Abstract
Temporomandibular joint osteoarthritis (TMJ-OA) is a complex disease that affects both cartilage and subchondral bone. It is accompanied by loss of extracellular matrix (ECM) and may be controlled by Bone Morphogenetic Protein 2 (BMP-2). We analyzed the effect of BMP-2 in both cartilage and subchondral bone in a TMJ-OA animal model that is deficient in Bgn and Fmod (Bgn−/−Fmod−/−). Whole mandibles were dissected from 3 week-old wild-type (WT) and Bgn−/− Fmod−/− mice, and incubated with and without 250 μg/ml BMP-2 for 2 days using an explant culture system. Condyle growth was measured by μCT and the expression levels of cartilage and bone related genes were analyzed using real time (RT)-PCR or by immunohistochemistry (IHC) from condyles that contained an intact cartilage/subchondral bone interface. Osteoclast activity was estimated by TRAP staining and by TRAP, Rankl, AdamTs4 mRNA expression levels. Our results showed that most parameters examined were slightly up-regulated in WT samples treated with BMP-2 and this up-regulation was significantly enhanced in the Bgn−/−Fmod−/− mice. The up-regulation of both catabolic and anabolic agents did not appear to positively affect the overall growth of Bgn−/−Fmod−/− condyles compared to WT controls. In summary, the up-regulation of both anabolic and catabolic genes in the WT and Bgn−/−Fmod−/− TMJs treated with BMP-2 suggests that BMP increases matrix turnover in the condyle and, further, that Bgn and Fmod could have protective roles in regulating this process.
Keywords: biglycan, fibromodulin, mineralization, growth factor, turnover, osteoclast
Introduction
Temporomandibular joint (TMJ) disorders afflict approximately 5–10% of the US population [Liu and Steinkeler 2013]. These disorders encompass a collection of at least 12 clinical phenotypes, one of which is TMJ degenerative joint disease (TMJ-DD) [Schiffman et al. 2014]. Individuals with TMJ-DD experience irreversible damage to the joint complex including degradation and loss of extracellular matrix proteins necessary for joint function. Unfortunately, in most cases, this irreversible damage occurs before symptoms present and the disease is diagnosed [Wang et al. 2015]. Therefore, efforts to identify markers and the molecular mechanisms characteristic of TMJ-DD in the early phases are needed in to detect and to develop treatments that can prevent the irreversible process of the disease.
The TMJ is a unique articulating joint comprised of the mandibular condyle which fits into the mandibular fossa of the temporal bone. The articular disc and the articular cartilage of the condyle are both fibrocartilagenous tissues that cover these two bones and promote articulation. The fibrocartilage of the mandibular condyle is comprised of extracellular matrix proteins and proteoglycans that provide the necessary properties to support the loads on the condyle during mastication and verbal communication. Within this extracellular matrix, there exists small leucine-rich proteoglycans (SLRPs) including biglycan (Bgn) and fibromodulin (Fmod), which play a role in sequestration of important signaling molecules and regulating collagen fibrillogenesis [Ameye and Young 2002]. Specifically, biglycan and fibromodulin modulate TGF super family signaling activity including bone morphogenetic proteins signaling (BMP) [Young et al. 2006, Wadhwa et al. 2004]. BMP signaling has well established roles in chondrogenesis and endochondral bone formation [Long and Ornitz 2013]. Abnormal activation of this signaling causes heterotopic cartilage and bone formation [Huegel et al 2015, Billings et al, 2008]. Specifically, excessive BMP-2 signaling has recently been shown to be involved in heterotopic cartilage formation and the pathogenesis of TMJ-OA [Bechtold et al. 2016, Albilia et al, 2013]. However, the mechanism of excessive BMP-2 signaling on the mandibular condylar cartilage and its implications in TMJ-OA are unknown.
We have previously developed a murine model with accelerated of TMJ-OA in mice double-deficient in biglycan and fibromodulin (Bgn−/−Fmod−/−). Using this model, we determined that Bgn−/−Fmod−/− mice develop TMJ-OA at 6–9 months of age whereas WT mice develop TMJ-OA at 12–18 months of age [Wadhwa et al. 2005a, Wadhwa et al, 2005b, Chen et al. 2009]. Consequently, the goal of this study was to examine BMP-2 signaling in Bgn−/−Fmod−/− mice. We hypothesized that BMP-2 treatment would promote over-activation of chondrogenic markers in the absence of Bgn and Fmod compared to WT mice due to dysregulation of the signaling pathway. Greater understanding of the early signaling events that mediate TMJ-OA is critical in the development of diagnostic tools and interventional therapeutics.
