Abstract
Purpose
We study CD38 levels in immunosuppressive CD4+CD25highFoxp3+ Tregs and further define immune modulating effects of a therapeutic CD38 monoclonal antibody (mAb) Isatuximab (Isa)/SAR650984 in multiple myeloma (MM).
Experimental Design
We evaluated percentages of CD38-expressing subsets in Tregs from normal donors and MM patients. PBMCs were then treated with Isa with or without Lenalidomide (Len) or Pomalidomide (Pom) to identify their impact on percentage and immunosuppressive activity of Tregs on CD4+CD25− T cells (Tcons). We investigated the mechanism of increased Tregs in MM patients in ex vivo cocultures of MM cells with PBMCs or Tcons.
Results
CD38 expression is higher on Tregs than Tcons from MM patients versus normal donors. CD38 levels and the percentages of CD38high Tregs are increased by Len and Pom. Isa preferentially decreases Treg and increases Tcon frequencies, which is enhanced by Pom/Len. Isa reduces Foxp3 and IL10 in Tregs and restores proliferation and function of Tcons. It augments MM cell lysis by CD8+ T and natural killer cells. Coculture of MM cells with Tcons significantly induces Tregs (iTregs), which express even higher CD38, CD25, and FoxP3 than natural Tregs. This is associated with elevated circulating CD38+ Tregs in MM patients vs. normal donors. Conversely, Isa decreases MM cell- and bone marrow stromal cell-induced iTreg by inhibiting both cell-cell contact and TGFβ/IL10. Finally, CD38 levels correlate with differential inhibition by Isa of Tregs from MM vs normal donors.
Conclusion
Targeting CD38 by Isa can preferentially block immunosuppressive Tregs and thereby restore immune effector function against MM.
Keywords: CD38, regulatory T (Treg), conventional T (Tcon), multiple myeloma (MM), Isatuximab (Isa) (SAR650984), tumor-induced Treg (iTregs), natural occurring Tregs (nTregs), PD1, CD107a, IFNγ, bone marrow stromal cells (BMSCs)
INTRODUCTION
Monoclonal antibodies (mAbs) targeting SLAMF7 and CD38 have become available to treat relapsed/refractory (RR) multiple myeloma (MM). Specifically, the first CD38 mAb daratumumab was approved in 2015 to treat RR MM (1) and is effective as a monotherapy (2,3). Isatuximab (Isa)/SAR650984 (4), another therapeutic CD38 mAb currently under clinical development, also shows significant clinical activity in heavily pretreated patients with RR MM, both as a monotherapy and combined with Lenalidomide (Len)/Dexamethasone (Dex) (5). In addition to Fc-dependent cytotoxicity mediated by IgG1-based CD38 mAbs, Isa induces direct killing of p53-mutated MM cells expressing high levels of CD38 in ex vivo cultures without effector cells and Fc cross-linking reagents (6). Isa significantly kills CD38high-expressing MM cells via induction of homotypic aggregation, leakage of lysosome-associated cathepsin B and lysosomal associated membrane protein-1 (LAMP-1), and generation of reactive oxygen species (6). Furthermore, apoptosis is significantly enhanced when Isa is combined with Pomalidomide (Pom)/Len (6). Since CD38 is widely expressed on hematopoietic cells, it is important to study whether Isa also has impact on these cells. To date, the effects of Isa on CD38-expressing immune cells and modulation of immune function is not defined.
Regulatory T cells (Tregs) play a crucial role in immune surveillance by suppressing activation, expansion, and function of target cells including CD4+CD25− conventional T cells (Tcons), cytotoxic CD8+ T cells, as well as Natural Killer (NK) cells (7). They inhibit both cellular and humoral immune responses (8,9). Two forms of Tregs, “natural” and “induced” have been reported (10,11). Naturally occurring Tregs (nTregs), which constitute 5%-10% CD4+ lymphocytes, originate in the thymus and disseminate to periphery. Induced Tregs (iTregs) are generated in the periphery by soluble cytokines and cell-cell contact (10–12).
In parallel, tumor cells have the capacity to avoid immune recognition, to induce immune cell dysfunction, and to escape from immune surveillance via Tregs (9,13). The proportions of Tregs are elevated in the circulation of patients with solid tumors and hematologic malignancies (14–19). Increasing levels of Tregs often correlate with tumor burden and disease progression (16,19–22). Accumulation of Treg in the tumor microenvironment is associated with reduced survival (15,17,18,23). Moreover, anti-tumor immune responses are enhanced in animal models after Treg depletion using mAbs against surface markers highly expressed on Tregs, i.e., CD25 (24), CTLA-4 and OX40 (25–27), and in transgenic DEREG mice (28). Thus, Tregs present a promising therapeutic target to restore anti-tumor immune responses.
CD38 is expressed on several lineages of hematopoietic cells including Tregs, and its levels correlate with the suppressive function of Tregs. CD38-knockout mice present with a loss of Foxp3+ regulatory T cells (29,30). Conversely, high CD38 expression may define a highly suppressive subset of Tregs (31–33). We here characterize the role of CD38 in Treg inhibitory function both on Tcons and other immune effector cells in MM. We then show that Isa, alone or with Len/Pom, modulates the frequency and function of Tregs dependent on CD38, thereby restoring immune effector function in MM.
MATERIALS AND METHODS
Cell lines, medium and reagents
Human multiple myeloma cell lines (U266, RPMI8226) were cultured in mycoplasma free condition and maintained in complete culture medium (RPMI 1640 medium supplemented by 10% FBS, 2 mM L-Glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin) in ventilated tissue culture flasks at 37°C in a 5% CO2 humidified incubator.
Peripheral blood nuclear cells (PBMCs) were collected from fresh buffy coat of healthy donors and MM patients after informed consent, in accordance with the Declaration of Helsinki and under the auspices of a Dana-Farber Cancer Institute Institutional Review Board approved protocol. PBMC were expanded in complete culture medium with 20 IU/ml rIL2 (Miltenyi Biotec, Germany). Isatuximab and its F(ab)’2 fragments were obtained from Sanofi (Cambridge, MA) (4,6). Len/Pom were purchased from Selectchem, anti-PD1/PD-L1 mAb from Biolegend (San Diego, CA), and Mitomycin C from Sigma-Aldrich (St Louis, MO).
