PSCs and fibrosis associated with stromal accumulation and deposition, both of which along with EMT are also known to contribute to the development of chemotherapeutic resistance of pancreatic cancer. Overall, these findings are consistent with our previous work which demonstrated DZNep to induce chemosensitizing properties in pancreatic cancer (19). Nonetheless, DZNep is a non-specific, global histone methylation inhibitor reported to cause several unintended toxicities (e.g., nephrotoxicity) in mice that halted the clinical development of the compound (30). Hence, by understanding the mechanistic aspects of DZNep, further efforts were made in this study to identify the molecular targets of DZNep responsible for its beneficial therapeutic actions. On this basis, these findings so far further support the utilities of histone demethylation agents for combined epigenetic-chemotherapeutic combination therapies in pancreatic cancer. Next logical steps include combination of miR-663a/miR-4787-5p and/or miR-202-5p with specific chemotherapeutic agents to improve the overall effectiveness of pancreatic cancer therapy. In fact, it seems single nanoparticle formulations combining a cocktail of miRNAs (here miR-663a/miR-4787-5p targeting TGF-β1 and miR-202-5p targeting TGFBR1 and TGFBR2) seems a plausible strategy to combat the aberrant TGF-β signaling in pancreatic cancers.
Figure 6.
Schematic representation showing the mechanism of DZNep in resisting TGF-β1-induced EMT in pancreatic cancer by upregulating miR-663 and miR-4787 that targets TGF-β1 and miR-202 that targets TGFBR1 and TGFBR2.
In summary, our studies identify a nucleoside-derived compound, DZNep, and specific DZNep-induced miRNAs to attenuate TGF-β signaling by acting at the ligand and/or receptor levels. These findings support the possibility of combining small molecule- (e.g., DZNep analogs) or large molecule- (e.g., miRNAs) based epigenetic reversal agents with conventional nucleoside analogs (e.g., gemcitabine, capecitabine) to improve the anti-metastatic potential of current pancreatic cancer therapy.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the award NIH 1R01CA188464 (RG)
Footnotes
The authors disclose no potential conflicts of interest
References
- 1.Mody H, Hung SW, Al-Saggar M, Griffin J, Govindarajan R. Inhibition of S-Adenosylmethionine-Dependent Methyltransferase Attenuates TGF-beta1-induced EMT and Metastasis in Pancreatic Cancer: Putative Roles of miR-663a and miR-4787-5p. Molecular cancer research: MCR. 2016 doi: 10.1158/1541-7786.MCR-16-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, et al. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Annals of surgical oncology. 2007;14(12):3527–33. doi: 10.1245/s10434-007-9540-3. [DOI] [PubMed] [Google Scholar]
- 3.Satoh K, Hamada S, Shimosegawa T. Involvement of epithelial to mesenchymal transition in the development of pancreatic ductal adenocarcinoma. Journal of gastroenterology. 2015;50(2):140–6. doi: 10.1007/s00535-014-0997-0. [DOI] [PubMed] [Google Scholar]
- 4.Smith BN, Bhowmick NA. Role of EMT in Metastasis and Therapy Resistance. Journal of clinical medicine. 2016;5(2) doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzillet C, de Gramont A, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, et al. Perspectives of TGF-beta inhibition in pancreatic and hepatocellular carcinomas. Oncotarget. 2014;5(1):78–94. doi: 10.18632/oncotarget.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology: official journal of the International Association of Pancreatology. 2007;7(5–6):423–35. doi: 10.1159/000108959. [DOI] [PubMed] [Google Scholar]
- 7.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFbeta in cancer. FEBS letters. 2012;586(14):1959–70. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Current opinion in oncology. 2013;25(1):76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 9.Massague J. TGFbeta signalling in context. Nature reviews Molecular cell biology. 2012;13(10):616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nature reviews Cancer. 2013;13(11):788–99. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whatcott C, Han H, Posner RG, Von Hoff DD. Tumor-stromal interactions in pancreatic cancer. Critical reviews in oncogenesis. 2013;18(1–2):135–51. doi: 10.1615/critrevoncog.v18.i1-2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell research. 2009;19(2):156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-beta - an excellent servant but a bad master. Journal of translational medicine. 2012;10:183. doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27(58):7274–84. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer cell. 2013;23(6):768–83. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Hung MC. Regulation and Role of EZH2 in Cancer. Cancer research and treatment: official journal of Korean Cancer Association. 2014;46(3):209–22. doi: 10.4143/crt.2014.46.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KH, Roberts CW. Targeting EZH2 in cancer. Nature medicine. 2016;22(2):128–34. doi: 10.1038/nm.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung SW, Mody H, Marrache S, Bhutia YD, Davis F, Cho JH, et al. Pharmacological reversal of histone methylation presensitizes pancreatic cancer cells to nucleoside drugs: in vitro optimization and novel nanoparticle delivery studies. PloS one. 2013;8(8):e71196. doi: 10.1371/journal.pone.0071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhutia YD, Hung SW, Krentz M, Patel D, Lovin D, Manoharan R, et al. Differential processing of let-7a precursors influences RRM2 expression and chemosensitivity in pancreatic cancer: role of LIN-28 and SET oncoprotein. PloS one. 2013;8(1):e53436. doi: 10.1371/journal.pone.0053436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer research. 2013;73(13):3927–37. doi: 10.1158/0008-5472.CAN-12-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon JJ, Nabinger SC, Vega Z, Sahu SS, Alluri RK, Abdul-Sater Z, et al. Pathophysiological role of microRNA-29 in pancreatic cancer stroma. Scientific reports. 2015;5:11450. doi: 10.1038/srep11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haqq J, Howells LM, Garcea G, Metcalfe MS, Steward WP, Dennison AR. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. European journal of cancer. 2014;50(15):2570–82. doi: 10.1016/j.ejca.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Zhang T, Hong H, Liu Q, Zhang H. miR-202 suppresses proliferation and induces apoptosis of osteosarcoma cells by downregulating Gli2. Molecular and cellular biochemistry. 2014;397(1–2):277–83. doi: 10.1007/s11010-014-2195-z. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Huang Z, Guo W, Ni S, Xiao X, Wang L, et al. microRNA-202-3p inhibits cell proliferation by targeting ADP-ribosylation factor-like 5A in human colorectal carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(5):1146–57. doi: 10.1158/1078-0432.CCR-13-1023. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zheng D, Xiong Y, Xue C, Chen G, Yan B, et al. miR-202 suppresses cell proliferation in human hepatocellular carcinoma by downregulating LRP6 post-transcriptionally. FEBS letters. 2014;588(10):1913–20. doi: 10.1016/j.febslet.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Wainwright EN, Jorgensen JS, Kim Y, Truong V, Bagheri-Fam S, Davidson T, et al. SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biology of reproduction. 2013;89(2):34. doi: 10.1095/biolreprod.113.110155. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Zang X, Ponnusamy M, Masucci MV, Tolbert E, Gong R, et al. Enhancer of Zeste Homolog 2 Inhibition Attenuates Renal Fibrosis by Maintaining Smad7 and Phosphatase and Tensin Homolog Expression. Journal of the American Society of Nephrology: JASN. 2016;27(7):2092–108. doi: 10.1681/ASN.2015040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun F, Lee L, Zhang Z, Wang X, Yu Q, Duan X, et al. Preclinical pharmacokinetic studies of 3-deazaneplanocin A, a potent epigenetic anticancer agent, and its human pharmacokinetic prediction using GastroPlus. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2015;77:290–302. doi: 10.1016/j.ejps.2015.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.