Materials and Methods
Animals
Female Bgn−/−Fmod−/− mice and their strain matched wild-type counterparts (C57BL/6) were used with approval from the Animal Care and Use Committee, National Institutes of Health (#NIDCR-12-655). The generation and genotyping of the Bgn−/−Fmod−/− have been reported previously [Wadhwa et al. 2005a].
Mandibular ex-vivo organ culture and treatment with BMP-2
Mandibular condyles were harvested from 3-week-old wild-type (WT) or Bgn−/− Fmod−/− mice (for histology) or 5-week-old mice (for mRNA), dissected in half and cultured in 24 well plate in DMEM and Pen/Strep antibiotics supplemented with 100 nmol/L ascorbic acid for 24 hours. The condyles were then incubated with or without 250 ng/ml human carrier-free recombinant BMP-2 (eBioscience). The left mandibular condyle was treated with BMP-2 and the right was treated with vehicle control. After 2 days, the explant cultures were collected and prepared for histology and quantitative real-time PCR (Fig. S1). For each sample, 5 condyles were combined and each experiment was repeated at least three times.
MicroCT
Treated mandibles were fixed and scanned using a μCT 50, Scanco Medical AG, (Bassersdorf, Switzerland). Scans were performed at 70 kV, 85 μA, 300 ms integration time, and at a resolution of 10 μm. After reconstruction, the images were stored in 3D arrays and used to measure the height of the mandible as shown in Figure 1A–B).
Figure 1.
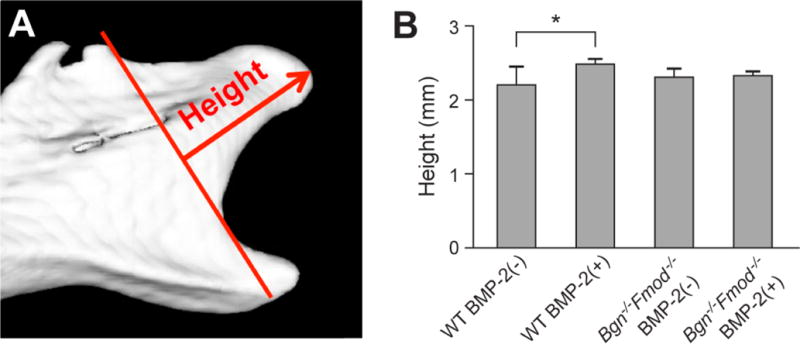
A) Representative microCT image of a 3 week-old mouse condyles after explant culture indicating the growth regions that were measured. B) Height of the condyles in WT or Bgn−/−Fmod−/− tissue with our without BMP-treatment.
Quantitative RT-PCR
Expression levels of cartilage and bone related genes were analyzed by RT-PCR using RNA obtained from condyles that contained both cartilage and subchondral bone. RNA was isolated using Trizol (Invitrogen) and purified with RNeasy. cDNA was generated by reverse transcribing total RNA using iScript from BioRad and subjecting it to real-time PCR using SYBER-mastermix (Applied Biosystem) in a Biorad MyiQ real-time PCR thermocycler. Each cDNA (5–10 ng) was amplified using the primers shown in Table S1. Gene expression was normalized to the housekeeping gene, S29. For each sample, 5 condyles were combined and each experiment was repeated at least three times.
Histology, Staining and Quantification
Paraffin-embedded sections were dewaxed in xylene washes and rehydrated through graded ethanol (100%, 95%, and 75% and water). Tissue sections were stained histochemically with hemotoxylin and eosin (H&E) or Safranin O. For immunohistochemistry, rehydrated sections were enzymatically treated with ABCase and incubated with primary antibodies at 4° C overnight including polyclonal rabbit anti-mouse aggrecan (1:100=5 mg/ml rabbit IgG cat # AB1031, ABCAM), polyclonal rabbit anti-mouse type I collagen (1:2000, rabbit antiserum, LF-68, gift from Dr. Larry Fisher). The broad spectrum immunoperoxidase AEC kit (Picture Plus, Zymed) was subsequently used to detect the immunoreactivity according to the manufacturers’ instructions. The sections were counterstained with hematoxylin. Images of the stained sections were captured and the area of positive staining accessed using software from Image Pro. Non-immune immunogloblulins of the same isotype as the primary antibody served as negative controls and showed no positive staining (not shown). Safranin O positive staining in the treated TMJs was also accessed using Image Pro software (S2). Osteoclast formation was estimated by counting the number of tartrate-resistant acid phosphatase (TRAP, 387A Sigma) positive multinucleated cells in 5–7 sections/sample.
