Abstract
Purpose
Evaluate the association between the use of phase 1 (P1) expansion cohorts (ECs) and drug performance in phase 2 (P2) as well as time to approval by the U.S. Food and Drug Administration (FDA).
Methods
We performed a systematic search of MEDLINE for single-agent dose-finding adult oncology P1 trials published 2006–2011 and subsequent P2 trials. Successful P2 trials were those that met their primary endpoints. Dates of approval were obtained from the Drugs@FDA website April 2014. A logistic regression model was used to determine the associations between variables and success in P2.
Results
We identified 533 P1 trials evaluating 381 drugs; 112 drugs had at least one P1 trial with an EC. P1 trials with ECs of 2–20 patients were associated with a higher rate of successful P2 trials than those with no EC (48% vs 27%, OR 2.1, 95%CI 1.1–4.0, p = 0.037). P2 success rates were the same for EC with 2–20 and more than 20 patients (48% vs 52%). Other positive associations were: disease-specific trials (OR 1.7, 95%CI 1.0–2.9, p=0.037), industry sponsorship (OR 2.9, 95%CI 1.5–5.7, p = 0.0024) and response rate of 6%-20% (OR 2.89, 95%CI 1.6–5.2, p = 0.0007). Drugs tested in P1 trials with ECs had a higher rate of 5-year-approval (19% vs 5%, HR 4.4, 95% CI 2.2–8.8, p < 0.001).
Conclusion
The use of ECs in P1 trials was associated with success of subsequent P2 trials. However, confounders may play a role in this association.
Keywords: Clinical Research, PhaseI-III Clinical Trials, Methodological Studies
INTRODUCTION
The need for improvement in the efficiency of cancer drug development has led to new innovative phase 1 trial designs.1, 2 One strategy is the use of expansion cohorts, in which additional patients are enrolled in a phase 1 trial after the maximum tolerated dose (MTD) or recommended phase 2 dose has been defined. The main goals of expansion cohorts are more accurate estimation of the MTD and more accurate assessment of drug activity. On the other hand, the use of expansion cohorts has been criticized3, 4 because many trials using expansion cohorts lack clear objectives, proper statistical design, and independent data and safety monitoring boards. The actual value of expansion cohorts for drug development remains unknown.
Manji et al5 reviewed all single-agent dose-finding phase 1 oncology trials in adults listed in the MEDLINE database during 2006–2011 and found that the proportion of trials with expansion cohorts increased over the study period, from 12% in 2006 to 38% in 2011. For this present study, we have expanded their database by systematically searching for the corresponding published phase 2 trials for all those phase 1 studies. We also searched the U.S. Food and Drug Administration (FDA) website for the approval status of each drug. Our goal was to evaluate the association between the use of phase 1 expansion cohorts and drug performance in phase 2 trials or eventual regulatory approval.
MATERIALS AND METHODS
Search Strategy and Study Selection
The search strategy for the phase 1 trials included in this study has been described elsewhere.5 In summary, we searched MEDLINE for trials published from January 1, 2006, through December 31, 2011, that were prospective, single-agent dose-finding phase 1 studies performed in adults and involving systemic administration of antineoplastic agents for the treatment of solid or hematologic malignancies. If an author defined its trial as “phase 1/2 “, it was included only if it had a dose-finding cohort.
There were no exclusion criteria based on therapeutic class (e.g., cytotoxic therapy, targeted therapy, or immunotherapy), but trials whose interventions included radiation therapy, surgery, or stem cell transplantation were excluded. We then used the Medical Subject Headings terms assigned to each report, the Drugs@FDA website (www.accessdata.fda.gov/scripts/cder/drugsatfda/), and the full text of the phase 1 articles to identify the experimental compound codes and chemical, generic, and trade names for each drug.
We used those terms to identify published phase 2 trials for each drug using the PubMed search engine with the “clinical trial” filter6 (January 1, 2005, to April 30, 2014). One reviewer (D.D.G.B.) analyzed all the trial abstracts to identify phase 2 studies. Studies were included if (1) they were not among the phase 1 trials identified; (2) they were classified as phase 1/2; phase 2 or had no available classification but enrolled more than 15 patients and had no dose escalation; (3) the abstract was available in English; (4) they evaluated single-agent systemic antineoplastic therapy; and (5) the study population consisted of adults with cancer, or results were reported separately for adult subgroups. We then excluded studies that (1) had duplicate publications; (2) were only published in conference proceedings; or (3) used interventions that included stem cell transplantation, radiation therapy, or surgery.
