Abstract
Women with atypical hyperplasia (AH) and lobular or ductal carcinoma in situ (LCIS/DCIS) are at increased risk of developing invasive breast cancer. Chemoprevention with selective estrogen receptor modulators or aromatase inhibitors can reduce breast cancer risk; however, uptake is estimated to be less than 15% in these populations. We sought to determine which factors are associated with chemoprevention uptake in a population of women with AH, LCIS, and DCIS. Women diagnosed with AH/LCIS/DCIS between 2007 and 2015 without a history of invasive breast cancer were identified (n=1719). A subset of women (n=73) completed questionnaires on breast cancer and chemoprevention knowledge, risk perception, and behavioral intentions. Descriptive statistics were generated and univariate and multivariable log-binomial regression were used to estimate the association between sociodemographic and clinical factors and chemoprevention uptake. In our sample, 29.3% had AH, 23.3% had LCIS, and 47.4% had DCIS; 29.4% used chemoprevention. Compared to women with AH, LCIS (RR: 1.43; 95% CI: 1.16–1.76) and DCIS (RR: 1.54; 95% CI: 1.28–1.86) were significantly associated with chemoprevention uptake, as was medical oncology referral (RR: 5.79; 95% CI: 4.80–6.98). Younger women were less likely to take chemoprevention (RR: 0.61; 95% CI: 0.42–0.87) and there was a trend towards increased uptake in Hispanic compared to non-Hispanic white women. The survey data revealed a strong interest in learning about chemoprevention, but there were misperceptions in personal breast cancer risk and side effects of chemoprevention. Improving communication about breast cancer risk and chemoprevention may allow clinicians to facilitate informed decision-making about preventative therapy.
Keywords: Chemoprevention, Atypical hyperplasia, Lobular carcinoma in situ, Ductal carcinoma in situ, Breast cancer
Introduction
Breast cancer is the most commonly diagnosed cancer among women in the United States, leading to over 40,000 deaths annually (1). The national costs of surveillance and treatment of breast cancer are expected to surpass $20 billion by 2020 (2). It is estimated that at least 15% of women, age 35–75 years, in the U.S. are considered high-risk for breast cancer, defined as having a greater than 1.67% 5-year risk or greater than 20% lifetime risk of developing invasive breast cancer according to the Gail Model (3). Factors that greatly increase the risk of invasive breast cancer development include atypical hyperplasia (AH), lobular carcinoma in situ (LCIS), or ductal carcinoma in situ (DCIS). It is estimated that AH increases the risk for invasive breast cancer by 3.7–5.3 times relative to women with non-proliferative breast disease (4). Coopey et al. reported that the 10-year risk of invasive and non-invasive breast cancer after a diagnosis of atypical ductal or lobular hyperplasia is 17.3% and 20.7%, respectively (5). LCIS is estimated to increase the risk of breast cancer by approximately 7–10 times the general population with an estimated 10-year breast cancer risk of 23.7% (5,6). DCIS also significantly increases the risk of invasive breast cancer with an estimated 11.2% of women developing a subsequent invasive breast cancer within 10 years (7). One preventative strategy available to these high-risk women is the use of chemoprevention with selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) to reduce the risk of estrogen-receptor positive breast cancer.
The U.S. Food and Drug Administration (FDA) approved the SERMs tamoxifen in 1999 and raloxifene in 2007 for the primary prevention of breast cancer among women who met high-risk criteria (8,9). In a randomized controlled, double-blind trial of tamoxifen for 5 years versus placebo, high-risk women who took tamoxifen had a relative risk (RR) of breast cancer of 0.57 (95% confidence interval [CI]: 0.46 to 0.70) (10). While raloxifene has only 81% of the efficacy of tamoxifen in reducing the risk of breast cancer, there is a lower risk of serious side effects, such as endometrial cancer and thromboembolism, and a decrease in the risk for osteoporotic fractures (11,12). In the randomized, double-blind, placebo-controlled trial of the AI, exemestane, for chemoprevention published in 2011, there was a 65% relative risk reduction in invasive breast cancer (hazard ratio [HR] 0.35, 95% CI: 0.18 to 0.70) when compared to placebo (13). The IBIS-II trial investigated the efficacy of anastrozole, another AI, in preventing breast cancer in high-risk postmenopausal women. Compared to placebo, there was a 50% risk reduction in invasive breast cancer (HR 0.50, 95% CI: 0.32 to 0.76) (14). The risk of serious side effects among women who take AIs is lower compared to tamoxifen (13,14). Among women with LCIS and AH, the data suggests that SERMs and AIs could afford greater benefits to these particularly high-risk populations (10,14). While not all of the chemoprevention trials included women with DCIS, three large randomized controlled trials demonstrated that adjuvant tamoxifen or anastrozole for 5 years significantly prevented subsequent breast cancers in women with DCIS undergoing lumpectomy plus radiation (15–17).
The U.S. Preventive Services Task Force has recommended that physicians discuss chemoprevention options with their high-risk patients (18). Despite the potential of these therapies to reduce the incidence of invasive breast cancer in the U.S., their uptake among high-risk women has been estimated to be lower than 15% (19). Multiple factors contribute to the low uptake of breast cancer chemoprevention, including concerns about side effects and lack of clinician knowledge about use of SERMs or AIs for breast cancer risk reduction (19,20). Limited research has been published analyzing the sociodemographic and clinical factors associated with chemoprevention uptake among high-risk women, including those with AH, LCIS, and DCIS (5,21). The objective of our study is to identify which demographic and clinical factors are associated with the decision to use chemoprevention among women with a history of AH, LCIS, or DCIS seen at an academic medical center. We also examined breast cancer risk perceptions, beliefs, and attitudes about chemoprevention decision-making among a subset of these high-risk women.