Statistical Analysis
Values are presented as the mean ± standard deviation. Statistical significance of differences among means was determined by two-way analysis of variance (ANOVA) with post hoc analysis by the Bonferonni method using SPSS. Statistical significance was defined as p< 0.05.
Results
The experimental strategy for examining BMP-2 function in ex-vivo explants is outlined in S1. Briefly, hemi-mandibles were dissected from the heads of WT and Bgn−/−Fmod−/− mice and included the entire condyle with its subchondral bone. Samples from 3 week-old mice were used for histology while 5 week-old mice were used for mRNA extraction. The hemi-mandibles were then cultured in the presence or absence of BMP-2 for 48 hours and processed for μCT, histology, and mRNA isolation for RT-PCR.
Hemi-mandibles from WT and Bgn−/−Fmod−/− incubated with or without BMP-2 were first analyzed by microCT to determine the relative height of the condyle after treatment (Figure 1A). This analysis showed that WT condyles responded with a slight but significant increase in length (Figure 1B), but the Bgn−/−Fmod−/− condyles did not. This finding prompted us to further examine the underlying cell and molecular foundation by histology, immunohistochemistry and quantitative RT-PCR.
The temporomandibular joint at 3 weeks of age is composed of a thick fibrocartilage surface that covers a layer of cartilage that can be clearly visualized by staining with safranin O (S2). The cartilage layer lies on top of the subchondral bone that composes the majority of the condyle structure. The area of safranin O positive staining in WT mandibles treated with BMP-2 was unchanged after the 48 h BMP treatment. However, the area of safranin O in condyles from Bgn−/−Fmod−/− mice was significantly increased (Fig. 2A, B). To confirm these observations, we next stained the sections for the expression of aggrecan (Acan) a known marker of differentiated cartilage tissue. This showed that BMP-2 slightly increased the extent of Acan staining in WT tissue, and more so in the Bgn−/−Fmod−/− condyles (Fig. 2C, D). When the relative expression of mRNA for Agan was examined with BMP-2 treatment we found that both WT and Bgn−/−Fmod−/− had increased Agan levels but that in the Bgn−/−Fmod−/− the BMP-2 response much was more robust that in the WT (Fig. 2E).
Figure 2.
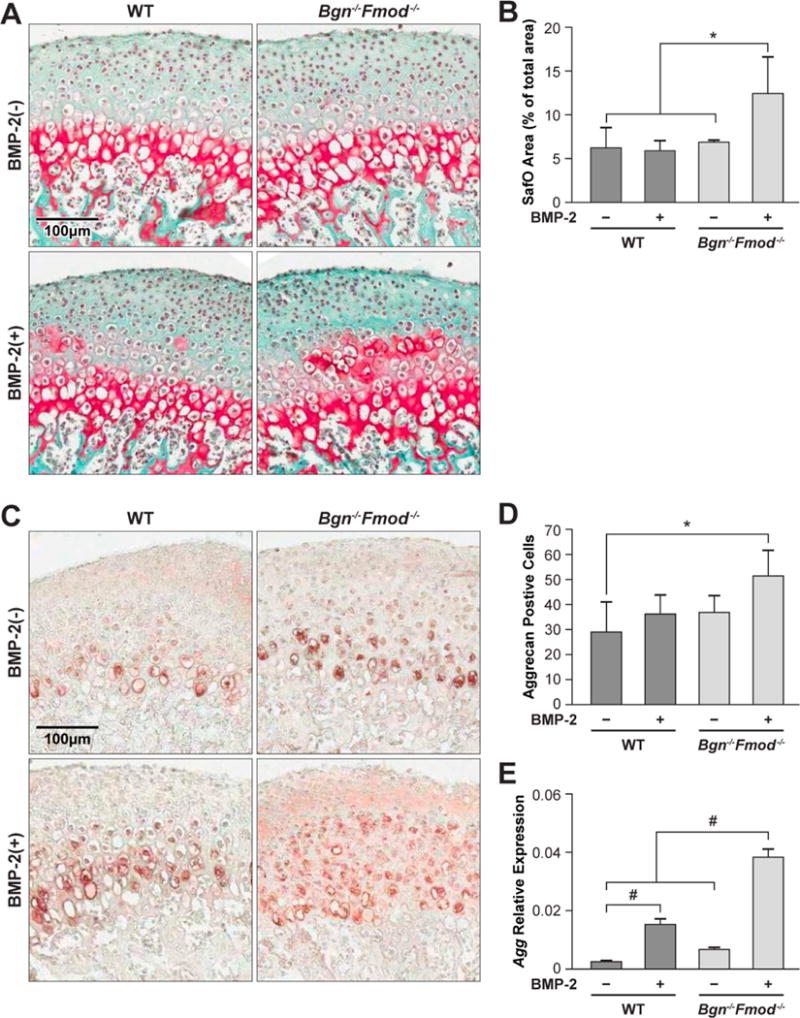
A) Safranin-O staining of WT (left panels) and Bgn−/−Fmod−/− condyles (right panels) treated without (top panel) or with (bottom panels) BMP-2. B) Histomorphometic quantitation of safranin-O positive area/total area. C) Immunohistochemistry for aggrecan (Acan) in WT (left panels) and Bgn−/−Fmod−/− condyles (right panels) treated without (top panel) or with (bottom panels) BMP-2 D) histomorphic evaluation of Agan positive cells. E) Relative expression of Acan mRNA in WT vs. Bgn−/−Fmod−/− with or without BMP-2. *p<0.05, #p<0.01.