Drug approval data were obtained from Drugs@FDA on April 30, 2014.7 We excluded any drug for which the date of electronic publication of the first phase 1 trial was 6 months after the first FDA approval for any indication for that drug. This exclusion was necessary because our search results included phase 1 studies of new indications for previously approved drugs for which the original phase 1 studies could not be found and because many trials did not specify the actual start date of the study. The study selection process is summarized in Figure 1.
Fig. 1.
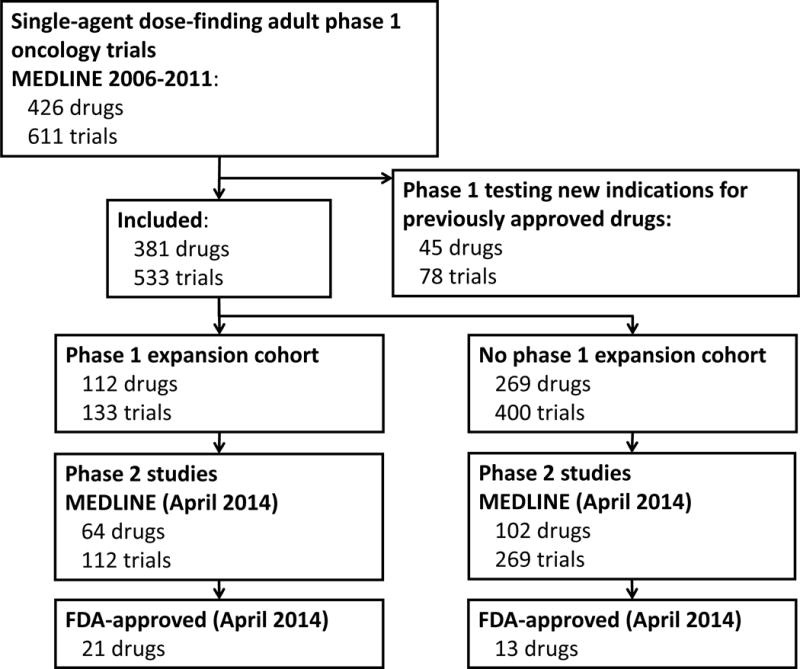
Flow diagram of study selection.
Endpoints of Interest
Drugs were classified in two groups depending on whether their phase 1 trials used expansion cohorts. Expansion cohorts were classified as any patients enrolled after the MTD or the recommended phase 2 dose had been defined. It was not possible to estimate how each individual phase 1 trial influenced the design of the corresponding phase 2 trials; therefore, we combined data for all phase 1 trials for each drug into single measurements. For example, if a drug was tested in three phase 1 trials and two of those had expansion cohorts, that drug was categorized in the expansion cohort group. The resulting “pooled trial” had patients from three trials in its dose escalation phase and from two trials in its expansion cohort.
Our primary endpoint was the probability of a drug’s success in phase 2. To be considered successful, drugs had to be tested in at least one phase 2 study meeting the following criteria: (1) the primary endpoint of the trial, as described in the methods section, included some measurement of drug efficacy; and (2) the results section stated that the primary endpoint was met. Trials without a clear efficacy endpoint were excluded.
The secondary endpoint was time to first FDA approval, measured from the date of electronic publication of the earliest phase 1 study of a drug to the date of the first FDA approval for treatment of a solid or hematologic malignancy. Date of first electronic publication was chosen because most phase 1 trials in the database did not report on date of first patient enrolled.
We also studied the impact of multiple variables on the two endpoints: therapeutic class, drug-specific trials, response rates, determination of an MTD, publication date, industry sponsorship, inclusion of multiple centers in the phase 1 trial, the number of patients enrolled, cancer type, the rate of grade 3–4 toxic effects, and occurrence of any grade 5 toxic effect.
Therapeutic classes were categorized as cytotoxic (traditional chemotherapy) or non-cytotoxic (immunotherapies, monoclonal antibodies, tyrosine kinase inhibitors, viral vectors, and vaccines). Response rates were based on the definitions in the different trials. The responses for the dose-escalation and dose expansion cohorts were combined and classified into four groups: 0% (no responders), >0% but <6% (average response rates for phase 1 single-agent oncology trials8–10), ≥6% but <20% and ≥20% (unusually high response rates). The rates of toxic effects were included in the analysis if the Common Terminology Criteria for Adverse Events version 3.0 or later had been used. We considered the MTD to have been reached if at least one phase 1 trial for a drug mentioned a value for the MTD.