Proliferation assay for Tregs and Tcons
PBMCs from normal donors were pretreated with or without 1μM Len/Pom for 3d, stained by 5μM CFSE (Invitrogen), and then plated in the presence or absence of indicated doses of Isa. Following 5d incubation, proliferating Tregs and Tcons were identified as CFSE-diluted subsets in CD4+CD25highFoxp3+ Tregs and CD4+CD25− Tcons, respectively. Unlabeled cells were used as a control.
Phenotyping and FACS analyses
Antibodies used for flow cytometry were as follows: CD4-Pacific Blue, CD25-PE, CD25-APC, CD127-FITC, Foxp3-PE, CD38-PE-Cy7, AnnexinV-PE, PD1-APC, CD8-FITC, CTLA4-PE-Cy7, CD44-FITC, CD62L-FITC, ICOS-FITC, GITR-PE, OX40-FITC, CD138-FITC, PD-L1-PE, and their isotype-matched mAbs (all from Biolegend). Intracellular staining of Foxp3, CTLA4, GITR, and OX40 were performed after fixation and permeabilization using cytofix/cytoperm kit (BD Biosciences), according to manufacturer’s protocol. Tregs were gated as CD4+CD25highFoxp3+ cells in CD4+ population and then sequential markers were assayed on Tregs, whereas CD4+CD25− cells were identified as Tcons. The remaining CD4+CD25low/intermediate subset was excluded in the current study because of their limited immunosuppressive activity compared with CD25high population (34). To avoid the effect of permeabilization when apoptosis assay was performed, CD4+CD25highCD127low/− cells were identified as Tregs (35). All flow cytometry was performed by BD FACS CantoII, and analyzed on FlowJo software version 10 (Treestar).
Cell purification and immunosuppressive function assay
Tregs were purified using CD4+CD25+ Regulatory T cell Isolation Kit (Miltenyi Biotec, Germany). The purity of isolated population was >95%. Tcons were used as target cells in immunosuppressive assays. In brief, Tcons were cultured alone or with autologous Tregs in 96-well tissue culture plates in the presence of Isa, alone or with Len/Pom, and stimulated with anti-CD3/CD28 beads (Miltenyi Biotec, Germany), according to the manufacturer’s recommendation. Proliferation was measured by [3H]-thymidine incorporation.
Activation of immune effector cells detected by degranulation (CD107a) and intracellular interferon gamma (IFNγ) production in response to MM cells
PBMCs from normal donors or MM patients were treated with serial doses of Isa and/or 1μM Len/Pom for 2–3 d, followed by addition of MM cells at E:T ratio of 10:1 together with CD107a antibody (36). After 1h incubation, protein transport inhibitors Brefeldin A and Monensin (BD Biosciences) were added for an additional 4 h. Cells were then harvested and stained with surface markers (CD3-Pacific Blue, CD56-FITC, CD8-APC) and fixed/permeabilized, followed by staining with anti-IFNγ mAb. All Abs used were from Biolegend.
Ex vivo co-culture in the generation of iTregs
MM cells were pre-treated with mitomycin C to block their proliferation, followed by 3 washes. They were next co-cultured with PBMCs or Tcons in tissue culture plates. PBMCs or Tcons alone were used as controls. Isa was added into co-cultures for 7d, followed by FACS analysis to determine frequency and phenotype of Tregs. Supernatants were also collected for cytokine assessment.
Statistical analyses
Results are shown as means ± SEM or ranges, as appropriate. The student’s t test was used to compare 2 groups. One-way ANOVA test was used to compare 3 or more groups. Two-way ANOVA test was used when there were two variables. Statistical analyses were carried out with Prism software (GraphPad Software, Inc). P < 0.05 was determined to be statistically significant.
Results
CD38 expression is higher on Tregs versus Tcons, and CD38high subsets are increased on Tregs of MM patients versus normal donors
We first examined CD38 expression on CD4+CD25highFoxp3+ Tregs and CD4+CD25− Tcons. Three subsets were seen in Tregs and Tcons: CD38 negative (-), low, and high expression populations. In a representative sample, Tregs have higher percentages of CD38-expressing subsets with increased CD38 expression versus Tcons (63.8 % vs 17.2%; median fluorescence intensity (MFI) of 466 vs 55.7) (Figure 1A, Supplement Figure 1A). Specifically, percentages of CD38high subsets are increased in Tregs vs Tcons from PBMCs of 8 normal donors (Figure 1B, left panel); conversely, frequencies of CD38low/− Tregs are decreased in Tregs compared with Tcons (Supplement Figure 1B, lower panel). MFIs of CD38 are higher for Tregs vs. Tcons (Supplemental Figure 1C). Significantly, percentages of CD38+subset (Supplement Figure 1D–E) and CD38high Tregs are increased in MM patients (n=11) vs normal donors (n=8) (Figure 1B, right panel; Figure 1C). Levels of CD38 correlate with Foxp3 in Tregs of MM patients (n=11, Figure 1D). Moreover, MM patient Tregs inhibit proliferation of autologous Tcons, as demonstrated by significantly decreased percentages of CFSE dilution (Figure 1E, Supplement Figure 1F).
Figure 1. CD38 level is higher on Treg versus Tcon and percentages of CD38high subset in Tregs are significantly increased in MM patients versus normal donors.