To deepen our understanding of the molecular basis for the observed changes that we saw in cartilage tissue morphology, the mRNA expression levels for additional key cartilage genes were determined. This analysis showed that BMP-2 increased the levels of type II collagen (Col2, Fig 3A) and type X collagen (Col10, Fig3B), Sox9, the master gene known to regulate cartilage formation (Fig 3C), and chondrocyte controlling gene ihh (Fig 3D) and that the relative level of induction was much greater in the Bgn−/−Fmod−/− condyles. Taken together these data showed that the Bgn−/−Fmod−/− condyles are more sensitive to the effects of BMP-2 causing expansion of the cartilage zone resulting in increased levels of safranin 0, Agan positive tissue as well in the expression of the cartilage related genes Col2, Col10, Sox9 and ihh.
Figure 3.
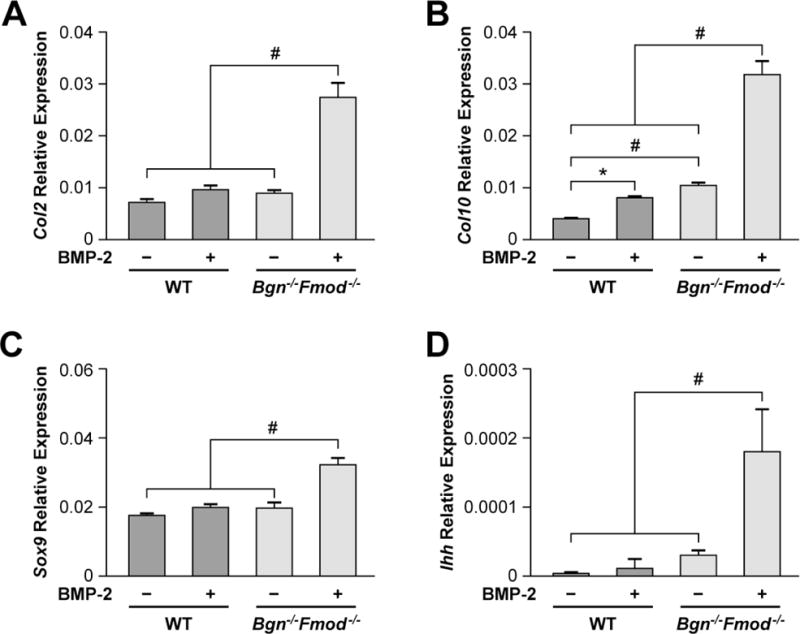
Real-time PCR of cartilage expressed genes in WT and Bgn−/−Fmod−/− treated without or with BMP-2. A) Type II collagen (Col2), B) Type X collagen (Col10) C) Sox9, D) ihh. #p<0.01.
We next examined the effects of BMP-2 on the proteins and mRNAs that are made by the surrounding fibrocartilage and bone. This analysis showed that the extent of type I collagen (Col1) in the fibrocartilage was slightly increased (but not significantly) in WT condyles treated with BMP-2 and that the Bgn−/−Fmod−/− condyles were much more responsive (Fig 4A). Real-time PCR of mRNA encoding Col1 mRNA showed a parallel significant increase of type I collagen in the Bgn−/−Fmod−/− condyles treated with BMP-2 compared with similarly treated WT explants (Fig 4B). The master gene, Runx2, known to regulate many bone enriched genes including type I collagen was found to be regulated in the same manner as type I collagen Fig 4 C). Osterix (Osx), the master regulatory factor operating down stream of Runx2 showed a different pattern compared to Runx2 whereby induction by BMP-2 in WT condyles was almost (but not completely) at the same level as the Bgn−/−Fmod−/− condyles (Fig 4D). A down-stream target of Wnt called Wisp1/Ccn4 that is known to control bone turnover was also examined and showed constitutive elevation in WT vs. Bgn−/−Fmod−/−, and that this increased expression was further enhanced by treatment with BMP-2 (Fig 4E).
