Abstract
Resting-state functional connectivity (rsFC) is widely used to examine cerebral functional organization. The ventral striatum (VS) is critical to motivated behavior, with extant studies suggesting functional hemispheric asymmetry. The current work investigated differences in rsFC between the left (L) and right (R) VS and explored gender differences in the extent of functional lateralization. In 106 adults, we computed a laterality index (fcLI) to query whether a target region shows greater or less connectivity to the L vs R VS. A total of 45 target regions with hemispheric masks were examined from the Automated Anatomic Labeling atlas. One-sample t test was performed to explore significant laterality in the whole sample and in men and women separately. Two-sample t test was performed to examine gender differences in fcLI. At a corrected threshold (p < 0.05/45 = 0.0011), the dorsomedial prefrontal cortex (dmPFC) and posterior cingulate cortex (pCC) showed L lateralization and the intraparietal sulcus (IPS) and supramarginal gyrus (SMG) showed R lateralization in VS connectivity. Except for the pCC, these findings were replicated in a different data set (n = 97) from the Human Connectome Project. Furthermore, the fcLI of VS—pCC was negatively correlated with a novelty seeking trait in women but not in men. Together, the findings may suggest a more important role of the L VS in linking saliency response to self control and other internally directed processes. Right lateralization of VS connectivity to the SMG and IPS may support attention and action directed to external behavioral contingencies.
Keywords: Ventral striatum, RsFC, Laterality, Hemisphericity, Sex difference
Introduction
There are hemispheric differences in the mesostriatal dopaminergic (DA) system, particularly with respect to the nigrostriatal circuit and its role in motor and spatial behavior (see Molochnikov and Cohen 2014 for a review). For instance, DA agonists elicited greater locomotor hyperactivity when injected into the right than left ventral striatum (VS; Belcheva et al. 1990). Studies also support functional asymmetry in the mesolimbic system. Cortical and hippocampal regulation of DA transmission in the nucleus accumbens appears to be stronger in the left than right hemisphere (Louilot and Le Moal 1994). 6-OHDA lesioning of the right but not left hemispheric mesocortical pathway results in bilateral reduction in DA content and an increase in DA turnover in the striatum (Sullivan and Szechtman 1995). Bilateral lesions of the prefrontal cortex reduced DA content and increased DA turnover only in the right nucleus accumbens (Sullivan and Szechtman 1995). Higher levels of dopamine D1 receptors were reported in the right striatum of female rats (Andersen et al. 2000), as confirmed by a recent study (Murphy and Fryxell 2015).
In humans, theta burst magnetic stimulation of the left but not right hemispheric dorsolateral prefrontal cortex inhibited dopamine release bilaterally in the caudate and unilaterally in the putamen (Ko et al. 2008). Right but not left hemispheric capsulotomy, disrupting cortical-striatal-thalamic circuit functions, has been associated with successful treatment of medication refractory obsessive compulsive disorder (Lippitz et al. 1999), presumably as a result of the modulation of right-hemispheric ventral striatal and amygdalar circuit (Sturm et al. 2003). Although not targeting the VS, other studies provided evidence linking hemispheric organization of the cortical limbic circuit to depression (Downar et al. 2014).
Much work of the VS has focused on its role in reward processing. In particular, numerous studies employed variants of the Monetary Incentive Delay Task to examine how the VS and corticostriatal circuit respond to reward anticipation and prediction. Although the bulk of this work did not distinguish right and left VS responses, a few studies did. For instance, right and left VS each showed a distinct influence of affective control on reward anticipation and prediction error (Staudinger et al. 2009). Gain involved predominantly right VS activation and less activation in major depressive disorder; in contrast, loss involved bilateral VS activation, with left VS activation restored by antidepressants (Stoy et al. 2012). Bilateral VS responded to reward anticipation with slightly greater activation in the left VS during immediate vs delayed reward and, compared to smokers, non-smokers demonstrated greater differences only in the right VS (Luo et al. 2011). Both gain and loss cue elicited greater activation of the right VS activation, in contrast to neutral cue, in control but not in alcoholic participants. Furthermore, right VS activation was negatively correlated with trait impulsivity in alcoholics but not controls (Beck et al. 2009). Reward anticipation elicited bilateral VS activation with ADHD patients showing less activation than controls only in the left VS (Strohle et al. 2008). Together, although it remains unclear how R and L VS differentially influences motivated behavior, these findings suggest potential functional lateralization of the VS and its relevance to a wide range of neuropsychiatric conditions.