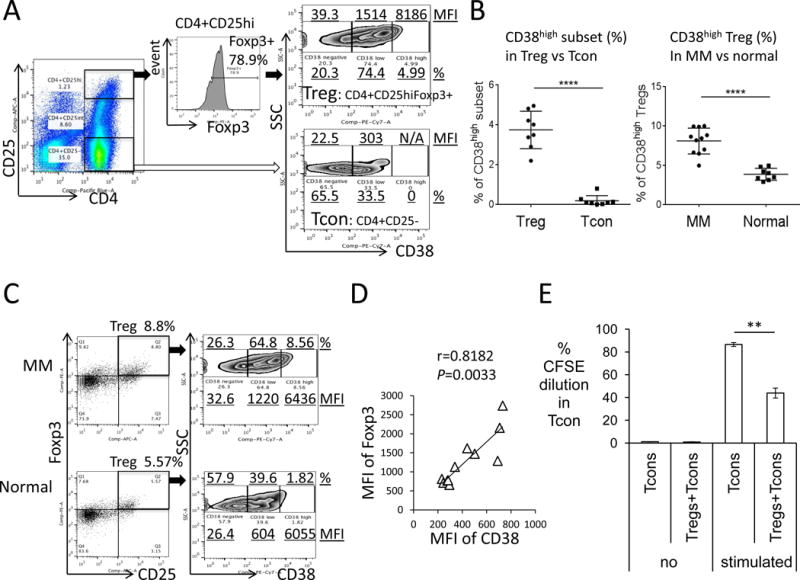
(A) The percentage and median fluorescence intensity (MFI) of CD38high, low, and negative (−) subsets were determined within CD4+CD25high(hi)Foxp3+Tregs (black arrow) and CD4+CD25− Tcons (open arrow). (B) Shown are summary (means ± SDs) of percentages of CD38high on Tregs versus Tcons from PBMCs of 8 normal donors (left panel). Right panel shows percentages of CD38high on Tregs from 11 MM patients and 8 normal donors. (C) Shown are plots of CD38 on Tregs from a representative MM patient and normal donor. (D) Levels of CD38 correlate with Foxp3 in Tregs of 11 MM patients. (E) Treg inhibits proliferation, assessed by CFSE dilution in Tcons, of autologous Tcons from MM patients. Stimulation is done using anti-CD3/CD28 beads. **P< 0.01, ****P< 0.001
CD38 expression on Tregs is upregulated by IMiDs
We next assessed the impact of Len/Pom on CD38 expression on viable Tregs. Low dose (1μM) len and pom significantly increase MFI of CD38 on Tregs after 3d and persisting until 10d; moreover, the higher percentage CD38+Tregs was maintained relative to control medium (Figure 2A). In addition to higher CD38 on Tregs from MM patients vs normal donors, Len or Pom enhances cell surface CD38 on Tregs of MM patients and normal donors (Supplemental Figure 2). Specifically, Len or Pom increases 2–3 fold the percentage of CD38high (CD38hi) subsets and the MFI of CD38 on Tregs in PBMCs from MM patients (Figure 2B–C). These results suggest that IMiDs may enhance sensitivity of viable Tregs to Isa.
Figure 2. CD38 expression is upregulated by IMiDs, associated with elevated CD38high Tregs.
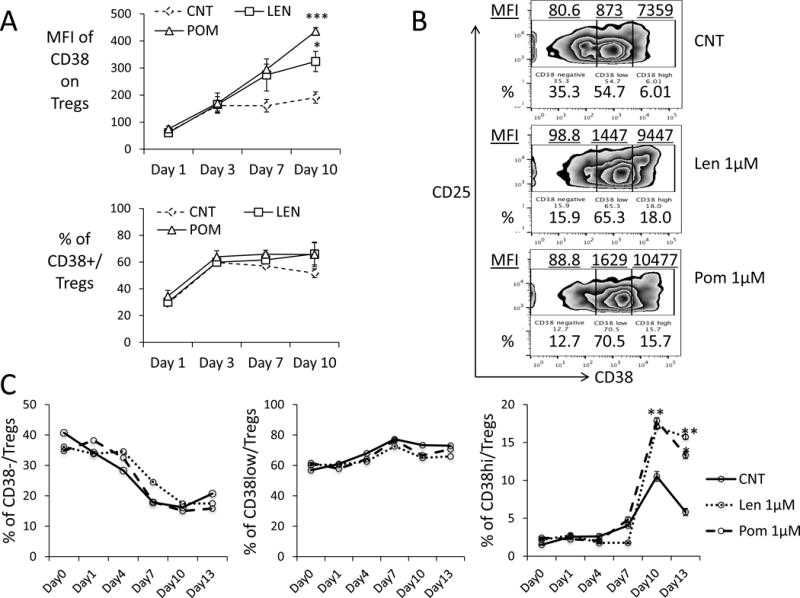
(A) PBMCs (n=3) were incubated with Len or Pom followed by flow cytometry analysis for CD38 levels (MFI) on Tregs and percentage of CD38-expressing Tregs. Shown are Means ± SEMs. (B) Shown are representative plots of CD38-expressing subsets in gated CD4+CD25hiFoxp3+ Tregs from a MM patient treated with indicated drugs for 7 days. MFI (underlined) and frequency of CD38−/low/high(hi) subsets are also indicated. (C) Frequencies of CD38−/low/hi Tregs of MM PBMCs were followed.
Isa decreases Treg frequencies and inhibits Treg-suppression of Tcon proliferation, which is enhanced by IMiDs
When PBMCs from normal donors (n=10) were treated with or without Isa (1μg/ml) for 3 days, the frequency of Tregs in CD4+ cells is reduced from 8.88±0.66% at baseline to 4.56±0.77% (p<0.01) (Figure 3A, Supplemental Figure 3A). In contrast, Tcons are increased from 63.70±7.51% to 75.83±5.87% (p<0.05), associated with decreased ratios of Tregs/Tcons. In MM patient samples (n=6), even 0.1 μg/ml of Isa decreases Tregs (16.35±1.62% at baseline to 7.34±1.78%, p<0.01) and stimulates Tcons (from 69.03±4.89% to 81.69±2.55%, p<0.05), associated with reduced Tregs/Tcons ratios (Figure 3B, Supplemental Figure 3B). Thus, higher CD38 expression on Tregs from MM patients versus normal donors correlates with increased Isa inhibition of MM Tregs. Notably, F(ab)’2 fragments of Isa are sufficient to decrease Tregs and stimulate Tcons (Figure 3C, Supplemental Figure 3C), confirming that the blockade of Tregs is CD38-specific and Fc-independent.
Figure 3. Isa reduces Treg frequencies and blocks Treg-inhibited proliferation of Tcons, which is enhanced by IMiDs.