Figure 4.
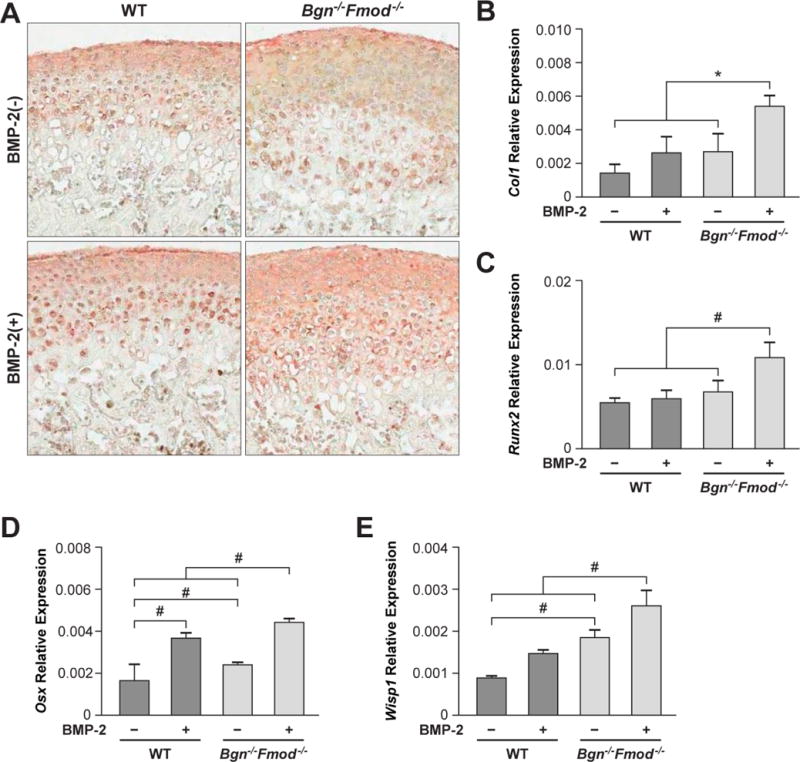
Immunohistochemistry (IHC) and real-time PCR of genes expressed in fibrocartilage and bone. A) IHC of type I collagen in WT (left panels) and bgn −/−, fmod−/− deficient condyles (right panels) treated without (top panel) or with (bottom panels) BMP-2. B) Real-time PCR of type I collagen (Col1), C) Runx2 D) osterix (Osx) and E) Wisp1 mRNA in WT and Bgn−/−Fmod−/− condyles treated with or without BMP-2. *p<0.05, #p<0.01
The enhancement of genes known to regulate bone and cartilage formation led us to question whether skeletal turnover in the Bgn−/−Fmod−/ condyles was differentially affected by BMP-2. To test this possibility, WT and Bgn−/−Fmod−/ condyles were treated with BMP-2 and stained for tartrate resistant acid phosphatase (TRAP) to identify osteoclasts. This analysis showed that the number of multinucleated TRAP positive cells was not significantly changed in BMP-2 treated WT condyles while in contrast Bgn−/−Fmod−/ condyles showed a higher number of TRAP positive cells that was increased with BMP-2 (Figure 5A). Analysis of mRNA for TRAP (Acp5) showed a similar outcome where BMP-2 treated Bgn−/−Fmod−/− condyles had much greater expression than BMP-2 treated WT samples (Fig 5 B). Interestingly, untreated Bgn−/−Fmod−/− condyles also had elevated TRAP mRNA expression compared to untreated WT condyles. Analysis of additional catabolic mRNAs Rankl (Fig. 5C) and Adamts4 (Fig 5D) showed a similar pattern to TRAP mRNA where the bgn−/0fmod −/− condyles had constitutively higher levels than WT condyles. BMP-2 treatment of Bgn−/−Fmod−/− condyles further increased Rankl and Adamts mRNA compared to WT controls.
Figure 5.