We postulate that functional lateralization may be reflected in cerebral connectivity of the VS. Analysis of resting-state fMRI data has proven to be a useful approach to characterizing functional architecture of a brain region. Specifically, low frequency blood oxygenation level dependent (BOLD) signal fluctuations reflect connectivity between functionally related brain regions (Biswal et al. 1995; Fair et al. 2007; Fox and Raichle 2007). Based on correlation in spontaneous BOLD activity, for instance, our recent studies have characterized whole-brain connectivity and the effects of age and medications for many cortical and subcortical areas, including the VS (Farr et al. 2014; Li et al. 2014; Manza et al. 2015; Zhang et al. 2015, 2012; Zhang and Li 2012, 2014). There has also been an accumulating literature to characterize VS rsFC during development (Fareri et al. 2015; Porter et al. 2015) and how VS rsFC is altered in neuropsychiatric illnesses, including depression (Leaver et al. 2015), autism (Rane et al. 2015), Alzheimer’s disease (Dennis and Thompson 2014), and Parkinson’s disease (Tahmasian et al. 2015).
Here, we examined rsFC of the VS in a cohort of 106 adults, focusing on hemispheric lateralization in cerebral connectivity and gender differences, and attempted to replicate the findings in an independent cohort of 97 adults. Following previous studies, we computed a laterality index (LI) for individual brain regions as identified from the automated anatomical labeling (AAL) atlas (Di et al. 2014; Liu et al. 2009), highlighted those that show a significant lateralization, and examined whether the lateralization in VS connectivity differs between men and women and varies with age and personality traits.
Methods
Data set
Resting-state fMRI scans of 106 healthy controls were obtained on a 3-Tesla Siemens Trio scanner at Yale University (43 men, 19–47 years of age with median = 27, and 63 women, 20–49 years of age with median = 25, and there was no age difference between men and women, p = 0.1; one scan per participant; duration: 10 min; TR = 2 s; eye closed). The replication sample comprised the “Beijing_Zang” data set from the Human Connectome Project (18–26 years of age; 31 men; one scan per participant; 8 min, TR = 2 s; eye closed). Individual subjects’ images were viewed one by one to ensure that the whole brain was covered. In our data set, 54 subjects were assessed with Cloninger’s Tridimensional Personality Questionnaire - Short Form (TPQ-Short). Derived from the 100-item long form of the TPQ (Cloninger 1987), the TPQ-Short demonstrated reliability and validity (Sher et al. 1995). It consists of 44 yes/no questions covering novelty seeking (NS; 13 items), harm avoidance (HA; 22 items) and reward dependence (RD; 9 items). Each personality subscale score was calculated by summing the item scores, reverse scoring where necessary. A higher subscore of each represents a higher level of NS, HA and RD. Because of the role of the VS in reward-related processes, we examined for correlations of lateralization with NS and RD scores across these individuals.
Imaging data processing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, UK). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Standard image pre-processing was performed. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Ashburner and Friston 1999; Friston et al. 1995). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
Additional pre-processing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Fair et al. 2007; Fox and Raichle 2007; Fox et al. 2005; Rombouts et al. 2003). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1 Hz contribute to regionally specific BOLD correlations (Cordes et al. 2001). Thus, we applied a temporal bandpass filter (0.009 Hz < f < 0.08 Hz) to the time course to obtain low-frequency fluctuations, as in previous studies (Fair et al. 2007; Fox and Raichle 2007; Fox et al. 2005; Lowe et al. 1998).
Head motion
As extensively investigated in Van Dijk et al. (2012), micro head motion (>0.1 mm) is an important source of spurious correlations in resting-state functional connectivity analysis (Van Dijk et al. 2012). Therefore, we applied a “scrubbing” method proposed by Power and colleagues (Power et al. 2012) and successfully applied in previous studies (Power et al. 2012; Smyser et al. 2010; Tomasi and Volkow 2014) to remove time points affected by head motions. Briefly, for every time point t, we computed the frame-wise displacement given by FD(t) = |Δdx(t)| + |Δdy(t)| + |Δdz(t)| + r|α(t)| + r|β(t)| + r|γ(t), where (dx, dy, dz) and (α, β, γ) are the translational and rotational movements, respectively, and r (=50 mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transforms rotations into displacements (Power et al. 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point that exceeded the head motion limit FD(t) >0.5 mm or DVARS(t) >0.5% (Power et al. 2012; Tomasi and Volkow 2014). On average, 1% of the time points were removed across subjects.