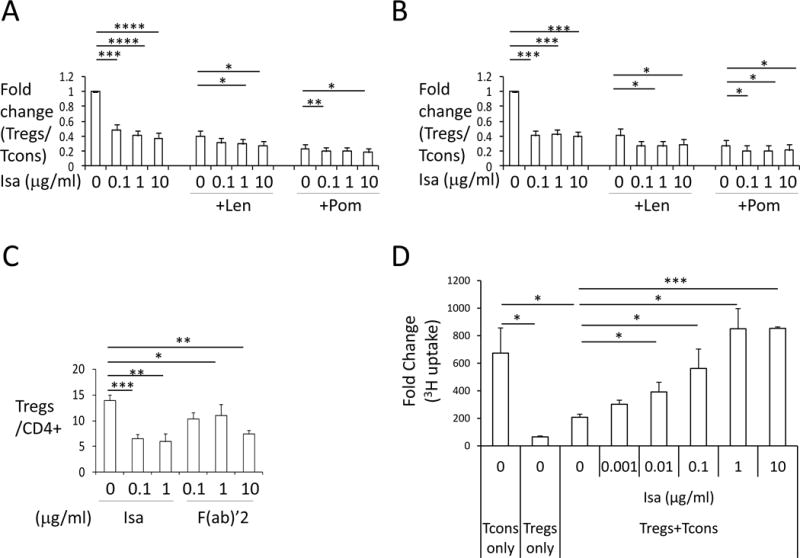
PBMCs from normal donors (A, n=10) or MM patients (B, n=7) were pretreated with 1μM Len or Pom for 3–5 days followed by incubation with Isa, alone or with Len/Pom, for another 3 days. Flow cytometry was used to determine the percentage of Tregs and Tcons in CD4+ lymphocytes. Tregs/Tcons is then normalized to untreated controls (lower panel) and fold changes (Means ± SEMs) are shown. Untreated cells are used as controls in all the setting. (C) PBMCs from 4 MM patients were treated with either Isa (whole IgG1) or its F(ab)’2 fragments and the percentage of Tregs is determined by flow cytometry. (D) Purified Tcons were incubated with or without autologous Tregs isolated from PBMCs at 1:1 ratio for 5 days in the presence of anti-CD3/CD28 activation beads, with indicated concentrations of Isa. Proliferation was assessed by [3H] thymidine incorporation added for the last 8h. Results are shown as fold changes relative to day 0. *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001.
Pom alone, more potently than Len, reduces Tregs and stimulates Tcons (Figure 3A–B, Supplemental Figure 3A–B). Consistent with upregulation of CD38 on Tregs, pre-treatment of PBMCs with Len/Pom for 3–5d enhances Isa-induced inhibition of Tregs from both normal donors and MM patients, evidenced by further decreased Tregs/Tcons ratios (Figure 3A–B).
We next determined whether Isa modulates the suppressive activity of Tregs on Tcons. Co-cultures of Tregs with autologous Tcons diminished proliferation of Tcons from 100% to 27.35±2.99%; conversely, Isa suppresses the inhibition of Tregs on Tcon proliferation in a dose-dependent manner (Figure 3D, Supplemental Figure 3D).
Isa induces apoptosis and inhibits proliferation and migration of Tregs
We next evaluated mechanisms of Isa-induced cytotoxicities against Tregs. Isa (0.1 μg/ml) induces approximately 2-fold higher percentage annexin V+ Tregs than control media (Figure 4A–B, A, normal donors; B, MM patients) or isotype IgG1 (data not shown), which is further enhanced by Len or Pom (Figure 4A). The impact of Isa on proliferation of Tregs was examined by staining PBMCs with CFSE for 5d, followed by flow cytometry analysis gated on Tregs. Isa decreases proliferation of Tregs in a dose-dependent manner, which is enhanced by Pom more potently than Len (Figure 4C). In contrast, Len or Pom alone slightly induce apoptosis and significantly decrease proliferation of Tregs in ex vivo culture.
Figure 4. Isa induces cytotoxicity and inhibits immunosuppressive molecules of Tregs.
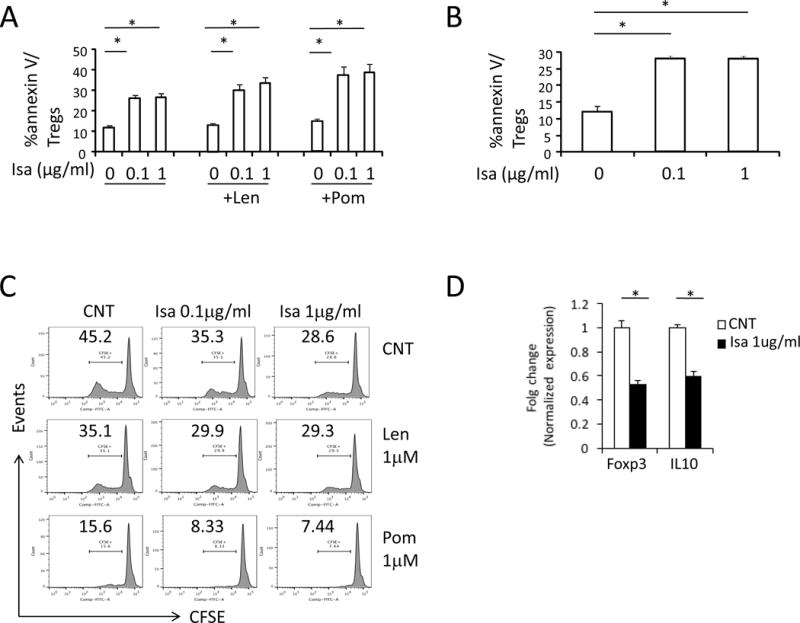
Isa-induced cytotoxicity in Tregs from normal donors (A) and MM patients (B) is shown as percentage of annexin V+ cells in Tregs. Shown are Means ± SEMs of 3 independent experiments. (C) PBMCs were stained with 5μM CFSE and incubated with indicated drugs for 5 days followed by flow cytometry analysis to determine percentage of diluted CFSE in Tregs. (D) Tregs were purified from PBMCs of normal donors treated with or without 1μg/ml Isa for 1d followed by real-time qRT-PCR assay for Foxp3 and IL10. Shown are fold changes relative to control groups after normalization with GAPDH control. *P< 0.05
Using real time qRT-PCR, Isa decreased Foxp3, a key immunosuppressive transcriptional factor, in Tregs from normal donors and MM patients (Figure 4D, Supplemental Figure 4). Inhibitory cytokine IL10 is also reduced in Isa-treated Tregs (Figure 4D).
Isa enhances CD8+ T and NK-mediated lysis of MM cells, which is further enhanced by Len/Pom
Since Tregs also influence NK and CD8+ T effector cells, we next assessed the effects of Isa on their function. PBMCs from 3 normal donors were treated for 3d with Isa, with or without Len/Pom (1μM), prior the addition of RPMI8226 MM cells and flow cytometry analysis. Upregulation of cell surface CD107a and interferon (IFN) γ production is associated with cytotoxicity induced in these effector cells (36). Isa increases percentages of CD107a and IFNγ in immune effector cells of normal donors (Supplemental Figure 5A–B), whereas an isotype control IgG1 mAb has no effects (data not shown). Isa (0.1 μg/ml) significantly increases the ability of these two immune effector cells to lyse target MM cells, confirmed by depletion of CD138+ MM cells in these cultures (data not shown). Although low dose Len or Pom (1 μM) alone for 3d minimally increased both CD107a and IFNγ, combined treatment of Isa with Pom, more potently than Len, further augments activation of these effector cells (Supplemental Figure 5A–B). Importantly, Isa, in a dose-dependent manner, upregulates effector function of CD8+ T and NK cells from MM patients (n=3) (Figure 5A–B), which is further augmented by Pom, more potently than Len.