Materials and Methods
Study Population and Selection Criteria
We conducted a retrospective cohort study of patients who received a diagnosis of AH, LCIS, or DCIS at Columbia University Medical Center (CUMC) in New York, NY between 2007 and 2015 in order to determine predictors of chemoprevention uptake. Inclusion criteria for the study included: 1) history of AH, LCIS, or DCIS without concurrent or prior invasive breast cancer; 2) for subjects with DCIS, evidence of estrogen receptor (ER)-positive and/or progesterone receptor (PR)-positive tumor status. Subjects with a history of bilateral mastectomy were excluded. All subjects were considered eligible for chemoprevention use based on their diagnosis of AH, LCIS, or ER+ and/or PR+ DCIS. This study was approved by the Institutional Review Board at CUMC and was conducted in accordance with recognized ethical guidelines.
Data Collection from the Electronic Health Record
Subject demographics, breast cancer risk factors, and medical information were collected through a chart review and data extraction of the electronic health record (EHR) at CUMC. The EHR captured data from diagnostic codes, breast pathology reports, and outpatient clinic notes, including referrals to breast oncology. The EHR data extraction also included the New York-Presbyterian Hospital tumor registry, which identified incident cases of LCIS and DCIS. All subjects with a diagnosis of AH or LCIS/DCIS were initially identified by their corresponding ICD-9/10 codes in these databases, 610.9/N60.99 and 233.0/D05.90, respectively. As LCIS and DCIS share the same ICD-9 and 10 codes, the NYP tumor registry and outpatient medical records were used to ascertain the appropriate diagnosis for each subject. If subjects had more than one diagnosis, they were classified by their most advanced breast lesion (DCIS > LCIS > AH). Any chart documentation of invasive breast cancer was identified with the ICD-9 code 174.9. Tumor registry and pathology reports were used to identify subjects who had invasive breast cancer prior to or concurrently with being diagnosed with AH, LCIS, or DCIS.
Other covariates collected included age, race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, and other), menopausal status, body mass index (BMI), hormone replacement therapy (HRT) use, family history of breast cancer (yes/no), history of hysterectomy, history of thromboembolism (deep vein thrombosis, pulmonary embolus, or stroke), history of uterine cancer, and medical oncology referral. Subjects who were missing information on their menopausal status were considered post-menopausal if they were over 55. Subjects missing information for their BMI were classified as unknown.
The primary outcome of interest was SERM or AI use as documented in the medication list of the EHR at any point in time and was dichotomized as yes/no ever use. Type of chemoprevention used was also identified and categorized as tamoxifen, raloxifene, AI (e.g., anastrozole, exemestane, letrozole), or multiple agents (i.e., patients may switch medications due to toxicities).
Data Collection from Patient-Administered Questionnaires
A subset of participants from the larger retrospective cohort were recruited to complete questionnaires during their first visit with a medical oncologist at CUMC. After providing informed consent, all subjects completed a baseline self-administered questionnaire in English or Spanish. Breast cancer knowledge was assessed with a 4-item scale, with adequate knowledge defined as >50% correct responses (22). Breast cancer risk perception was assessed by asking subjects to rate their chance of developing breast cancer and how it compares to other women (23). Breast cancer worry was a composite of patient responses to two questions on a 7-point Likert scale (24,25). Subjects were presented with 12 reasons for taking preventive action to lower risk for breast cancer and asked to rate how true each statement was on a 5-point Likert scale (26). Chemoprevention behavioral intention and decision satisfaction were assessed using items defined by Korfage et al.(27). Knowledge and worry about chemoprevention side effects were assessed using items defined by Fagerlin et al.(28). Acculturation, health literacy, and numeracy were assessed using brief validated measures of each construct (29–31).
Statistical Analysis
Subjects were stratified according to whether or not they had ever taken chemoprevention and descriptive statistics of sociodemographic and clinical characteristics were generated. Chi-square test, or Fisher’s exact test for cell ranges below 5, were used to compare the distribution of risk factors between those who did and did not take chemoprevention. Univariate analysis was conducted to give an unadjusted estimate of the risk associated with each variable on the outcome of chemoprevention use. Because the primary outcome of chemoprevention uptake was relatively common in our study population, log-binomial regression was used to calculate and report relative risk rather than odds ratios (32). A multivariable model was constructed using log-binomial regression to assess the relationship between breast disease (AH, LCIS, DCIS) and chemoprevention uptake when adjusted for other variables. The model was adjusted a priori for breast disease, age, and race/ethnicity. Variables that had an association of p<.05 in univariate analysis were also included in the final model. Menopausal status was excluded from the final model because it is highly correlated with age. For the subset of subjects who received the patient-administered questionnaire, descriptive statistics of sociodemographic and clinical characteristics, as well as survey responses, were generated. All statistical analysis was conducted using SAS version 9.4 (SAS Institute, Cary, NC) and a p-value of <.05 was considered statistically significant.
Results
Results of EHR analysis
During the study period of January 2007 to December 2015, approximately 2933 subjects with an ICD-9/10 code for AH or LCIS/DCIS were initially identified through the EHR. Of these subjects, 1719 (58.6%) met all inclusion criteria and were included in our final analysis. Of the 1214 subjects excluded from the original dataset, 1066 (87.8%) had evidence of invasive breast cancer either before or concurrently with their diagnosis of AH, LCIS, or DCIS. An additional 58 (4.8%) were excluded due to history of bilateral mastectomy or ER/PR-negative DCIS, and 90 (7.4%) were excluded because there was no clarification of whether they had LCIS or DCIS in their medical record. Figure 1 depicts a CONSORT diagram describing our study population.