Seed based correlation and group analyses
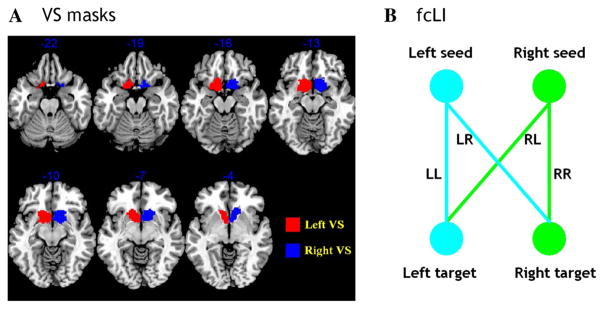
The left and right VS masks were generated using both cytoarchitectonic and topographical criteria (Fig. 1a; see details in our previous study: Li et al. 2014). The BOLD time courses were averaged spatially over each of the left and right VS seeds. For individual subjects, we computed the correlation coefficient between the averaged time course of each seed region and the time courses of all other brain voxels. To assess and compare the resting-state functional connectivity, we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Berry and Mielke 2000; Jenkins and Watts 1968): z = 0.5 loge[(1 + r)/(1 − r)]. The Z maps were used in group random effect analyses. We performed one-sample t test each on the Z maps of left and right VS and paired-sample t test comparing the two Z maps.
Fig. 1.
a Seed regions: right and left ventral striatum (VS). b A schematic to show how the functional connectivity lateralization index (fcLI) is computed. Please see text for a detailed explanation
Functional connectivity laterality index (fcLI)
A few considerations distinguished the computation of functional connectivity laterality index (fcLI) from the laterality index employed conventionally to characterize lateralization of cerebral activations to cognitive challenges: (L − R)/(L + R). First, in the latter, negative connectivity of a brain region to the L (or R) seed cannot be distinguished from positive connectivity to the R (or L) seed in the contribution to laterality. Second, target regions in the same hemisphere of the seed region will always have stronger functional connectivity than their hemispheric counterparts (please see “Results” below). To manage these issues, therefore, we followed previous studies (Di et al. 2014; Liu et al. 2009) to compute the fcLI based on connectivities of paired seed and target regions between the hemispheres. Briefly, the fcLI was computed as follows:
where LL is the functional connectivity between the L seed and L target region; RR is the functional connectivity between the R seed and R target region; RL is the functional connectivity between the R seed and L target region; and LR is the functional connectivity between the L seed and R target region (Fig. 1b). As computed, a positive fcLI indicates left lateralization; i.e., the target region, irrespective of its hemisphericity, is more connected to the L than R seed region. By contrast, a negative fcLI indicates right lateralization. The value of fcLI ranges from −1 (R lateralization) to +1 (L lateralization), with lager value indicating greater lateralization in the connectivity between the seed and target. In the current study, we computed the fcLI with each of the 45 brain regions with both L and R hemispheric masks from the AAL atlas as target regions.
Results
Differences in whole-brain connectivity between right and left VS
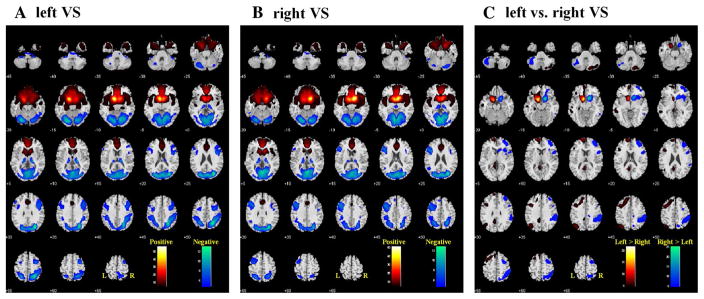
Figure 2a, b each shows the whole-brain rsFC of the left and right VS, at p < 0.05, corrected for whole-brain voxelwise family-wise error (FWE) of multiple comparisons. In general, the left and right VS each showed greater positive connectivity to cortical and subcortical regions in the same hemisphere and negative connectivity in the opposite hemisphere. A direct contrast between these maps clearly demonstrated these differences (Fig. 2c).
Fig. 2.