Figure 5. Isa augments CD8+ T and NK-effector cell-mediated cytotoxicity against MM cells, which is further enhanced by Len/Pom.
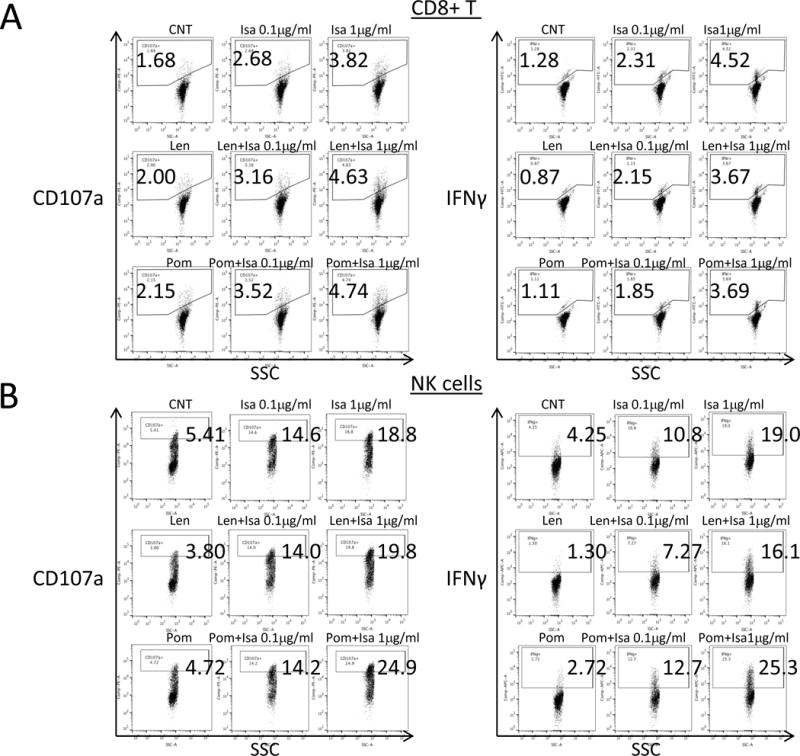
PBMCs from MM patients were pretreated with Isa, alone or with Len/Pom for 2–3 days, and then RPMI8226 MM cells were added at E:T ratio of 10:1 for 5h, followed by flow cytometry analysis for cell membrane CD107a and intracellular IFNγ. Dot plots are shown for CD107a (left panel) and IFNγ (right panel) in CD3+CD8+ T cells (A) and NK cells (B) of MM patient PBMCs treated as indicated.
Tumor cells induce generation of iTregs
We found that circulating Tregs in MM patients are significantly higher compared with normal donors (Figure 6A), as in previous reports (20,22,37). This could be due to generation of Tregs in the periphery, designated as tumor-induced Tregs (iTregs) derived from naïve CD4+ cells or CD4+CD62L+ central memory cells (38–41). To study whether such a mechanism leads to elevated percentage Tregs in MM patients, PBMCs were co-cultured with MM cells for 7d, followed by flow cytometry analysis. Using the same CD4+CD25highFoxp3+ gating strategy, the frequency of Tregs in CD4+ populations significantly increases when co-cultured with either RPMI8226 or U266 MM cells (Supplemental Figure 6A, left panels). To further identify the origin of these iTregs, purified Tcons were co-cultured with MM cells. MM cells induce even more iTregs from Tcons than from whole PBMC populations (Supplemental Figure 6A, right panels): fold increases in iTreg induced by MM cells were significantly higher from Tcons (n=11) than PBMCs (n=5) (Supplemental Figure 6B). We found that iTregs in cocultures of PBMCs with MM cells increase expression of CD38 with time (Supplemental Figure 6C). Fold induction in CD38 levels is significantly higher in iTregs vs nTregs in ex vivo co-cultures containing low dose IL-2 (Figure 6B). Importantly, proliferation of autologous Tcons is inhibited by iTregs induced by MM cells in these ex vivo cocultures (Figure 6C). Whether the source of iTreg is PBMCs or Tcons, their phenotype is characterized by higher CD38, CD25, Foxp3, CD44, ICOS, and PD1, as well as lower CD127 expression, compared with nTregs (Supplemental Figure 6C–E). Changes in these cell surface proteins are even more significant on iTregs derived from Tcons vs PBMCs, following culture with multiple CD38low/−MM cell lines (Supplemental Figure 6C–E).
Figure 6. MM cells induced-Tregs (iTregs) highly express CD38 and are blocked by Isa.