Figure 1.
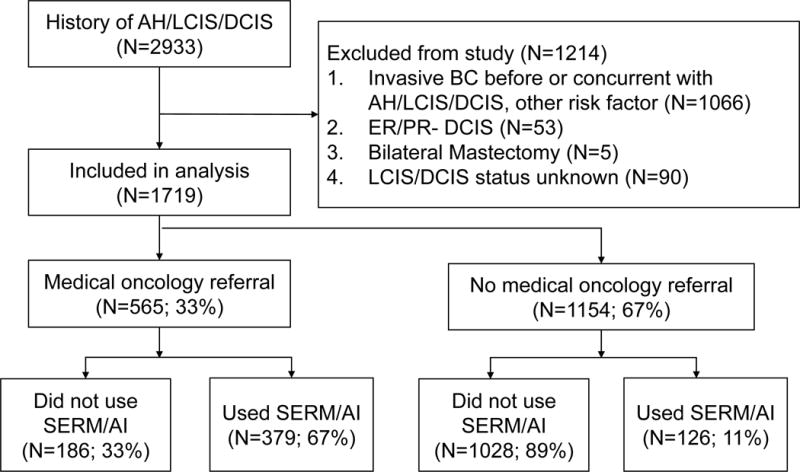
CONSORT diagram of subjects by eligibility for analysis and chemoprevention use. Abbreviations: AI=aromatase inhibitor; AH=atypical hyperplasia; BC=breast cancer; DCIS=ductal carcinoma in situ; ER=estrogen receptor; LCIS=lobular carcinoma in situ; PR=progesterone receptor; SERM=selective estrogen receptor modulator.
Table 1 describes the baseline characteristics of the study population. The mean age of our sample was 60 years, with a range of 21 to 98 years, and over two-thirds were postmenopausal. Our sample was racially and ethnically diverse with 44.9% non-Hispanic white, 9.2% non-Hispanic black, 23.1% Hispanic, 5.9% Asian, and 16.9% other. In our total sample, 815 (47.4%) had DCIS, 401 (23.3%) had LCIS, and 503 (29.3%) had AH. About a third of these women had been seen by a medical oncologist. Relatively few subjects had chart documentation of hysterectomy (2.6%), HRT use (2.9%), history of thromboembolism (2.2%), or uterine cancer (0.6%).
Table 1.
Baseline characteristics of women diagnosed with atypical hyperplasia and lobular or ductal carcinoma in situ at Columbia University Medical Center, New York, NY (2007–2015)
| Characteristic | No Chemoprevention (n = 1214; 70.62%) |
Chemoprevention (n = 505; 29.38%) |
Total (n = 1719, %) |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Breast Histology | Atypical Hyperplasia | 420 | (34.60) | 83 | (13.44) | 503 | (29.26) | |
| Lobular Carcinoma in situ | 294 | (24.22) | 107 | (21.19) | 401 | (23.33) | ||
| Ductal Carcinoma in situ | 500 | (41.19) | 315 | (62.38) | 815 | (47.41) | <.0001 | |
|
| ||||||||
| Age | Mean Age - Years [SD] | 60.11 | (12.61) | 60.48 | (9.95) | 60.22 | (11.88) | |
| <45 Years | 114 | (9.39) | 23 | (4.55) | 137 | (7.97) | ||
| 45–54 Years | 328 | (27.02) | 118 | (23.27) | 446 | (25.95) | ||
| 55–64 Years | 351 | (28.91) | 185 | (36.63) | 536 | (31.18) | ||
| 65–74 Years | 255 | (21.00) | 128 | (25.35) | 383 | (22.28) | ||
| 75+ | 166 | (13.67) | 51 | (10.10) | 217 | (12.62) | <.0001 | |
|
| ||||||||
| Menopause | No | 420 | (34.60) | 120 | (23.76) | 540 | (31.41) | |
| Yes | 794 | (65.40) | 385 | (76.24) | 1179 | (68.59) | <.0001 | |
|
| ||||||||
| Race and Ethnicity | Non-Hispanic White | 551 | (45.39) | 221 | (43.76) | 772 | (44.91) | |
| Non-Hispanic Black | 98 | (8.07) | 60 | (11.88) | 158 | (9.19) | ||
| Hispanic | 255 | (21.00) | 142 | (28.12) | 397 | (23.09) | ||
| Asian | 60 | (4.94) | 41 | (8.12) | 101 | (5.88) | ||
| Other | 250 | (20.59) | 41 | (8.12) | 291 | (16.93) | <.0001 | |
|
| ||||||||
| Body Mass Index [BMI] (kg/m2) | Mean BMI - Score [SD] | 27.06 | (6.16) | 28.05 | (6.20) | 27.39 | (6.19) | |
| <18.5 | 34 | (2.80) | 8 | (1.58) | 42 | (2.44) | ||
| 18.5–24.99 | 384 | (31.63) | 167 | (33.07) | 551 | (32.05) | ||
| 25–29.99 | 301 | (24.79) | 154 | (30.50) | 455 | (26.47) | ||
| 30+ | 264 | (21.75) | 162 | (32.08) | 426 | (24.78) | ||