Data Extraction
Three authors (D.D.G.B., D.L.F.J., and A.Z.) reviewed full-text versions of all the phase 1 studies and collected information on response rates, toxic effects, and MTD. One author (D.D.G.B.) reviewed all full- text publications for discrepancies. The remaining information from the phase 1 trials had been previously extracted.5
A single author (D.D.G.B.) extracted the data for the phase 2 studies from their abstracts and obtained the date of first approval from the Drugs@FDA website.
Data Synthesis and Statistical Analysis
Study factors were compared between the expansion cohort and non-expansion cohort groups using chi-squared tests. A logistic regression model was used to determine the associations between each of the study variables and the probability of success in subsequent phase 2 trials. The cutoff for “total number of patients in phase 1 trials” was based on values that would create a more balanced distribution; cutoffs for “response rate” and “toxicity rate” were based on the literature and clinical judgment. For size of the expansion cohorts, subgroups were created based on visual analysis of a scatterplot with smooth fitted lines of the probability of success in phase 2 relative to the size of the expansion cohort (Figure 2).
Fig. 2.
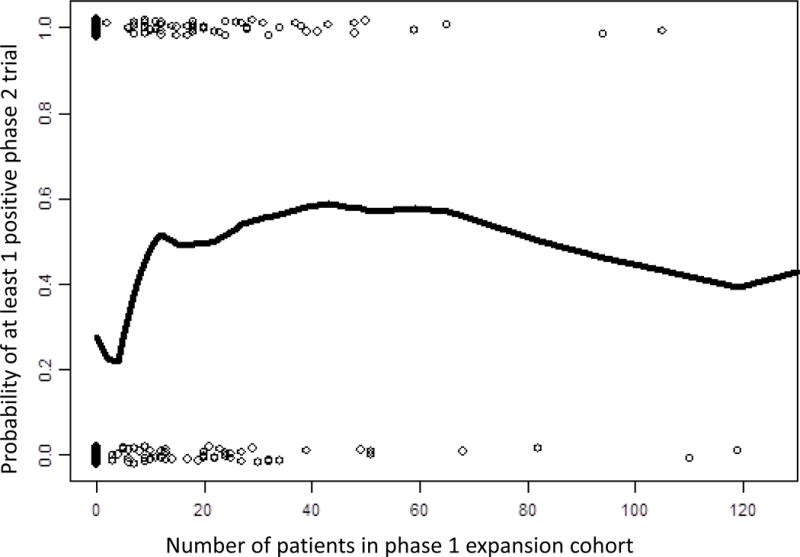
Scatterplot of probability of success in a phase 2 trial relative to the size of the phase 1 expansion cohort.
Cox proportional hazards regression models were used to evaluate the associations between independent variables and time to first FDA approval. Data were censored on April 30, 2014. The probabilities of FDA approval as a function of time since the publication of the phase 1 study were estimated using the Kaplan-Meier method.
All statistical analyses were performed using Spotfire S+ 8.2 for Windows (TIBCO Software Inc., Palo Alto, CA). Unless otherwise specified, the significance threshold was set at 0.05.
RESULTS
Study Selection
The systematic review by Manji et al5 included 426 drugs tested in 611 unique phase 1 clinical trials. In our analysis, we excluded 45 of those 426 drugs because their first phase 1 trial was published more than 6 months after the date of their first FDA approval. Of the remaining 381 drugs, 112 (29%) were tested in at least one phase 1 expansion cohort. Our search strategy for phase 2 trials yielded 1660 abstracts. After review, we identified 381 phase 2 studies evaluating 166 drugs (Figure 1).
Drug Characteristics
Table 1 summarizes drug characteristics and performance in phase 1 trials. Drugs in the expansion cohort group were less likely to be cytotoxic (12% vs 25%, p = 0.006), more likely to have been tested in at least 1 trial that was multicenter (82% vs 64%, p < 0.001), or industry-sponsored (89% vs 72%, p < 0.001), and more likely to have a first publication during 2009–2011 rather than 2006–2008 (63% vs 49%, p = 0.013). These drugs were also more likely to have been tested in two or more phase 1 trials (36% vs 17%, p = 0.002) and more likely to have enrolled 47 or more patients in all phase 1 trials combined (63% vs 21%, p < 0.001).
Table 1.