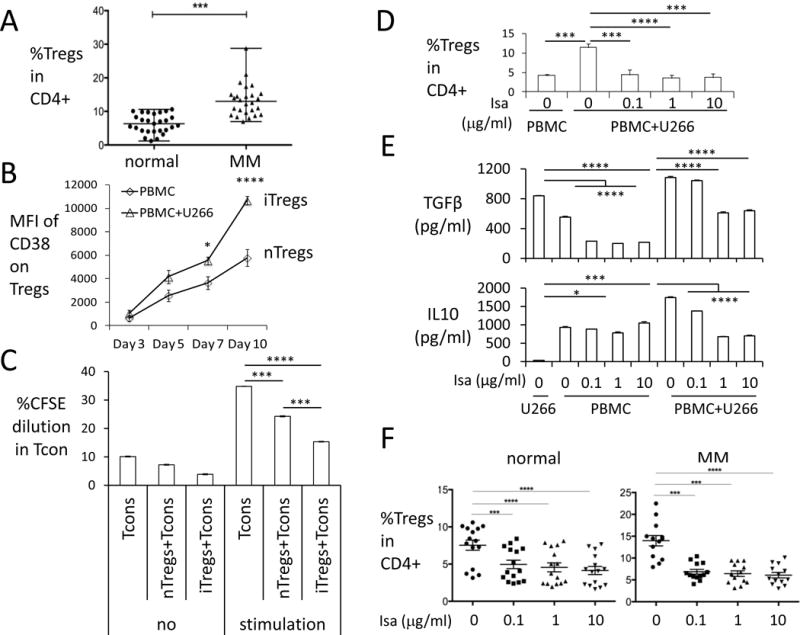
(A) Percentages of Tregs in CD4+ lymphocytes were determined in normal donors (n=27) and MM patients (n=26). Shown are median ± ranges. (B) CD38 levels were examined at indicated time periods in iTregs (triangle) vs. nTreg (square) in PBMC cocultured with or without CD38low/− U266 cells in low dose IL-2-containing culture media. (C) CFSE-labeled Tcons cocultured with either nTregs isolated from normal donors or iTregs isolated from MM cell induction in ex vivo coculture system were stimulated with (stimulation) or without (no) anti-CD3/CD28 beads for 6 d. Proliferation were determined by percentage of CFSE dilution in Tcons. (D) Percentages of iTregs were determined in co-cultures of MM cells with PBMCs (n=4) treated with indicated doses of Isa for 7 days. Shown are Means ± SEMs. (E) Supernatants of ex vivo co-cultures (in D) were assayed for TGFβ (upper panel) and IL10 (lower panel) by ELISA. (F) PBMCs from healthy donors (n=15) and MM patients (n=13) were treated with Isa, and percentage Tregs in CD4+ lymphocytes was measured by flow cytometry analysis. Shown are Means ± SEMs. *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
We next studied mechanisms of iTregs generation. PD-L1 is concurrently increased on MM cells in these cocultures (Supplemental Figure 7A). Conditioned media from MM cells increase frequency of iTregs (Supplemental Figure 7B), indicating the importance of cytokines secreted from MM cells in the induction of iTregs. Specifically, both TGFβ and IL10 are significantly increased in supernatants from co-cultures of MM cells with either PBMCs or Tcons (Supplemental Figure 7C, Figure 6E). Stronger induction of both cytokines from Tcons vs PBMCs correlates with greater extent of iTregs generation from Tcons vs. PBMCs (Supplemental Figure 6B & 7C). Neutralizing anti-TGFβ, -PD1, and -PD-L1 mAb partially blocked iTregs induction (Supplemental Figure 7D–E), supporting their roles in generation of iTregs. Combined with decreased induction of iTregs when using transwell plates to separate Tcons from MM cells, these data indicate that cell-to-cell contact also contributes to the generation of iTregs (data not shown).
Isa also significantly inhibits tumor- and bone marrow stromal cell-induced Tregs (iTregs) which highly express CD38
We next determined whether Isa inhibits MM cell-induced iTregs which express high levels of CD38 (Figure 6B, Supplemental Figure 6C–E). Isa blocks induction of iTregs from PBMCs (Figure 6D) and from Tcons (Supplemental Figure 8A) in ex vivo co-cultures with U266 MM cells. Similar levels of apoptosis were observed in both Isa-treated and untreated CD38-negative U266 MM cells, evidenced by annexin V/PI-based flow cytometry analysis (Supplementary Figure 8B), excluding the possibility that failure of U266 cells to induce iTregs was due to tumor cell killing by Isa. TGFβ and IL10 were significantly elevated in supernatants from cocultures of PBMCs with U266 MM cells and significantly reduced by Isa (Figure 6E). Since these cytokines are critical in iTregs generation, we also examined whether BM stromal cells (BMSCs) induce generation of iTregs. Cocultures of PBMCs with adherent BMSCs from MM patients increased the percentage of Tregs, which was also inhibited by Isa (Supplementary Figure 8C). These results indicate that CD38 mAb is capable of blocking iTregs in the BM microenvironment. Finally, Isa decreases the percentage of Tregs from MM patients more efficiently than those from normal donors: MM patients baseline 15.98±1.19% reduced to 5.87±0.54% at 0.1μg/ml Isa, n=13; normal donors baseline 7.54±0.68% decreased to 4.96±0.57% at 0.1μg/ml Isa, n=15 (Figure 6F).
DISCUSSION
Tregs, as regulatory elements, actively suppress immune responses and represent a predominant tolerance-inducing modality. Conversely, blockade of Tregs may reverse the suppressive immune environment via promoting T cell activation and cytotoxicity, thereby allowing the immune system to efficiently attack the tumor. We here demonstrate that targeting CD38 by Isa, as recently reported by daratumumab,(32) preferentially blocks Tregs greater than Tcons due to increased CD38 levels on Tregs. Since CD38high Tregs exhibit even higher immunosuppressive ability (32,42), targeting CD38 can abrogate this subset more effectively than CD38low or negative subsets, thereby relieving the immunosuppressive BM microenvironment. We show that blockade of Tregs by Isa restores Tcons and upregulates cytolysis of MM cells mediated by cytotoxic T and NK cells, which is further enhanced by IMiDs.
Our studies show that Isa reduces the frequency of Tregs and blocks their suppressive function on Tcons from both normal donors and MM patients. The increased proportion of Tcons after Isa treatment is due to significantly increased proliferation of Tcons. Correspondingly, the ratio of Tregs to Tcons (Tregs/Tcons) significantly decreased following Isa treatment. Fc-independent mechanisms including apoptosis and decreased proliferation could account for Tregs inhibition, in addition to antibody-dependent cytotoxicity (ADCC) and antibody-dependent phagocytosis mediated by Fc-expressing NK and macrophages. Our studies show that Isa preferentially induces apoptosis of Tregs greater than Tcons due to increased percentages of CD38+ Tregs with higher CD38 expression than Tcons. CD38high Tregs, which have even greater immunosuppressive capacity than CD38−/low Tregs (32), are most sensitive to CD38 targeting. In addition, Isa decreases Foxp3 and IL10 in viable Tregs, further targeting the immunosuppressive function of Tregs. It remains to be determined whether such differential effects of Isa on Tregs vs Tcons can increase its therapeutic window.
Besides blocking the suppressor cell component, Isa spares Tcons, CD8+ T cells, and NK cells, consistent with the limited toxicity observed in clinical trials (5,43). Isa significantly increases CD8+ T- and NK cell-mediated anti-MM immune response, with enhanced induction of CD107a and IFNγ. Inhibition of immune effector cells by suppressor Tregs is blocked following treatment of PBMCs with Isa for at least 2d. Considering the underlying immune deficiency of MM patients, targeting Tregs to restore effective anti-tumor response represents a promising treatment strategy. Importantly, Isa targets CD38high Tregs in an Fc-independent manner, even in MM patients with a highly impaired immune system.