| Unknown | 231 | (19.03) | 14 | (2.77) | 245 | (14.25) | <.0001 | |
|
| ||||||||
| Family History of Breast Cancer | No | 1120 | (92.60) | 368 | (72.87) | 1488 | (86.56) | |
| Yes | 94 | (7.74) | 137 | (27.13) | 231 | (13.44) | <.0001 | |
|
| ||||||||
| Hysterectomy | No | 1183 | (97.45) | 491 | (97.23) | 1674 | (97.38) | |
| Yes | 31 | (2.55) | 14 | (2.77) | 45 | (2.62) | 0.7959 | |
|
| ||||||||
| Comorbiditiesa | No | 1177 | (96.95) | 494 | (97.82) | 1671 | (97.21) | |
| DVT/PE/Stroke | 30 | (2.47) | 8 | (1.58) | 38 | (2.21) | ||
| Uterine Cancer | 7 | (0.58) | 3 | (0.59) | 10 | (0.58) | 0.5223 | |
|
| ||||||||
| HRT Useb | No | 1204 | (99.18) | 466 | (92.28) | 1670 | (97.15) | |
| Yes | 10 | (0.82) | 39 | (7.72) | 49 | (2.85) | <.0001 | |
|
| ||||||||
| Medical Oncology Referral | No | 1028 | (84.68) | 126 | (24.95) | 1154 | (67.13) | |
| Yes | 186 | (15.32) | 379 | (75.05) | 565 | (32.87) | <.0001 | |
DVT = Deep Vein Thrombosis, PE = Pulmonary Embolism
HRT = Hormone Replacement Therapy
Among the 1719 subjects included in our final analysis, 505 (29.4%) had a history of ever using SERMs or AIs. Approximately 16.5% of patients with AH used a SERM or AI, compared to 26.7% of patients with LCIS, and 38.7% of patients with DCIS. The breakdown of chemoprevention used was 274 (54.3%) tamoxifen, 78 (15.4%) raloxifene, 97 (19.2%) aromatase inhibitors, and 56 (11.1%) used multiple agents. Figure 2 describes the distribution of type of chemoprevention stratified by breast histology type.
Figure 2.
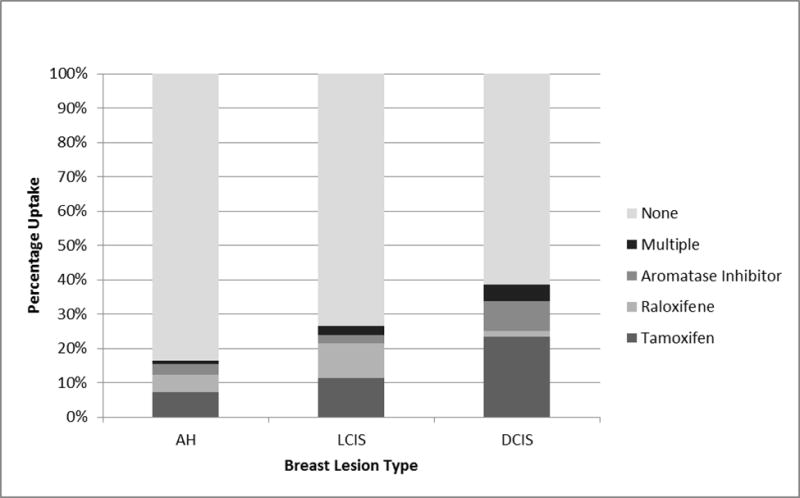
Distribution of chemoprevention uptake by breast lesion type. Abbreviations: AH=atypical hyperplasia; DCIS=ductal carcinoma in situ; LCIS=lobular carcinoma in situ.
In univariate analysis (Table 2), type of breast disease, age, menopausal status, race/ethnicity, BMI, family history of breast cancer, HRT use, and medical oncology referral were associated with chemoprevention uptake. Our multivariable model was adjusted for age, race/ethnicity, family history, breast disease, HRT use, and medical oncology referral. Compared to women with AH, those with a history of LCIS were 1.43 (95% CI: 1.16 to 1.76) times as likely and subjects with DCIS were 1.54 (95% CI: 1.28 to 1.86) times as likely to take chemoprevention. Age was also significantly associated with chemoprevention uptake. Women less than 45 years old and those over age 75 were also less likely to initiate chemoprevention. Race and ethnicity was no longer significantly associated in the multivariable model; however, the association between Hispanic women and chemoprevention use approached significance (p=0.075). The strongest predictor of chemoprevention uptake was medical oncology referral. Subjects who were seen by a medical oncologist were 5.79 times as likely to take chemoprevention when compared to those who did not receive a referral (95% CI: 4.80 to 6.98).
Table 2.