Drug and Trial Characteristics According to Whether At Least One Expansion Cohort was used in phase 1
| Characteristic | No. of Drugs (%)
|
p | |
|---|---|---|---|
| Not Tested in an Expansion Cohort (Total = 269) | Tested in an Expansion Cohort (Total = 112) | ||
|
| |||
| Publication year of first phase 1 trial | 0.013 | ||
| 2006–2008 | 136 (51) | 41 (37) | |
| 2009–2011 | 133 (49) | 71 (63) | |
| Disease-Specific | 91 (34%) | 48 (43%) | 0.12 |
| Cytotoxic drug class | 68 (25) | 14 (12) | 0.006 |
| Industry sponsorship | 195 (72) | 100 (89) | <0.001 |
| Involvement of multiple centers in a phase 1 trial | 171 (64) | 92 (82) | <0.001 |
| No. of patients in all phase 1 trials | <0.001 | ||
| 5–24 | 122 (45) | 7 (6) | |
| 25–46 | 92 (34) | 35 (31) | |
| 47–289 | 55 (21) | 70 (63) | |
| Malignancy | 0.28 | ||
| Solid tumor | 188 (70) | 70 (62) | |
| Hematologic | 35 (13) | 21 (19) | |
| Mixed | 46 (17) | 21 (19) | |
| Pooled phase 1 response rate | 0.005 | ||
| Not available | 17 (6) | 3 (3) | |
| 0% | 137 (51) | 28 (25) | |
| >0% and <6% | 40 (15) | 34 (30) | |
| ≥6% and <20% | 56 (21) | 29 (26) | |
| ≥20% | 19 (7) | 18 (16) | |
| Pooled grade 3–4 toxic effect rate | 0.24 | ||
| Not available | 18 (7) | 6 (5) | |
| ≥0% and <10% | 146 (54) | 52 (47) | |
| ≥10% and <30% | 61 (23) | 34 (30) | |
| ≥30% | 44 (16) | 20 (18) | |
| Any trial with at least one grade 5 toxic effect | 21 (8) | 23 (21) | <0.001 |
| MTD reached | 133 (49) | 82 (73) | <0.001 |
Abbreviation: MTD, maximum tolerated dose.
Compared with the drugs in the non-expansion cohort group, drugs in the expansion cohort group had higher response rates in the phase 1 trials (72% vs 43%% with any response, p = 0.005). Despite similar rates of grade 3–4 toxic effects between the groups, the drugs tested in expansion cohorts were more likely to lead to at least one event of grade 5 toxicity (21% vs 8%, p < 0.001) and to have an MTD defined in a phase 1 trial (73% vs 49%, P < 0.001).
Success Rates
Of the 381 drugs included in our study, 166 were tested in phase 2 trials, and 132 had at least one successful phase 2 trial. Drugs in the expansion cohort group were more likely to have successful phase 2 trials (51% vs 28%, p < 0.001; Figure 3). The analysis of the scatterplot suggests that larger expansion cohorts were associated with a higher probability of success in phase 2, but no additional benefit was seen for cohorts larger than 20 patients (Figure 2).
Fig. 3.
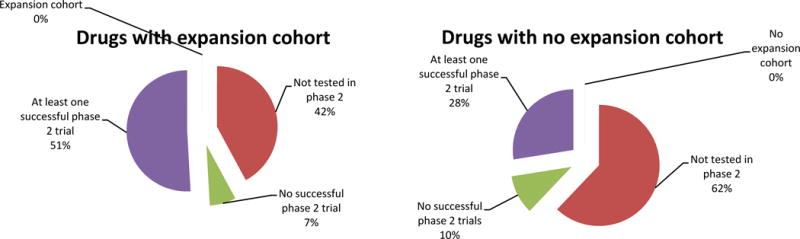
Phase 2 outcomes for drugs tested in phase 1 trials with expansion cohorts compared to drugs tested in phase 1 trials without expansion cohorts.
Multivariate analysis showed that phase 1 trials with expansion cohorts of 2–20 patients were associated with a higher rate of successful phase 2 trials than phase 1 trials with no expansion cohorts (odds ratio [OR] 2.1, 95% confidence interval [CI] 1.1–4.0, p = 0.037). Other factors associated with successful phase 2 trials were industry sponsorship (OR 2.9, 95% CI 1.5–5.7, p = 0.0024), phase 1 response rates 6%-77% compared with a response rate of 0% (OR 2.9, 95% CI 1.6–5.2, p = 0.0007) and disease-specific phase 1 trials (OR 1.7, 95% CI 1.0–2.9, p=0.037) (Table 2).