IMiDs inhibit proliferation and function of Tregs in vitro (44,45). However, reports of Treg frequency in patients treated with len are variable. In CLL patients, len treatment reduced proportion of Tregs (46). Conversely, a delayed increase of Tregs after treatment with len has been reported in MM in the setting of induction, maintenance, or salvage treatment with Len or Pom (22,35,47,48). Elevated Tregs in vivo after immune stimulation by Len may represent a negative feedback loop to maintain immune homeostasis. Indeed, our data shows that IMiDs reduce Tregs and stimulate Tcons in vitro. Importantly, low dose Len/Pom with Isa enhances suppression of Tregs and induces immune effector cell-mediated MM cell lysis in vitro. Mechanistically, Len and Pom up-regulate CD38 levels on viable Tregs and increase the percentage of CD38high Tregs, thereby conferring further sensitivity to Isa treatment.
Increasing evidence indicates that patients with cancer may have higher proportions of Tregs, which may serve as a predictor for survival. Importantly, increased circulating functional Tregs have been noted in MM patients compared with normal donors (22,37). Increased Tregs in patients with cancer can be derived from naïve CD4+ T cells by stimulation with tumor cells and tumor bystander cells. Using an ex vivo coculture system to mimic the in vivo BM microenvironment, we showed that MM cells are able to induce generation of iTregs from both PBMC and Tcons. Specifically, iTregs are induced when co-culturing MM cells with purified Tcons in the absence of antigen-presenting cells. Furthermore, when compared with nTregs, iTregs express even higher cell surface CD38, Foxp3, CD25, CD44, ICOS and PD1, as well as lower levels of CD127. This suggests an even greater suppressive function of iTregs, further supporting targeting CD38 to block these highly immunosuppressive Tregs derived from Tcons. In addition, PD-L1 is increased on MM cells in these cocultures, which may further promote differentiation of Tcons into Tregs via ligation with PD1 (49). PD-L1 upregulation on MM cells mediates adherence to BM stromal cells and induction of MM-related cytokines, which further increases immunosuppression in the BM microenvironment (42). These results suggest the utility of combining Isa with PD-L1 mAb to enhance anti-MM immunity.
Both soluble cytokines and cell-to-cell contact are critical in the generation of iTregs. On one hand, inhibitory cytokines IL10 and TGFβ in the culture supernatant are significantly increased when tumor cells are present. Indeed, a blocking anti-TGFβ mAb partially inhibits generation of iTregs. Conditioned media from MM cells also induces Tregs from PBMCs, supporting a soluble cytokine mechanism. On the other hand, separating Tcons and MM cells by transwell plates attenuates induction of iTregs. Moreover, membrane PD-L1 expression is upregulated on MM cells in parallel with increased PD1 receptor on Tregs. Either anti-PD1 mAb or anti-PD-L1 mAb can inhibit induction of iTregs, indicating a role of surface receptor-ligand interaction in this process. Our current findings are consistent with the notion that induction of CD25+Tregs from CD25-Tcons was partially abrogated when Tcons were separated from tumor cells (40), indicating a role for cell-cell contact.
Our data show that Isa prevents induction of iTregs by MM cells and bone marrow microenvironment cells, associated with reduced TGFβ and IL10 in the coculture supernatants. Potential mechanisms whereby Isa attenuates iTregs generation include: (1) iTregs express higher levels of CD38 than nTregs, making them even more sensitive to Isa; and (2) soluble cytokines contribute to generation of iTregs, and Isa may reduce IL10 production through blocking CXCL12/CXCR4 signaling. For example, CXCL12 costimulates IL10 secretion by a diverse population of CD45RA− T cells, including Tregs (50). Blocking CD38 impairs CXCL12/CXCR4 signaling pathway in CLL cells (51). Whether this occurs in Tregs remains to be determined.
In summary, Isa suppresses the inhibitory function of Tregs which highly express CD38 by decreasing their cell number, inhibiting immunosuppressive cytokines, and blocking their trafficking. Isa enhances NK− and CD8+ T effector cell-mediated anti-tumor immune responses, which can be further enhanced by IMiDs. Furthermore, increased circulating iTregs in MM patients are derived from Tcons in both cell-cell contact-dependent and -independent manners. Importantly, Isa blocks these processes. Targeting CD38 with Isa may therefore induce immunomodulatory effects which both relieve immunosuppression and trigger anti-MM immunity.
Supplementary Material
Translational Relevance.
CD38 mAb Isatuximab (Isa), as a monotherapy and in combinational use, have shown significant clinical activities against human multiple myeloma (MM). Here we characterize that CD38 levels are higher in CD4+CD25highFoxp3+ Treg versus Tcon (CD4+CD25−) and MM patients have increased percentages of CD38high Tregs than normal donors. Importantly, Isa inhibits suppressive function of Tregs highly expressing CD38 by decreasing their percentages, reducing immune inhibitory cytokines (TGFβ, IL10), and blocking their trafficking. Isa further enhances NK− and CD8+ T effector cell-mediated anti-tumor immune responses, which can be further enhanced by IMiDs. In ex vivo cocultures, MM cells significantly induce functional Treg (iTreg) highly expressing CD38 and suppressing Tcon. Furthermore, increased circulating iTreg in MM patients are derived from Tcons in both cell-cell contact-dependent and -independent manners. Importantly, Isa blocks these processes. Targeting CD38 with Isa may therefore induce immunomodulatory effects which both relieve immunosuppression and trigger anti-MM immunity.
Key points.
CD38 levels are elevated in regulatory T cells versus conventional T cells in MM patients versus normal donors
CD38 mAbs may overcome immunosuppression via inhibiting Treg while restoring immune effector cell function
Acknowledgments
The authors thank Dr. Francisco Adrian, Zhili Song at Sanofi for providing reagents and Dr. Hua Jiang, and Alireza Kalbasi for helpful input and excellent technical assistance. The authors thank all clinical and laboratory members of the Jerome Lipper Multiple Myeloma Center of the Dana-Farber Cancer Institute for support and help for this study.
Funding
National Institutes of Health Grants RO1CA050947, RO1CA207237, RO1100707, and DF/HCC SPORE in Multiple Myeloma P50CA100707; K.C.A. is an American Cancer Society Clinical Research Professor.
Footnotes
AUTHOR CONTRIBUTIONS
X.F., Y.-T.T., K.C.A. conceptualized research and formed the hypothesis of this paper; X.F., C.A., G.A., K.W., L.Z. designed, performed experiments, collected, and analyzed data; C.A., G.A., L.Q. provided reagents and analytic tools; N.M., K.C.A. provided MM patient samples; X.F., Y.-T.T. wrote the manuscript; Y.-T.T., K.C.A. critically evaluated and edited the manuscript.