Log-binomial univariate and multivariable model of the association between sociodemographic and clinical factors and chemoprevention use; multivariable model adjusted for age, race/ethnicity, family history, hormone replacement therapy use, and medical oncology referral
| Characteristic | Univariate RR (95% CI) |
p-value | Multivariable RR (95% CI) |
p-value | |||
|---|---|---|---|---|---|---|---|
| Breast Histology | Atypical Hyperplasia | Reference | |||||
| Lobular Carcinoma in situ | 1.62 | (1.25 – 2.09) | 0.0002 | 1.43 | (1.16 – 1.76) | 0.0009 | |
| Ductal Carcinoma in situ | 2.34 | (1.89 – 2.90) | <.0001 | 1.54 | (1.28 – 1.86) | <.0001 | |
|
| |||||||
| Age | <45 Years | 0.63 | (0.42 – 0.95) | 0.0272 | 0.61 | (0.42 – 0.87) | 0.0069 |
| 45–54 Years | Reference | ||||||
| 55–64 Years | 1.29 | (1.07 – 1.58) | 0.0072 | 1.27 | (1.10 – 1.47) | 0.0012 | |
| 65–74 Years | 1.26 | (1.02 – 1.56) | 0.0289 | 1.22 | (1.05 – 1.43) | 0.0116 | |
| 75+ | 0.89 | (0.67 – 1.18) | 0.4163 | 1.06 | (0.85 – 1.31) | 0.6119 | |
|
| |||||||
| Menopause | No | Reference | |||||
| Yes | 1.47 | (1.23 – 1.76) | <.0001 | ||||
|
| |||||||
| Race and Ethnicity | Non-Hispanic White | Reference | |||||
| Non-Hispanic Black | 1.33 | (1.06 – 1.67) | 0.0153 | 0.99 | (0.85 – 1.16) | 0.9076 | |
| Hispanic | 1.25 | (1.05 – 1.48) | 0.0114 | 1.10 | (0.99 – 1.22) | 0.075 | |
| Asian | 1.42 | (1.09 – 1.84) | 0.0087 | 0.99 | (0.82 – 1.18) | 0.8942 | |
| Other | 0.49 | (0.36 – 0.67) | <.0001 | 0.92 | (0.72 – 1.19) | 0.5403 | |
|
| |||||||
| Body Mass Index [BMI] (kg/m^2) | <18.5 | 0.63 | (0.33 – 1.19) | 0.1524 | |||
| 18.5–24.99 | Reference | ||||||
| 25–29.99 | 1.12 | (0.93 – 1.34) | 0.2303 | ||||
| 30+ | 1.25 | (1.05 – 1.50) | 0.0112 | ||||
| Unknown | 0.19 | (0.11 – 0.32) | <.0001 | ||||
|
| |||||||
| Family History of Breast Cancer | No | Reference | |||||
| Yes | 2.40 | (2.09 – 2.76) | <.0001 | 0.98 | (0.87 – 1.09) | 0.6526 | |
|
| |||||||
| Hysterectomy | No | Reference | |||||
| Yes | 1.06 | (0.68 – 1.65) | 0.7935 | ||||
|
| |||||||
| Comorbiditiesa | No | Reference | |||||
| DVT/PE/Stroke | 0.71 | (0.38 – 1.32) | 0.2833 | ||||
| Uterine Cancer | 1.01 | (0.39 – 2.62) | 0.9758 | ||||
|
| |||||||
| HRT Useb | No | Reference | |||||
| Yes | 2.85 | (2.43 – 3.35) | <.0001 | 1.04 | (0.90 – 1.19) | 0.5941 | |
|
| |||||||
| Medical Oncology Referral | No | Reference | |||||
| Yes | 6.14 | (5.16 – 7.32) | <.0001 | 5.79 | (4.80 – 6.98) | <.0001 | |
DVT = Deep Vein Thrombosis, PE = Pulmonary Embolism
HRT = Hormone Replacement Therapy
Results of patient-administered questionnaires
A subset of 73 women completed validated questionnaires after an initial visit with a medical oncologist. The subset was slightly younger than the full cohort (mean age 53.5 years and 54.8% postmenopausal) and the distribution of race/ethnicity was similar to that of the full cohort. Thirty-five women (47.8%) had AH, 17 (23.3%) had LCIS, and 21 (28.77%) had ER+ and/or PR+ DCIS. Thirty-one (42.8%) subjects opted for chemoprevention, with 54.8% of those patients taking tamoxifen, 25.8% taking aromatase inhibitors, 9.7% taking raloxifene, and 9.7% taking multiple medications.
The sample generally showed high levels of acculturation, health literacy, and numeracy. Scores on a breast cancer knowledge index were relatively high, with 61.4% of subjects demonstrating adequate breast cancer knowledge. When these high-risk women were asked to rate their chance of developing breast cancer, 49.3% of subjects rated their chance as “moderately” or “very” high. Similarly, 52.2% of subjects considered their chance of developing breast cancer to be much higher than their peers. The majority of respondents agreed or strongly agreed with statements that reflected personal reasons for seeking out treatment for lowering breast cancer risk. “I want to improve my health” (93.1%), “I want to live longer” (95.8%), and “I want to avoid getting breast cancer treatment” (85.9%) were the most commonly cited reasons for taking action to reduce risk. Less common responses were those that related to family or friends getting breast cancer or encouragement from others to take action. With respect to side effects of chemoprevention, 71.6% of subjects were very worried or extremely worried about the side effects and 56.9% thought the side effects were very serious or extremely serious. Approximately 50.7% of subjects reported that they did not want to take a pill every day. Despite discussing chemoprevention with a medical oncologist, only 50% thought the benefits of preventative therapy were worth the risks.
After initial consultation with a medical oncologist, 52.9% of subjects felt they had enough information about chemoprevention to make a decision on whether or not to take it. The majority of participants (78.6%) indicated that they would be very or extremely likely to speak with their healthcare provider about chemoprevention drugs in the future. Among subjects who had taken chemoprevention, 50% indicated that they were very or extremely satisfied with their decision and an additional 37.5% were moderately satisfied.
Discussion
A prior systematic review reported chemoprevention uptake to be approximately 14.8% among high-risk women who are offered SERM or AI therapy; however, chemoprevention uptake was close to 30% in our cohort (19). Factors found to be associated with chemoprevention uptake in our study include referral to a medical oncologist and higher risk breast histology (DCIS > LCIS > AH). Age less than 45 years was inversely associated with chemoprevention uptake.