Table 2.
Univariate and Multivariate Logistic Regression Models of Covariates Associated with Probability of Success in Phase 2 Trials
| Variable | No. of Drugs (Total = 381) | No. of Successful Phase 2 Trials (Total = 130) | OR (95% CI)
|
|||
|---|---|---|---|---|---|---|
| Univariate Analysis | p | Multivariate Analysis | p | |||
|
| ||||||
| Total no. of patients in expansion cohorts | ||||||
| 0 | 269 | 74 | 1 (reference) | 1 (reference) | ||
| 2–20 | 62 | 30 | 2.47 (1.40–4.35) | 0.002 | 2.13 (1.14–3.98) | 0.019 |
| ≥20 | 50 | 26 | 2.85 (1.54–5.29) | 0.001 | 1.91 (0.97–3.753) | 0.063 |
| Disease-specific | ||||||
| No | 242 | 70 | 1 (reference) | 1 (reference) | ||
| Yes | 139 | 60 | 1.87 (1.21–2.89) | 0.0050 | 1.72 (1.04–2.85) | 0.037 |
| Cytotoxic drug class | ||||||
| Yes | 82 | 25 | 0.81 (0.48–1.37) | 0.43 | ||
| No | 299 | 105 | 1 (reference) | |||
| Publication year of first phase 1 trial | ||||||
| 2006–2008 | 177 | 59 | 1 (reference) | |||
| 2009–2011 | 204 | 71 | 1.07 (0.70–1.63) | 0.77 | ||
| Malignancy | ||||||
| Hematologic | 56 | 26 | 1.59 (0.89–2.85) | 0.12 | ||
| Mixed | 37 | 13 | 0.44 (0.29–0.85) | 0.015 | ||
| Solid tumor | 258 | 91 | 1 (reference) | |||
| Industry sponsorship | ||||||
| Yes | 295 | 111 | 2.13 (1.21–3.73) | 0.009 | 2.88 (1.46–5.66) | 0.0024 |
| No | 86 | 19 | 1 (reference) | 1 (reference) | ||
| Involvement of multiple centers in a phase 1 trial | ||||||
| Yes | 263 | 91 | 1.07 (0.68–1.70) | 0.77 | 0.63 (0.36–1.09) | 0.098 |
| No | 118 | 39 | 1 (reference) | 1 (reference) | ||
| Total no. of patients in phase 1 trials | ||||||
| 5–24 | 129 | 29 | 1 (reference) | |||
| 25–46 | 127 | 45 | 1.89 (1.09–3.28) | 0.024 | ||
| 47–289 | 125 | 56 | 2.80 (1.63–4.82) | 0.002 | ||
| Pooled phase 1 response rate | ||||||
| 0% | 165 | 35 | 1 (reference) | 1 (reference) | ||
| >0% and <6% | 74 | 29 | 2.39 (1.32–4.35) | 0.0045 | 1.92 (1.02–3.62) | 0.043 |
| ≥6% and <20% | 85 | 39 | 3.15 (1.79–5.55) | <0.001 | 2.87 (1.57–5.24) | 0.0007 |
| ≥20% | 37 | 19 | 3.92 (1.86–8.26) | <0.001 | 2.46 (1.07–5.64) | 0.035 |
| Pooled rate of grade 3–4 toxic effects in phase 1 trials | ||||||
| ≥0% and <10% | 198 | 65 | 1 (reference) | |||
| ≥10% and <30% | 95 | 29 | 0.90 (0.53–1.52) | 0.69 | ||
| ≥30% | 64 | 28 | 1.59 (0.89–2.83) | 0.11 | ||
| MTD reached | ||||||
| No | 166 | 49 | 1 (reference) | |||
| Yes | 215 | 81 | 1.44 (0.94–2.22) | 0.097 | ||
| Any grade 5 toxic effect | ||||||
| Yes | 44 | 14 | 0.89 (0.45–1.74) | 0.73 | ||
| No | 337 | 116 | 1 (reference) | |||
Abbreviations: OR, odds ratio; CI, 95% confidence interval; MTD, maximum tolerated dose.
Drug Approval
The median time from first publication to FDA approval was 66 months (50–92 months) for the 34 drugs (9%) that were approved (Supplemental Table 1). Univariate analysis showed that drugs in the expansion cohort group had a higher 5-year probability of approval (19% vs 5%, hazard ratio [HR] 4.4, 95% CI 2.2–8.8, p < 0.001).