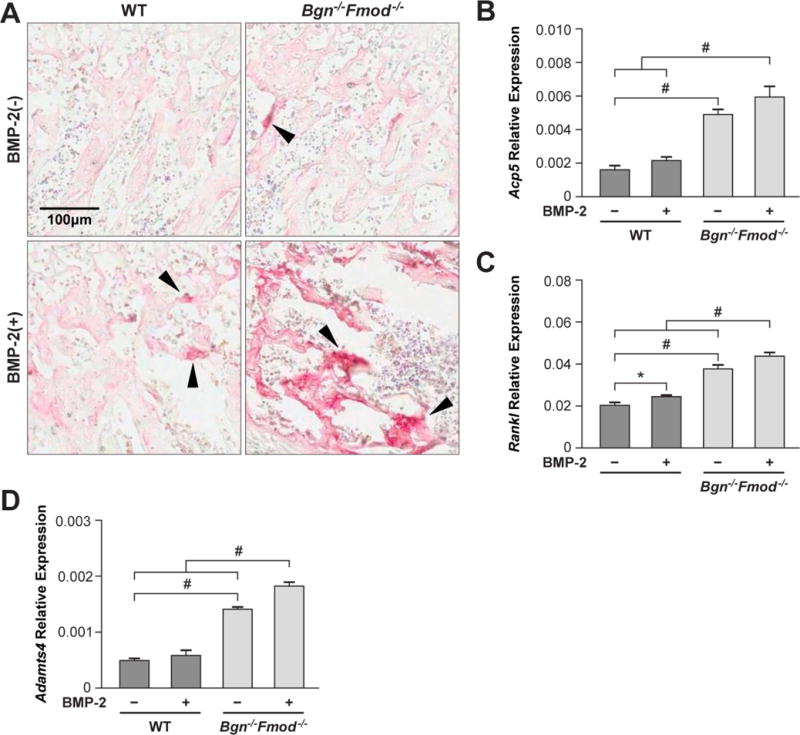
Assessment of bone resorption A) Tartrate Resistant Acid Phosphatase (TRAP) staining of osteoclasts in WT (left panels) and Bgn−/−Fmod−/− deficient condyles (right panels) treated without (top panel) or with (bottom panels) BMP-2. Real-time PCR to measure levels of B) TRAP (Acp5) C) Rankl D) and AdamTS4 mRNA.
Discussion
For patients who experience TMJ-OA, irreversible degeneration of the joint often occurs prior to detection or diagnosis. Thus, early detection and diagnosis with biomarkers could lead to early intervention and reduce the incidence of irreversible damage to the TMJ. The goal of this study was to determine whether excessive BMP-2 signaling results in over-activation of chondrogenesis in a Bgn /−Fmod −/− model of TMJ-OA. Results from this study illustrate that BMP-2 treatment potentiated the induction of Acan, Col2, Col 10, and Ihh in Bgn−/−Fmod−/− mice. Excess BMP-2 signaling has also been implicated in the pathogenesis of accelerated TMJ-OA in Pgr4-KO mice [Bechtold et al. 2016]. In contrast, absence of BMP signaling in transgenic mice with Bmp1Ra conditionally deleted in neural crest cells led to failure of articular disc separation from the hypoplastic condyle and under development of the mandibular condylar cartilage [Gu et al. 2014]. Taken together, the results suggest that a threshold of BMP-2 is needed for normal TMJ development.
The mandibular condylar cartilage is unique in that it is derived from the periosteum and undergoes endochondral ossification 1. In other growth plates, it is believed that there are a finite number of progenitor cells and once these cells are depleted, growth stops [Hinton et al. 2015]. A similar mechanism may occur in the TMJ. For example, in mice the mandibular condylar cartilage rapidly undergoes endochondral ossification until 2 months of age [Ghassemi et al. 2016]. Afterwards, the mandibular condylar cartilage becomes senescent and phenotypically resembles other articular cartilages [Levne et al. 1985]. Therefore, in the absence of Bgn and Fmod, excessive BMP-2 signaling may cause accelerated progenitor cell depletion resulting in accelerated age-related TMJ degeneration. Future studies will focus on delivering BMP-2 inhibitors to young (growing) and adult (non-growing) mice to examine if they can inhibit age related TMJ-OA in the Bgn−/−Fmod−/− mice in order to further test this hypothesis.