Whole brain functional connectivity of the left (a) and right (b) ventral striatum (VS), one-sample t test, p < 0.05, FWE corrected. Warm color positive correlation, cool color negative correlation. c two-sample t test: left vs right VS, p < 0.05 FWE corrected. Warm color left > right, cool color right > left. Neurological orientation: R = right
Therefore, to examine whether the L and R VS shows lateralized cerebral connectivity, one needs to go beyond this intrinsic, “biased” pattern of connectivity. An important question to ask is whether a given brain region is more connected to the L or R VS irrespective of its hemisphericity. To this end, we followed previous studies (Di et al. 2014; Liu et al. 2009) to derive a lateralization index of each of the 45 brain regions with both L and R hemispheric masks from the AAL atlas.
fcLI identified lateralized regional connectivities to the VS
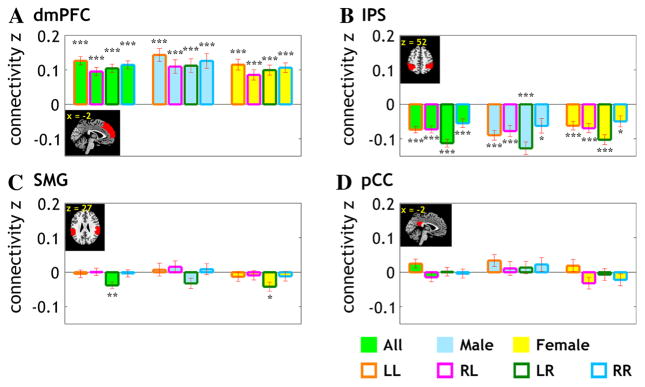
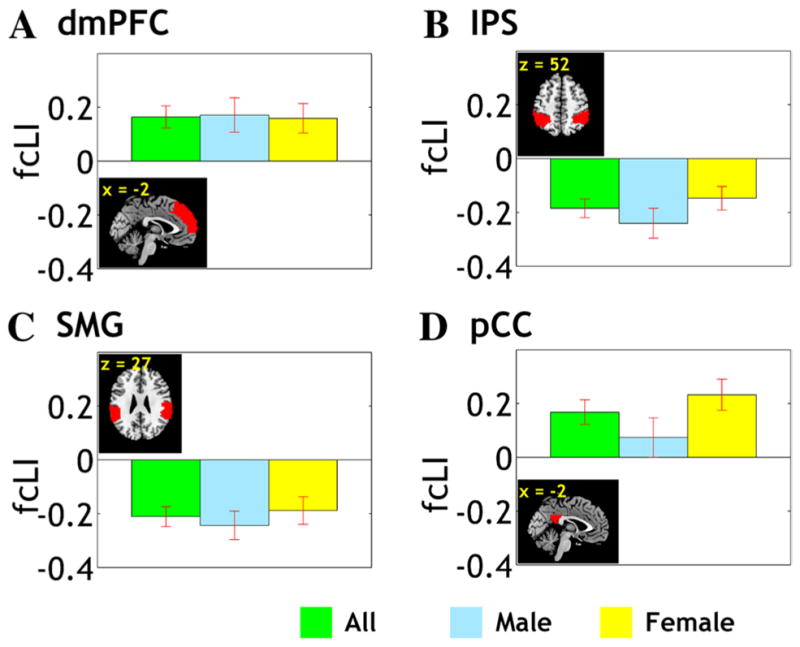
The results showed that, at a corrected threshold (p < 0.05/45 = 0.0011), the dorsomedial prefrontal cortex (dmPFC) and the posterior cingulate cortex (pCC) showed L lateralization and the intraparietal sulcus (IPS) and supra-marginal gyrus (SMG) showed R lateralization in VS connectivity (Fig. 3). The following analysis thus focused on these four regions of interest.
Fig. 3.