Expansion cohorts with more than 20 patients were not associated with significantly higher drug approval rates than cohorts with 2–20 patients (22% vs 15%, HR 1.1, 95% CI 0.33–2.47, p = 0.84; Figure 4). Many other variables were associated with higher probability of approval over time, including industry sponsorship (HR 4.9, 95% CI 1.2–20, p = 0.005), enrollment of 47 or more patients in the phase 1 trials (HR 4.7, 95% CI 2.3–9.7, p < 0.001), testing in patients with hematologic malignancies (HR 2.9, 95% CI 1.4–6.0, p = 0.0065), disease-specific trials (HR 7.1, 95% CI 3.3–15.2, p<0.001), any response seen in the phase 1 trials (HR 9.2, 95% CI 2.8–30.2, p < 0.001), and determination of an MTD in the phase 1 trials (HR 3.1, 95% CI 1.4–7.1, p = 0.008). However, because of the low number of events (approvals), we were not able to perform a multivariate analysis of study factors and time to approval.
Fig. 4.
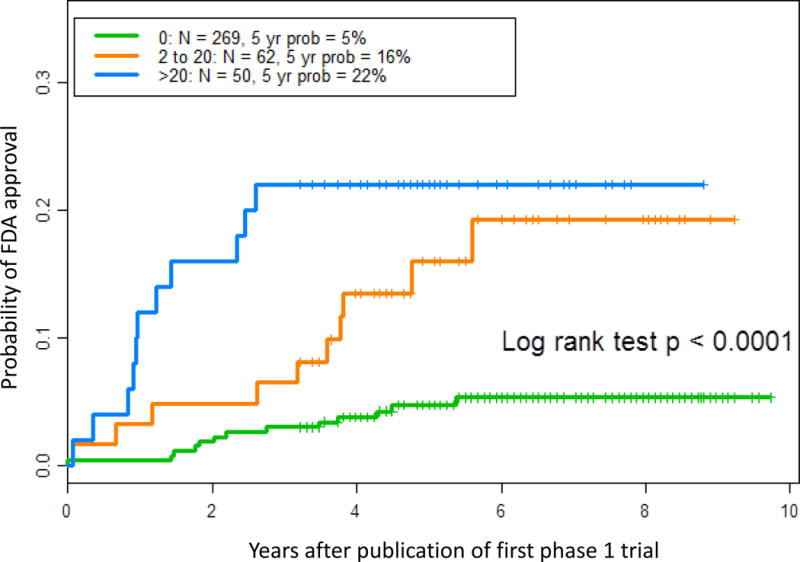
Probability (prob) of FDA approval as a Kaplan-Meier failure function of time to first FDA approval and the number of patients in the phase 1 expansion cohort.
DISCUSSION
We reviewed 381 oncologic drugs and identified their first phase 1 trials, their success rates in subsequent phase 2 trials, and the status of their approval by the FDA. There was a positive association between the use of an expansion cohort in phase 1 and probability of success in phase 2, but drugs with expansion cohorts larger than 20 did not seem to do better than drugs with expansion cohorts of 2–20 patients.
There is much discussion about the value of expansion cohorts for characterizing the safety profiles of drugs. In the systematic review by Manji et al,5 54% of the phase 1 trials with expansion cohorts in the database (which we also used in the current review) identified a new toxic effect that had not been reported in the dose escalation phase, and 13% led to a change in the recommended phase 2 dose. Our study showed that the proportion of drugs causing grade 5 toxic effects and reaching a defined MTD was higher in the expansion cohort group. Even though we did not differentiate between toxic effects occurring during the dose escalation phase and those occurring during the dose expansion phase, this finding suggests that expansion cohorts allow early identification of toxic effects that would otherwise be identified only in phase 2.
To our knowledge, the current study is the first to evaluate the correlation between the size of expansion cohorts and an efficacy endpoint—the probability of success in phase 2 trials. Interestingly, our analysis showed no benefit of increasing the size of an expansion cohort above 20 patients. This finding is similar to the previously reported results of a series of mathematical simulations suggesting that a cohort size of 20 patients would have a 96% probability of identifying a new dose-limiting toxic effect11, 12 and an up to 50% likelihood of resulting in a change in the MTD.13 Those results are consistent with the study by Jardim et al,14 which showed a direct correlation between the size of a phase 1 trial and its ability to predict toxic effects in phase 3 trials but demonstrated that sample sizes above 60 rarely provide additional information.