It is well known that aggrecan is a major proteoglycan in cartilage and that it has important roles in regulating cartilage integrity. In the present study, we focused on Bgn and Fmod because we found they are both abundantly expressed and localized in the articular cartilage of the mandibular condyle [Wadhwa et al. 2001]. Interestingly in the Bgn−/−Fmod−/− condyles the expression of aggrecan is first increased at 3 weeks of age, and then, at 5 weeks of age decreased with further reduction at 24 weeks of age compared to WT condyles. We believe this is due, in part, to overactive TGF-beta production leading to an up-regulation proteolytic enzymes that degrade aggrecan including ADAMTS4 and ADAMTS5 [Embree et al. 2010]. In this context, we cannot exclude the possibility that some of the effects we see in the BMP-2 responsiveness in the Bgn−/−Fmod−/− mice could, in fact, be from alterations in aggrecan levels and function.
In our previous study we found that TGF-ß signaling was elevated in Bgn−/−Fmod−/− mice. Our results with BMP-2 are similar; however, in this study, we found that BMP-2 also caused an increase in Rankl [Embree et al. 2010]. Increased Rankl in the Bgn−/−Fmod−/− mice was confirmed by our previous published microarray data [Embree et al. 2011], suggesting that osteoclastogenesis may be specific for BMP-2 signaling pathway rather than the TGF-ß signaling pathway.
Bgn and Fmod have a highly ordered structure that is composed of a core protein with extensive post-translational modifications including the addition of chontroitin/dematan sulafate (Bgn) or keratin sulfate glycosaminoglycan (Fmod) glycosaminoglycan chains. Currently it is not known what region of these small proteoglycans is important for the functions we show they have in the present study. An important next step will be to test the role of the core protein compared to the proteoglycan form (with GAG chains) to see what part of these SLRP molecules is biologically active. In this regard, it’s interesting to note that three different families with point mutations in the core protein of in biglycan have bone shortening suggesting the overall importance of the core protein in biglycan function (Cho et al, Am J Hum Genet 2016 98(6):1243-8). Clearly more experiments are needed to fully understand SLRPs mechanism of action requiring a comprehensive and systematic test all the parts of Bgn and Fmod’s structure either by gain of function or “knockin” genetic manipulation.
Considering how many SLRPs populate skeletal tissues it is likely there is some overlap in their functions including in the ability to bind and regulate growth factors such as the TGF-beta family members. When we examined either decorin or Fmod single deficient mice we did not see any major bone phenotype so did not pursue the single decorin KO or fmod KO further in this context. With regards to cartilage, the single Bgn KO and Fmod KO both display mild osteoarthritis and because Fmod and Bgn are so highly co-expressed in the cartilage of the condyle we suspected they had overlapping and possible compensatory functions there. By making mice deficient in both Fmod and Bgn we subsequently uncovered new roles for these SLRPS that were masked by a compensation effect. This was evident in the Bgn−/−Fmod−/− which had much earlier and severe OA compared to the single bgn and fmod deficient mice [Ameye et al. 2002]. Because of the nature of the genetic model used we cannot tell whether Bgn or Fmod dominates in the functions we discovered namely SLRP control of BMP-2 induced bone and cartilage turnover.
As mentioned earlier Bgn and Fmod are members of the small leucine-rich repeat proteoglycans. Members of this family have been linked to OA by their action in mediating extracellular collagen organization, mediating TGF-ß signaling and/or regulating subchondral bone turnover (for review see [Ni et al. 2014]). Therefore, decreased expression of Bgn and Fmod may be a biomarker for the early stages of TMJ-OA and/or potentially indicate a higher probability of developing TMJ-OA.
The detection of TMJ-OA usually occurs after there is irreversible damage to the joint. Therefore, efforts to detect and prevent and the disease are needed. In our model, there is excessive BMP-2 signaling. Therefore, monitoring the expression of BMP-2, Bgn and Fmod may be beneficial in the early detection of TMJ-OA. Furthermore, manipulating the expression of these proteins may be beneficial in preventing the progression of the disease process.
Supplementary Material
Table S1. Primer sequences used for Real-Time PCR
S1. Diagram showing an outline of experimental approach used to treat and analyze the TMJ from WT Bgn−/−Fmod−/− mice.
S2. Low power view of condyle showing fibrocartilage, cartilage and subchondral bone layers. The Safranin O red stained area was used to quantify the relative area of cartilage present in treated and untreated WT and Bgn−/−Fmod−/− condyles.
Acknowledgments
We would like to thank A. Donald and L. Li for technical assistance. This work was supported in part by the intramural program of the NIDCR (VK, SS, TMK, MFY) and by a fellowship from the Japanese Promotion for the Advancement of Science (MS).