The strongest predictor of chemoprevention uptake was a medical oncology referral. Physician recommendation for chemoprevention has been found to be associated with uptake in several studies (21). Lack of physician knowledge has been cited as an important factor in influencing low chemoprevention uptake (20). Additionally, insufficient reimbursement to internal medicine physicians, family medicine physicians, and obstetricians/gynecologists has been shown to be a barrier for chemoprevention counseling (33). About a third of our study population was seen by a breast oncologist, who may be more knowledgeable about the risks and benefits of chemoprevention and therefore more willing to prescribe SERM or AI therapy as compared to a primary care physician or gynecologist. Our study was conducted at a tertiary care academic medical center with access to a high-risk breast clinic. However, patients seen in community practices may not have access to specialized risk counseling. In order to increase uptake of chemoprevention for breast cancer risk reduction in all settings, interventions should be targeted at primary care providers who may not be aware of a woman’s high-risk status or have experience with prescribing SERMs or AIs for chemoprevention. One such intervention developed by our group is the Breast cancer risk NAVigation (BNAV) tool, which is a web-based tool for primary care providers. BNAV serves as a repository of information and resources to help providers in the primary care setting assess breast cancer risk and understand the risks and benefits of chemoprevention (34). We also developed a patient-facing decision aid, RealRisks, for women found to be high risk for breast cancer. A randomized clinical trial evaluating these patient and provider decision support tools on chemoprevention uptake is currently underway (NCT03069742).
Our results also indicated that chemoprevention uptake is relatively high among women with high-risk breast lesions compared to other high-risk populations (i.e., 5-year Gail risk score ≥1.67% or strong family history of breast cancer) (19). As DCIS and LCIS are stronger risk factors for breast cancer than AH, it is possible that women with higher risk breast lesions are more likely to be recommended chemoprevention by their physician. Tamoxifen has been part of the standard of care for patients with DCIS since the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 trial were first published in 1999 (16). Data from the NSABP B-35 trial supporting the use of anastrozole for postmenopausal women with hormone receptor-positive DCIS was only recently published in 2016 (15). The Breast Cancer Prevention Trial (BCPT) of tamoxifen for primary prevention also found that women with AH and LCIS have a greater risk reduction from tamoxifen than women without high-risk breast lesions (10,35). In the BCPT study, use of tamoxifen reduced the risk of invasive breast cancer by 86% in women with history of AH and 56% in women with history of LCIS (35). Similarly, the International Breast Cancer Intervention Study (IBIS-II) published in 2014 found that while at-risk women saw a 53% breast cancer risk reduction (HR 0.47, 95% CI: 0.32 to 0.68) with anastrozole compared to placebo, the subgroup of AH and LCIS patients saw a risk reduction of 69% (HR 0.31, 95% CI: 0.12 to 0.84) (14). Given that women with AH and LCIS may have a higher baseline risk of breast cancer [10-year risk ranging from 20–23% (5,6)], they will likely derive a greater absolute risk reduction from chemoprevention use compared to other high-risk women. Therefore, targeted interventions to increase chemoprevention uptake specifically in these high-risk populations may be an effective public health strategy to reduce breast cancer incidence.
Chemoprevention uptake varied by age as well. Younger women were less likely to take chemoprevention compared to older women. Older age has been found to be associated with chemoprevention uptake in a number of studies, although there is evidence that younger women are more likely to adhere to the 5-year course of therapy (36–38). The increased uptake of chemoprevention among older women can potentially be explained by the fact that tamoxifen is the only FDA-approved chemoprevention medication for high-risk premenopausal women, while those who have experienced menopause can also be prescribed raloxifene or aromatase inhibitors (39). While the majority of patients in this study used tamoxifen as the chemopreventive agent of choice, this may be a result of the timeframe of the study (2007–2015). Aromatase inhibitors were not introduced for primary prevention of breast cancer or treatment of DCIS until after 2011 (13–15). It remains to be seen whether aromatase inhibitors for breast cancer chemoprevention will gain wider acceptance compared to SERMs.
We additionally found a trend towards increased chemoprevention uptake in Hispanic women (p=0.075). This is in contrast to another study published by Kaplan et al. that found that Latinas had the lowest proportion of willingness to take chemoprevention with tamoxifen when compared to Whites, Asians, and African Americans (22). Of note, the Gail model, which is frequently used to determine eligibility for chemoprevention, may underestimate breast cancer risk among Hispanic women affecting their eligibility for chemoprevention use (40–42). While all of our subjects were eligible for chemoprevention due to their diagnosis of AH, LCIS, or DCIS, our finding suggests that Hispanic women may have greater interest in initiating chemoprevention despite the fact that the Gail model may underestimate their breast cancer risk. Additional research should be done to further validate breast cancer risk models for Hispanic women and to investigate chemoprevention use in diverse populations.
Socioeconomic status (SES) including educational level, income, and medical insurance coverage were not collected in this study due to lack of availability within the EHR. Our group has previously published findings on the impact of these factors on chemoprevention uptake among a similar population of high-risk women with self-reported data on SES. Among 316 high-risk women eligible for chemoprevention seen in our breast clinic, chemoprevention uptake was 51% and educational level, insurance status, and annual household income were not significant predictors of chemoprevention uptake (21). Cost of chemoprevention agents and lack of insurance coverage have also been shown to be barriers to uptake, particularly among those of lower income (43,44).