Therefore, it is reasonable to limit the size of expansion cohorts to 20–30 patients when a phase 2 or 3 trial is planned. However, when no further trials are planned and investigators want to obtain efficacy date from a phase 1 study, probably more patients are needed. This could explain why expansion cohorts with more than 20 patients were not associated with more positive phase 2: it is possible that they were not followed by further trials and drugs were either approved or abandoned.
There is some concern that expansion cohorts may either stimulate researchers to move from phase 1 to phase 3 or simply delay the initiation of phase 2 trials, therefore prolonging the total period of drug development15. We found that drugs in the EC group were actually more likely to be tested in P2 trials than drugs in the no-EC group (58% vs 38%), suggesting that for most cases, P2 trials were performed, despite the expansion cohorts.
In a review of all phase 1 trials and corresponding phase 2 trials published in the Journal of Clinical Oncology during 2004–2014, Behtaj et al17 also described a positive association between the use of expansion cohorts, industry sponsorship, and study drugs being non-cytotoxic agents. However, those authors found no association between the use of expansion cohorts and FDA approval. Comparison between both studies is challenging, because Behtaj et al17 used a narrower search strategy (positive phase 1 studies are more likely to be published in high-impact journals such as the Journal of Clinical Oncology18–20) and included drug-combination phase 1 studies (which usually have higher response-rates and, therefore, approval rates8).
Our study has 2 main limitations. First, there might have been publication bias. It is possible that drugs that are more active are more likely to be tested in expansion cohorts and that expansion cohorts are more likely to be published than small, negative studies. This could have led to an over-estimation of the outcomes in the expansion cohort group.
The second limitation is the fact that we were unable to determine the actual starting date or causality between the phase 1 dose escalation cohorts, dose expansion cohorts and phase 2 trials. Therefore, we had to make choices in data analysis that can lead to bias. Drugs that are more active are more likely to be enrolled in multiple trials and, therefore, more likely to have at least one trial with an expansion cohort and to lead to successful phase 2 studies. By doing a pooled analysis of all phase 1 and phase 2 trials, this might also have overestimated the outcomes in the expansion cohort group. To ameliorate this effect, we corrected our analysis for response rates, but the effect will always remain a significant source of bias in our analysis.
In conclusion, our study has demonstrated an association between the use of expansion cohorts in phase 1 trials and successful drug performance in phase 2 trials. Our findings also suggest that, when the objective of the expansion cohort is identifying toxicity and obtaining information for the design of a phase 2 trial, a sample size of 20 could be adequate. Future research should focus on optimizing sample size of multiple expansion-cohorts or of biomarker-driven cohorts and on incorporating efficacy data from expansion cohorts in the design of phase 2 trials.
Supplementary Material
Statement of translational relevance.
Phase I expansion cohorts are common in drug development, especially in oncology. However, two main questions remain unanswered: what is the optimal design for a phase I expansion cohort and what is their real impact in the drug development process.
In this manuscript, we have reviewed 381 cancer drugs, focusing on their original phase 1 trials, subsequent phase 2 studies and eventual FDA approval. After correcting for possible confounders, we have shown that phase 1 expansion cohorts are associated with a higher likelihood of success in phase 2 and that in general a sample size of 20 patients is enough for defining the maximum tolerated dose (MTD) and providing enough efficacy information for adequate design of phase 2 trials.
Therefore, this manuscript supports the role of phase-1 expansion cohorts in drug development and provides important information on their optimal size and, therefore, it is of interest of the readers of this journal.
The authors
Acknowledgments
This study was supported by the NCATS grant UL1 TR000371 and NIH/NCI grant P30 CA016672to the University of Texas MD Anderson Cancer Center. We would like to thank Johnique Atkins for editorial review and formatting.
Financial support This study was supported by the NCATS grant UL1 TR000371 and NIH/NCI grant P30 CA016672 to the University of Texas MD Anderson Cancer Center
Contributor Information
Diogo D. G. Bugano, Hospital Israelita Albert Einstein, São Paulo, Brazil
Kenneth Hess, The University of Texas MD Anderson Cancer Center, Houston, TX.
Denis L. F. Jardim, Centro de Oncologia do Paraná, Curitiba, Brazil
Alona Zer, Princess Margaret Cancer Centre, Toronto, Canada.
Funda Meric-Bernstam, The University of Texas MD Anderson Cancer Center, Houston, TX.