fcLI of the dorsomedial prefrontal cortex (dmPFC), intraparietal sulcus (IPS), supramarginal gyrus (SMG), and posterior cingulate cortex (pCC). VS connectivity to the dmPFC and pCC is left lateralized (fcLI > 0) whereas connectivity to the IPS and SMG is right lateralized (fcLI < 0)
So far, the analysis has focused on fcLI as a measure of the lateralization of VS connectivity to the brain regions irrespective of their hemisphericity. For example, the dmPFC “as a whole” is more connected to the L vs R VS. However, the fcLI is computed through VS connectivity to the right- and left-hemispheric dmPFC. It would be of interest to examine whether the lateralization of VS connectivity to dmPFC (and other brain regions) is mediated primarily through greater connectivity of the L VS to L dmPFC and/or less connectivity of the R VS to R dmPFC, etc. To this end, we extracted the connectivity z values for L and R VS to each hemispheric mask for a three-way (L/R VS × L/R target region × gender) ANOVA (Fig. 4). The results showed that, as expected, all regions showed a significant VS × target region interaction (all p’s < 10−6). However, the three way interactions were not significant for any of the four regions (all p’s > 0.26). Thus, we removed the gender factor and conducted a two-way (L/R VS × L/R target region) ANOVA for the 4 target regions of interest. In addition to a significant interaction (all p’s < 10−6), the results showed a significant main effect for VS hemisphericity for 3 target regions (p < 0.007, pCC; p < 0.000005, IPS; p < 0.0005, SMG) but not for the dmPFC (p = 0.15) and a marginally significant main effect of target region hemisphericity for the SMG (p = 0.05).
Fig. 4.
Mean ± standard error for the connectivity z value for the four target regions that showed a significant lateralization in VS connectivity. The data are broken down according to gender (men vs women), VS (L vs R), and target region (L vs R). Significance of the connectivity were further examined by one-sample t test against zero for z values and marked with *** for p < 0.0001, ** for p < 0.001, and * for p < 0.01
Thus, the lateralization is accounted by a higher connectivity to the hemispheric VS for the pCC, SMG, and IPS. That is, L lateralization of the pCC results from higher connectivity of both L and R target region to the L VS and R lateralization of the IPS and SMG results from higher connectivity of both L and R target region to the R VS. For the SMG, the R lateralization may also result from higher connectivity of the R and L VS to the R SMG.
The effect of age and personality traits on fcLI
We examined whether age influences lateralization of these rsFC, as our earlier work showed age-related changes in cerebral connectivity to the dorsal striatum (Manza et al. 2015). None of these regions demonstrated an fcLI in correlation with age for the entire sample (all p’s > 0.27) or when men (all p’s > 0.20) and women (all p’s > 0.21) were examined separately.
We also examined whether personality traits influence lateralization of these rsFC in a subset of the participants (n = 54, 17 males) who were assessed with the Tridimensional Personality Questionnaire (TPQ). We performed 36 correlations—3 TPQ subscores × 3 samples (all, male, female) × 4 regions of interest—and used a p value of 0.0014 (0.05/36) as the threshold of statistical significance. Only the fcLI of pCC appeared to show an overall trend in correlating negatively with novelty seeking (r = −0.47, p = 0.004).
Findings from the replication sample
In an independent sample of 97 young adults (18–26 years of age; 31 men) scanned under similar conditions, we performed identical analyses to examine the fcLI. The results showed that the dmPFC (p < 0.0011, uncorrected) was significantly left-lateralized in VS connectivity, and SMG (p < 0.006), and IPL (p < 0.030) were significantly right-lateralized in VS connectivity. However, the pCC did not show a significant fcLI (p = 0.1).
Results based on different pre-processing approaches
A recently study compared three different pre-processing approaches, where unfiltered spurious variance signals such as head motion estimates are regressed out of time series after voxelwise bandpass filtering (BpReg), with bandpass filtering after regression (RegBp) and simultaneous filtering (Simult), and suggested that the Simult approach is superior (Hallquist et al. 2013). Furthermore, Power et al. (2014) also suggested that motion artifact can influence time points before and after the main component of motion. An additional consideration is the smoothing kernel. Therefore, we reprocessed the data with Simult, 4 mm smoothing kernel, and removal of time points before and after the main component of motion. The results showed that the fcLI findings were largely the same. Under a threshold of p < 0.05/45 = 0.0011, SMG and IPL remained significant in lateralization. The pCC (p = 0.0025) and dmPFC (p = 0.0015) came out slightly short at the threshold.
Discussion
Resting-state functional connectivity (rsFC) of the VS
The patterns of rsFC of the VS are very similar to those shown in earlier reports. For instance, the VS shows positive connectivity to an incentive-based learning and motivation circuit that includes the amygdala, hippocampus, medial prefrontal cortex, and insula, although not every study reported VS connectivity to these structures (Cohen et al. 2009; Delgado et al. 2000; Di Martino et al. 2008; Hare et al. 2008; Knutson et al. 2001, 2005; Leotti and Delgado 2011a, b; Li and Daw 2011; Li et al. 2011; O’Doherty 2004; Wimmer et al. 2012). In contrast, as could be visualized from Fig. 2, the VS exhibited a distributed pattern of negative connectivities with parietal regions commonly associated with spatial and temporal attentional selection and with lateral frontal cortical regions that subserve working memory and cognitive control (Coull et al. 2003; Di Martino et al. 2008; Fernandez-Duque et al. 2000; Lepsien and Nobre 2006; Nobre et al. 2004). These similarities support the general validity of the current findings.