From the analysis of the questionnaire data in a subset of women with AH, LCIS, and DCIS, we found that only about 50% of these high-risk women perceived their personal risk of developing breast cancer to be higher than an average-risk woman. Additionally, over 70% of the survey participants were worried about the side effects of chemoprevention. Our findings concur with the findings in previous studies that demonstrate inaccurate risk perception is associated with an overestimation of the side effects of chemoprevention (45,46). Concern about side effects is often cited as a major factor in decision-making about chemoprevention and many high-risk women believe the benefits of tamoxifen are not worth the risks of thromboembolism and uterine cancer (47–49). Our findings suggest that future interventions developed to increase chemoprevention uptake among high-risk women should in part aim to improve risk perception so that patients can make more informed decisions about the risks and benefits of SERM and AI use.
Consistent with our results, a focus group study of women at risk for breast cancer found that risk awareness is only one of many factors that are involved in chemoprevention decision-making (50). Holmberg et al. found that women’s decisions to participate in the Study of Tamoxifen and Raloxifene (STAR) was most often based on personal experiences and few women mentioned risk estimates unless they were specifically prompted (50). Similarly, some of the major reasons cited by our subjects for wanting to take preventative action included wanting to “feel better” and “be there for their family”. This suggests that decision-making about chemoprevention is a highly personal choice based on more than just risk numbers, even among women who would benefit most from use based on risk status. The most commonly cited reason for taking action to lower breast cancer risk was a desire to “live longer.” While chemoprevention can reduce the incidence of breast cancer, no survival benefit has been shown in randomized controlled trials (10,11,13,14). This finding indicates that there are also misconceptions about the benefits of chemoprevention.
There are several limitations to our study. We conducted a single institution study in an urban academic medical center with access to a high-risk clinic, therefore, our results may not be generalizable to community practices or more rural settings. Given the retrospective nature of the cohort study, there was about 20% missing data for race/ethnicity and BMI. Race/ethnicity was significant in univariate analysis, but was no longer significant in multivariable analysis. While we included current use of HRT in our model, it was not found to be significant in multivariable analysis. However, we did not have data on history of prior HRT use and this limitation likely resulted in under-reporting of HRT use. Additionally, we did not have data on chemoprevention adherence, persistence, discontinuation rates, and reasons for discontinuation. Some of our subjects likely had significant comorbidities that may have superseded the need for chemoprevention, but we did not include a measure of these comorbidities in our analysis. Selection bias in the survey study may have been introduced since only subjects seen by a medical oncologist were recruited. Participants who followed through with a medical oncology consultation were specifically counseled on breast cancer risk and use of chemoprevention.
Our study has several strengths, including having a racially and ethnically diverse population of women with AH, LCIS, and DCIS. Age and race/ethnicity were all well-distributed within our cohort. Our retrospective cohort study also had a large sample size and assessed the uptake of chemoprevention among a high-risk population that would benefit most from SERM or AI use. From the survey data, we were also able to capture information on perceived breast cancer risk and chemoprevention knowledge using validated measures before subjects had made a decision about whether or not to use chemoprevention.
Chemoprevention agents have been shown in randomized controlled trials to dramatically reduce the incidence of breast cancer for high-risk women. Our study provides evidence that women with AH, LCIS, or DCIS may take chemoprevention at a higher rate than other high-risk populations and that consultation with a medical oncologist also increases chemoprevention uptake. Concern about the frequency and severity of side effects may limit the number of women who are willing to take chemoprevention. Improving communication about breast cancer risk, as well as the risks and benefits of chemoprevention, may facilitate informed decision-making about SERM or AI therapy for breast cancer risk reduction.
Acknowledgments
Financial Support: K.D. Crew and R. Kukafka, NIH, NCI R01 CA177995-01A1
References
- 1.SEER Cancer Stat Facts: Female Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html. Accessed May 23, 2017.
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. Journal of the National Cancer Institute. 2003;95(7):526–32. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 4.Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25(19):2671–7. doi: 10.1200/JCO.2006.09.0217. [DOI] [PubMed] [Google Scholar]
- 5.Coopey SB, Mazzola E, Buckley JM, Sharko J, Belli AK, Kim EM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast cancer research and treatment. 2012;136(3):627–33. doi: 10.1007/s10549-012-2318-8. [DOI] [PubMed] [Google Scholar]
- 6.Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23(24):5534–41. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Falk RS, Hofvind S, Skaane P, Haldorsen T. Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast cancer research and treatment. 2011;129(3):929–38. doi: 10.1007/s10549-011-1531-1. [DOI] [PubMed] [Google Scholar]
- 8.Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013;8(2):135–55. doi: 10.2174/1574884711308020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. The Lancet Oncology. 2015;16(1):67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005;97(22):1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 11.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer prevention research. 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickerham DL, Cecchini RS, Vogel VG, Costantino JP, Cronin WM, Bevers TB, et al. Final updated results of the NRG Oncology/NSABP Protocol P-2: Study of Tamoxifen and Raloxifene (STAR) in preventing breast cancer. Paper presented at: ASCO Annual Meeting 2015; Chicago, IL. [Google Scholar]