CONFLICT OF INTEREST
N.M. serves on advisory boards to Millennium, Celgene, Novartis, and a scientific founder of Oncopep. K.C.A. serves on advisory boards to Onyx, Celgene, Gilead, and is a scientific founder of Acetylon, Oncopep, and C4 Therapeutics. The remaining authors declare no competing financial interests.
References
- 1.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373(13):1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 2.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–60. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 3.McKeage K. Daratumumab: First Global Approval. Drugs. 2016;76(2):275–81. doi: 10.1007/s40265-015-0536-1. [DOI] [PubMed] [Google Scholar]
- 4.Deckert J, Wetzel MC, Bartle LM, Skaletskaya A, Goldmacher VS, Vallee F, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20(17):4574–83. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 5.Sondergeld P, van de Donk NW, Richardson PG, Plesner T. Monoclonal antibodies in myeloma. Clin Adv Hematol Oncol. 2015;13(9):599–609. [PubMed] [Google Scholar]
- 6.Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399–408. doi: 10.1038/leu.2015.240. [DOI] [PubMed] [Google Scholar]
- 7.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10(3):222–9. doi: 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–71. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol. 2013;4:190. doi: 10.3389/fimmu.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frassanito MA, Ruggieri S, Desantis V, Di Marzo L, Leone P, Racanelli V, et al. Myeloma cells act as tolerogenic antigen-presenting cells and induce regulatory T cells in vitro. Eur J Haematol. 2015;95(1):65–74. doi: 10.1111/ejh.12481. [DOI] [PubMed] [Google Scholar]
- 13.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 14.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13(7):2075–81. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 16.Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, et al. CD4(+)CD25(+)CD127(low/−) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131(1):109–18. doi: 10.1016/j.clim.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 18.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of Regulatory T Cells Is Increased in Peripheral Blood and Tumor Microenvironment of Patients with Pancreas or Breast Adenocarcinoma. The Journal of Immunology. 2002;169(5):2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 19.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65(6):2457–64. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 20.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108(3):804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–12. [PubMed] [Google Scholar]
- 22.Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One. 2012;7(10):e47077. doi: 10.1371/journal.pone.0047077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannopoulos K, Kaminska W, Hus I, Dmoszynska A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: detailed characterisation of immune status in multiple myeloma. Br J Cancer. 2012;106(3):546–52. doi: 10.1038/bjc.2011.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008;3(4):e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123(6):2447–63. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92(6):475–80. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 27.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 28.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70(20):7788–99. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Chen YG, Reifsnyder PC, Schott WH, Lee CH, Osborne M, et al. Targeted Disruption of CD38 Accelerates Autoimmune Diabetes in NOD/Lt Mice by Enhancing Autoimmunity in an ADP-Ribosyltransferase 2-Dependent Fashion. The Journal of Immunology. 2006;176(8):4590–9. doi: 10.4049/jimmunol.176.8.4590. [DOI] [PubMed] [Google Scholar]
- 30.Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. 2010;207(12):2561–8. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110delta regulates expression of CD38 on regulatory T cells. PLoS One. 2011;6(3):e17359. doi: 10.1371/journal.pone.0017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai YT, Anderson KC. A new era of immune therapy in multiple myeloma. Blood. 2016;128(3):318–9. doi: 10.1182/blood-2016-06-719856. [DOI] [PubMed] [Google Scholar]
- 34.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24(7):1169–77. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 35.Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother. 2015;64(10):1271–86. doi: 10.1007/s00262-015-1729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai YT, Horton HM, Kong SY, Pong E, Chen H, Cemerski S, et al. Potent in vitro and in vivo activity of an Fc-engineered humanized anti-HM1.24 antibody against multiple myeloma via augmented effector function. Blood. 2012;119(9):2074–82. doi: 10.1182/blood-2011-06-364521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107(10):3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 38.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116(9):2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor Evasion of the Immune System by Converting CD4+CD25− T Cells into CD4+CD25+ T Regulatory Cells: Role of Tumor-Derived TGF. The Journal of Immunology. 2007;178(5):2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 40.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111(11):5359–70. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Chang Li X, Xiao X, Sun R, Tian Z, Wei H. CD4(+)CD62L(+) central memory T cells can be converted to Foxp3(+) T cells. PLoS One. 2013;8(10):e77322. doi: 10.1371/journal.pone.0077322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An G, Acharya C, Feng X, Wen K, Zhong M, Zhang L, et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood. 2016;128(12):1590–603. doi: 10.1182/blood-2016-03-707547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Donk NW, Moreau P, Plesner T, Palumbo A, Gay F, Laubach JP, et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood. 2016;127(6):681–95. doi: 10.1182/blood-2015-10-646810. [DOI] [PubMed] [Google Scholar]
- 44.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Idler I, Giannopoulos K, Zenz T, Bhattacharya N, Nothing M, Dohner H, et al. Lenalidomide treatment of chronic lymphocytic leukaemia patients reduces regulatory T cells and induces Th17 T helper cells. Br J Haematol. 2010;148(6):948–50. doi: 10.1111/j.1365-2141.2009.08014.x. [DOI] [PubMed] [Google Scholar]
- 47.Minnema MC, van der Veer MS, Aarts T, Emmelot M, Mutis T, Lokhorst HM. Lenalidomide alone or in combination with dexamethasone is highly effective in patients with relapsed multiple myeloma following allogeneic stem cell transplantation and increases the frequency of CD4+Foxp3+ T cells. Leukemia. 2009;23(3):605–7. doi: 10.1038/leu.2008.247. [DOI] [PubMed] [Google Scholar]
- 48.Busch A, Zeh D, Janzen V, Mugge LO, Wolf D, Fingerhut L, et al. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin Exp Immunol. 2014;177(2):439–53. doi: 10.1111/cei.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kremer KN, Kumar A, Hedin KE. Haplotype-independent costimulation of IL-10 secretion by SDF-1/CXCL12 proceeds via AP-1 binding to the human IL-10 promoter. J Immunol. 2007;178(3):1581–8. doi: 10.4049/jimmunol.178.3.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaisitti T, Aydin S, Rossi D, Cottino F, Bergui L, D’Arena G, et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia. 2010;24(5):958–69. doi: 10.1038/leu.2010.36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