In the below, we discuss the findings in a broad context of functional specialization of the individual brain regions that demonstrated lateralized connectivity to the VS. Note that the literature specifically on lateralized connectivity of the VS is non-existent. Therefore, our discussion focused on the functions of these individual brain regions to support a role each of the L VS in linking saliency response to self control and other internally directed processes and the R VS in attention and action directed to external behavioral contingencies. Further, in view of the exploratory nature of the study, we referenced widely previous studies of VS connectivity in the context of individual differences and clinical implications.
Hemispheric lateralization of VS rsFC
The dorsomedial prefrontal cortex (dmPFC) is critical to cognitive motor control and the posterior cingulate cortex (pCC) has been implicated in internally directed attention and cognition (Leech and Sharp 2014). In imaging studies, the dmPFC was shown to respond to proactive control, response inhibition, and post-error slowing in the stop signal task (Cai et al. 2014a; Chao et al. 2009; Duann et al. 2009; Hendrick et al. 2010; Hu et al. 2015; Ide and Li 2011). Lesion of the dmPFC in humans led to deficits in control in the presence of response conflicts (Nachev et al. 2007). Direct current stimulation of the pre-supplementary motor area (pre-SMA) facilitated response inhibition (Yu et al. 2015). The pCC is part of the default mode network (DMN). Although the DMN has conventionally been characterized as a “task-negative” network, deactivating to external challenges, it increases activity when attention is internally directed, such as during episodic memory retrieval and planning for the future (Spreng 2012). Thus, the findings of left lateralized VS connectivity to the dmPFC and pCC may suggest a more important role of the L VS in linking saliency response to internally directed processes such as self control.
In contrast, the supramarginal gyrus (SMG) and intra-parietal sulcus (IPS) of the inferior parietal lobule are each part of the ventral and dorsal attention system and positively connected to somatomotor cortical and subcortical structures (Zhang and Li 2014). The SMG responded to sudden onsets in “odd-ball” paradigms across multiple sensory modalities (Huang et al. 2005) and to saccadic eye movements to a visual target (Perry and Zeki 2000), and mediated bottom-up process linking attention to memory retrieval (Burianova et al. 2012; Ciaramelli et al. 2008). A study with concurrent electroencephalography and fMRI highlighted the role of the IPS in biasing task-related visual cortical activation during cued spatial attention (Liu et al. 2014). In task-related regional interaction, cognitive control was associated with increased connectivity of the dorsome-dial prefrontal cortex with the IPS (Harding et al. 2015). Thus, right lateralization of VS connectivity to the inferior parietal cortex may support action priming and externally triggered behavioral contingencies.
Animal studies
Whereas there were no functional connectivity studies, previous work has suggested structural and functional lateralization of the VS in animal models. For instance, nucleus accumbens core volume was larger in the right than left hemisphere across male and female rats (Wong et al. 2016), although findings from humans seemed less than consistent (Ahsan et al. 2007; Mamah et al. 2007; Neto et al. 2008; Tamagaki et al. 2005). Dopamine concentration (Rosen et al. 1984; and Schwarting 2001) and D2 receptor binding with [3H] spiroperidol (Schneider et al. 1982) appeared to be higher on the right mesolimbic striatum in rats. However, this hemispheric asymmetry may be only true in animals with right paw preference (Budilin et al. 2008; Cabib et al. 1995). VS function is regulated by prefrontal cortical structures. As discussed earlier, 6-OHDA lesioning of the right but not left hemispheric mesocortical pathway results in bilateral reduction in DA content and an increase in DA turnover in the striatum (Sullivan and Szechtman 1995). Bilateral lesions of the prefrontal cortex reduced DA content and increased DA turnover only in the right nucleus accumbens (Sullivan and Szechtman 1995). Another study reported hemispheric effects of prefrontal cortical lesions on VS DA concentration and escape behavior elicited by foot shock (Carlson et al. 1996). Along with these findings, the current results suggest hemispheric asymmetry in subcortical and prefrontal cortical regulation of VS DA and behavior supported by the VS.