- 13.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. The New England journal of medicine. 2011;364(25):2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 15.Margolese RG, Cecchini RS, Julian TB, Ganz PA, Costantino JP, Vallow LA, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387(10021):849–56. doi: 10.1016/S0140-6736(15)01168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. The Lancet Oncology. 2011;12(1):21–9. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(8):604–14. doi: 10.7326/0003-4819-158-8-201304160-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–5. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer prevention research. 2010;3(6):686–8. doi: 10.1158/1940-6207.CAPR-10-0100. [DOI] [PubMed] [Google Scholar]
- 21.Reimers LL, Sivasubramanian PS, Hershman D, Terry MB, Greenlee H, Campbell J, et al. Breast Cancer Chemoprevention among High-risk Women and those with Ductal Carcinoma In Situ. The breast journal. 2015;21(4):377–86. doi: 10.1111/tbj.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan CP, Kim SE, Wong ST, Sawaya GF, Walsh JM, Perez-Stable EJ. Willingness to use tamoxifen to prevent breast cancer among diverse women. Breast cancer research and treatment. 2012;133(1):357–66. doi: 10.1007/s10549-012-1960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipkus IM, Kuchibhatla M, McBride CM, Bosworth HB, Pollak KI, Siegler IC, et al. Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiol Biomarkers Prev. 2000;9(9):973–5. [PubMed] [Google Scholar]
- 24.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729–36. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 25.Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Annals of internal medicine. 1991;114(8):657–61. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 26.Mochari-Greenberger H, Mills T, Simpson SL, Mosca L. Knowledge, preventive action, and barriers to cardiovascular disease prevention by race and ethnicity in women: an American Heart Association national survey. J Womens Health (Larchmt) 2010;19(7):1243–9. doi: 10.1089/jwh.2009.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korfage IJ, Fuhrel-Forbis A, Ubel PA, Zikmund-Fisher BJ, Greene SM, McClure JB, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast cancer research: BCR. 2013;15(5):R74. doi: 10.1186/bcr3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagerlin A, Dillard AJ, Smith DM, Zikmund-Fisher BJ, Pitsch R, McClure JB, et al. Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast cancer research and treatment. 2011;127(3):681–8. doi: 10.1007/s10549-011-1450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94. [PubMed] [Google Scholar]
- 30.Marín G, Sabogal F, VanOss Marín B, Otero-Sabogal F, Pérez-Stable EJ. Development of a short acculturation scale for Hispanics. Hispanic Journal of Behavioral Sciences. 1987;9:183–205. [Google Scholar]
- 31.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 32.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–3. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan CP, Haas JS, Perez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med. 2005;41(1):7–15. doi: 10.1016/j.ypmed.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 34.Yi H, Xiao T, Thomas PS, Aguirre AN, Smalletz C, Dimond J, et al. Barriers and Facilitators to Patient-Provider Communication When Discussing Breast Cancer Risk to Aid in the Development of Decision Support Tools. AMIA Annual Symposium proceedings/AMIA Symposium AMIA Symposium. 2015;2015:1352–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 36.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2016;27(4):575–90. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roetzheim RG, Lee JH, Fulp W, Matos Gomez E, Clayton E, Tollin S, et al. Acceptance and adherence to chemoprevention among women at increased risk of breast cancer. Breast. 2015;24(1):51–6. doi: 10.1016/j.breast.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Land SR, Cronin WM, Wickerham DL, Costantino JP, Christian NJ, Klein WM, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the National Surgical Adjuvant Breast and Bowel Project P-1 Breast Cancer Prevention Trial. Cancer prevention research. 2011;4(9):1393–400. doi: 10.1158/1940-6207.CAPR-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crew KD. Addressing barriers to uptake of breast cancer chemoprevention for patients and providers. American Society of Clinical Oncology educational book/ASCO American Society of Clinical Oncology Meeting. 2015:e50–8. doi: 10.14694/EdBook_AM.2015.35.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuno RK, Costantino JP, Ziegler RG, Anderson GL, Li H, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. Journal of the National Cancer Institute. 2011;103(12):951–61. doi: 10.1093/jnci/djr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gail MH, Costantino JP, Pee D, Bondy M, Newman L, Selvan M, et al. Projecting individualized absolute invasive breast cancer risk in African American women. Journal of the National Cancer Institute. 2007;99(23):1782–92. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 42.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of the National Cancer Institute. 1989;81(24):1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 43.Heisey R, Pimlott N, Clemons M, Cummings S, Drummond N. Women’s views on chemoprevention of breast cancer: qualitative study. Can Fam Physician. 2006;52:624–5. [PMC free article] [PubMed] [Google Scholar]
- 44.Cyrus-David MS, Strom SS. Chemoprevention of breast cancer with selective estrogen receptor modulators: views from broadly diverse focus groups of women with elevated risk for breast cancer. Psychooncology. 2001;10(6):521–33. doi: 10.1002/pon.547. [DOI] [PubMed] [Google Scholar]
- 45.Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. American Society of Clinical Oncology educational book/ASCO American Society of Clinical Oncology Meeting. 2012:85–90. doi: 10.14694/EdBook_AM.2012.32.152. [DOI] [PubMed] [Google Scholar]
- 46.Smith BL, Gadd MA, Lawler C, MacDonald DJ, Grudberg SC, Chi FS, et al. Perception of breast cancer risk among women in breast center and primary care settings: correlation with age and family history of breast cancer. Surgery. 1996;120(2):297–303. doi: 10.1016/s0039-6060(96)80301-1. [DOI] [PubMed] [Google Scholar]
- 47.Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Annals of family medicine. 2005;3(3):242–7. doi: 10.1370/afm.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salant T, Ganschow PS, Olopade OI, Lauderdale DS. “Why take it if you don’t have anything?” breast cancer risk perceptions and prevention choices at a public hospital. Journal of general internal medicine. 2006;21(7):779–85. doi: 10.1111/j.1525-1497.2006.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–5. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 50.Holmberg C, Waters EA, Whitehouse K, Daly M, McCaskill-Stevens W. My Lived Experiences Are More Important Than Your Probabilities: The Role of Individualized Risk Estimates for Decision Making about Participation in the Study of Tamoxifen and Raloxifene (STAR) Med Decis Making. 2015;35(8):1010–22. doi: 10.1177/0272989X15594382. [DOI] [PMC free article] [PubMed] [Google Scholar]