Abbreviations used in this paper
- ECM
extracellular matrix
- TMJ-OA
temporomandibular joint osteoarthritis
- Bgn
biglycan
- Fmod
fibromodulin
- WT
wild-type
- SLRPs
small leucine-rich proteoglycans
- BMP-2
Bone Morphogenetic Protein 2
Footnotes
There are no conflicts of interest to disclose.
References
- Albilia JB, Tenenbaum HC, Clokie CM, et al. Serum levels of BMP-2, 4, 7 and AHSG in patients with degenerative joint disease requiring total arthroplasty of the hip and temporomandibular joints. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(1):44–52. doi: 10.1002/jor.22182. [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12(9):107r–116r. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Bechtold TE, Saunders C, Mundy C, et al. Excess BMP Signaling in Heterotopic Cartilage Forming in Prg4-null TMJ Discs. Journal of dental research. 2016;95(3):292–301. doi: 10.1177/0022034515613508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, et al. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2008;23(3):305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gupta T, Barasz JA, et al. Analysis of microarchitectural changes in a mouse temporomandibular joint osteoarthritis model. Archives of oral biology. 2009;54(12):1091–1098. doi: 10.1016/j.archoralbio.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree MC, Kilts TM, Ono M, et al. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol. 2010;176(2):812–826. doi: 10.2353/ajpath.2010.090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree M, Ono M, Kilts T, et al. Role of subchondral bone during early-stage experimental TMJ osteoarthritis. Journal of dental research. 2011;90(11):1331–1338. doi: 10.1177/0022034511421930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi Nejad S, Kobezda T, Tar I, Szekanecz Z. <t>Development of temporomandibular joint arthritis: The use of animal models. Joint Bone Spine. 2016 doi: 10.1016/j.jbspin.2016.05.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gu S, Wu W, Liu C, et al. BMPRIA mediated signaling is essential for temporomandibular joint development in mice. PloS one. 2014;29(8):e101000. doi: 10.1371/journal.pone.0101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RJ, Jing J, Feng JQ. Genetic Influences on Temporomandibular Joint Development and Growth. Curr Top Dev Biol. 2015;115:85–109. doi: 10.1016/bs.ctdb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Huegel J, Enomoto-Iwamoto M, Sgariglia F, Koyama E, Pacifici M. Heparanase stimulates chondrogenesis and is up-regulated in human ectopic cartilage: a mechanism possibly involved in hereditary multiple exostoses. The American journal of pathology. 2015;185(6):1676–1685. doi: 10.1016/j.ajpath.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Steinkeler A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent Clin North Am. 2013;57(3):465–479. doi: 10.1016/j.cden.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Livne E, von der Mark K, Silbermann M. Morphologic and cytochemical changes in maturing and osteoarthritic articular cartilage in the temporomandibular joint of mice. Arthritis and rheumatism. 1985;28(9):1027–1038. doi: 10.1002/art.1780280910. [DOI] [PubMed] [Google Scholar]
- Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harbor perspectives in biology. 2013;5(1):a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni GX, Li Z, Zhou YZ. The role of small leucine-rich proteoglycans in osteoarthritis pathogenesis. Osteoarthritis and cartilage / OARS, Osteoarthritis. Research Society. 2014;22(7):896–903. doi: 10.1016/j.joca.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. Journal of oral & facial pain and headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XDd, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. Journal of dental research. 2015;94(5):666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree MC, Bi Y, Young MF. Regulation, regulatory activities, and function of biglycan. Crit Rev Eukaryot Gene Expr. 2004;14(4):301–315. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.50. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree MC, Kilts TM, Young MF, Ameye LG. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis and cartilage / OARS. Osteoarthritis Research Society. 2005;13(9):817–827. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree M, Ameye L, Young MF. Mice deficient in biglycan and fibromodulin as a model for temporomandibular joint osteoarthritis. Cells, tissues, organs. 2005;181(3–4):136–143. doi: 10.1159/000091375. [DOI] [PubMed] [Google Scholar]
- Young MF, Bi Y, Ameye L, et al. Small leucine-rich proteoglycans in the aging skeleton. J Musculoskelet Neuronal Interact. 2006;6(4):364–365. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences used for Real-Time PCR
S1. Diagram showing an outline of experimental approach used to treat and analyze the TMJ from WT Bgn−/−Fmod−/− mice.
S2. Low power view of condyle showing fibrocartilage, cartilage and subchondral bone layers. The Safranin O red stained area was used to quantify the relative area of cartilage present in treated and untreated WT and Bgn−/−Fmod−/− condyles.