Lillian L. Siu, Princess Margaret Cancer Centre, Toronto, Canada
Albiruni Razak, Princess Margaret Cancer Centre, Toronto, Canada.
David S. Hong, The University of Texas MD Anderson Cancer Center, Houston, TX
References
- 1.Dahlberg SE, Shapiro GI, Clark JW, et al. Evaluation of statistical designs in phase i expansion cohorts: The Dana-Farber/Harvard cancer center experience. J Natl Cancer Inst. 106:2014. doi: 10.1093/jnci/dju163. [DOI] [PubMed] [Google Scholar]
- 2.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101:708–20. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iasonos A, O’Quigley J. Clinical trials: Early phase clinical trials—are dose expansion cohorts needed? Nat Rev Clin Oncol. 2015;12:626–628. doi: 10.1038/nrclinonc.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theoret MR, Pai-Scherf LH, Chuk MK, et al. Expansion Cohorts in First-in-Human Solid Tumor Oncology Trials. Clin Cancer Res. 2015;21:4545–4551. doi: 10.1158/1078-0432.CCR-14-3244. [DOI] [PubMed] [Google Scholar]
- 5.Manji A, Brana I, Amir E, et al. Evolution of clinical trial design in early drug development: Systematic review of expansion cohort use in single-agent phase i cancer trials. J Clin Oncol. 2013;31:4260–4267. doi: 10.1200/JCO.2012.47.4957. [DOI] [PubMed] [Google Scholar]
- 6.Hoogendam A, de Vries Robbé PF, Stalenhoef AFH, et al. Evaluation of PubMed filters used for evidence-based searching: validation using relative recall. J Med Libr Assoc. 2009;97:186–193. doi: 10.3163/1536-5050.97.3.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner EH. How to access and process FDA drug approval packages for use in research. Br Med J. 2013;5992:f5992. doi: 10.1136/bmj.f5992. [DOI] [PubMed] [Google Scholar]
- 8.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 9.Roberts TG, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda Y, Huang E, Finnigan S, et al. Risk and benefits of phase 1 trials, 2001 through 2012. J Clin Oncol. 2014;32(suppl) abstr 2552. [Google Scholar]
- 11.Penel N, Fournier C, Bérille J. Proposal for size justification of expanded cohort at phase-2- recommended dose. Invest New Drugs. 2011;29:713–715. doi: 10.1007/s10637-009-9385-7. [DOI] [PubMed] [Google Scholar]
- 12.Boonstra PS, Shen J, Taylor JMG, et al. A Statistical Evaluation of Dose Expansion Cohorts in Phase I Clinical Trials. JNCI J Natl Cancer Inst. 2015;107:dju429–dju429. doi: 10.1093/jnci/dju429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iasonos A, O’Quigley J. Design considerations for dose-expansion cohorts in phase I trials. J Clin Oncol. 2013;31:4014–4021. doi: 10.1200/JCO.2012.47.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jardim DL, Hess KR, LoRusso P, et al. Predictive value of phase i trials for safety in later trials and final approved dose: Analysis of 61 approved cancer drugs. Clin Cancer Res. 2014;20:281–288. doi: 10.1158/1078-0432.CCR-13-2103. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell S, Sima C, Jameson G, et al. Factors Influencing Time to Determination of the Recommended Phase 2 Dose in Phase 1 Clinical Trials. Am J Clin Oncol. 2012;36:1. doi: 10.1097/COC.0b013e31824370a3. [DOI] [PubMed] [Google Scholar]
- 16.Kaitin KI. Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther. 2010;87:356–361. doi: 10.1038/clpt.2009.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behtaj M, Fu P, Sharma N, et al. Evaluating the role of phase I expansion cohorts in drug development. J Clin Oncol. 2015;33:e13585. [Google Scholar]
- 18.Paulson K, Saeed M, Mills J, et al. Publication bias is present in blood and marrow transplantation: an analysis of abstracts at an international meeting. Blood. 2011;118:6698–6701. doi: 10.1182/blood-2011-08-367466. [DOI] [PubMed] [Google Scholar]
- 19.Suñé P, Suñé JM, Montoro JB. Positive Outcomes Influence the Rate and Time to Publication, but Not the Impact Factor of Publications of Clinical Trial Results. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0054583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang P, Pond GR, Welch S, et al. Factors associated with publication of randomized phase iii cancer trials in journals with a high impact factor. Curr Oncol. 2014;21:564. doi: 10.3747/co.21.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