Potential clinical implications
Patients with major depressive disorder showed increased VS connectivity to part of the default mode network, including the precuneus and pCC, and electroconvulsive therapy remediated this hyperconnectivity (Leaver et al. 2015). Patients with depression showed reduced activation for wins compared with losses in multiple structures including bilateral VS and the pCC in a monetary reward task (Satterthwaite et al. 2015). Furthermore, rsFC within this network, most notably connectivity strength in the left VS, was also diminished in proportion to depression severity. Hypoconnectivity of the VS to pCC is reported in individuals with high social anhedonia (Wang et al. 2015b) and adolescents with internet addiction disorder (Lin et al. 2015), who frequently showed comorbid depression. VS connectivity to the dmPFC was also implicated in the etiological processes of many neuropsychiatric conditions, including depression (Grimm et al. 2009), anorexia nervosa (Via et al. 2015) and other eating disorders (Dunlop et al. 2015; Val-Laillet et al. 2015) that frequently involve comorbid depression.
In substance use disorders, lateralization of responses to impulsive decision making and drug craving has recently been noted in a meta-analysis (Gordon 2016). Prefrontal regulation of VS activity is implicated in control of craving in cigarette smokers (Kober et al. 2010). Abstinence from smoking was associated with reduced functional connectivity between the left VS and superior frontal gyrus in cigarette smokers (Sweitzer et al. 2016). The right anterior insula plays an important role in monitoring and integrating environmental stimuli (Cai et al. 2014b; Chen et al. 2015). In a study of the effects of a distractor on attention, high vs low reward distractors activated the anterior insula and this effect could be predicted by the changes in functional connectivity between the anterior insula and VS (Wang et al. 2015a). Nicotine-addicted individuals demonstrated decreased urges and craving to smoke following unilateral insula damage, with apparently a stronger effect in the right hemisphere (Naqvi et al. 2007). In another study, cocaine addicts demonstrated increased anticorrelations (more negative rsFC in addicts than in controls) between right VS and pCC, as well as between right VS and IPS (Wilcox et al. 2011). In cocaine addicts methylphenidate reduced connectivity of the right VS with the IPS extending to the angular gyrus and precuneus (Konova et al. 2013). Duration of prescription opioid dependence positively correlated with the functional connectivity between VS and mPFC (Upadhyay et al. 2010). Overall, these studies suggest the clinical relevance in delineating lateralization of VS connectivity, which is widely implicated in mood disorders and addiction.
The rsFC between the dmPFC and VS was also associated with personality traits such as rejection sensitivity (Powers et al. 2013) and negative urgency (Muhlert and Lawrence 2015). “Type A” behavioral pattern, characterized by competitiveness and hostility, time urgency and impatience, is associated with greater rsFC between the left VS and the left ventromedial prefrontal cortex (Wang et al. 2014). VS-dmPFC connectivity was negatively correlated with impulsivity in individuals with antisocial personality disorder (Kolla et al. 2016). Together, these studies suggest the importance in differentiating hemispheric functional asymmetry in understanding cerebral function and dysfunction (Nielsen et al. 2013).
Conclusions and limitations
The focus of the study is on the ventral striatum (VS) and the lateralization of VS cerebral connectivity. The results showed a predominant pattern of right- and left-hemispheric lateralization each for externally and internally directed processes. These results are to be considered with the hypothesis of prefrontal lateralization of cognitive and affective processing, where right- and left-hemispheric activities are often linked to pleasant stimuli/approach behavior and unpleasant stimuli/avoidance behavior (Miller et al. 2013; Spielberg et al. 2013). However, our data set do not contain behavioral measures and more research is needed to further explore this hypothesis. Further, because of the exploratory nature of the work, we did not formulate specific hypotheses to test against the datasets. The findings from the current work, however, raised the question whether right- and left-lateralization of rsFC each for externally and internally directed processes would extend to other seed regions. An additional limitation concerns non-replication for VS lateralization in connectivity with the pCC. This issue requires further investigation in a larger data set.
Acknowledgments
Supported by NIH Grants DA023248, DA026990, AA021449, and K25DA040032, and the Peter McManus Charitable Trust. The funding agencies otherwise have no role in the conceptualization of the study, data collection and analysis, or the decision to publish these results.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-016-1358-y) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- Ahsan RL, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. NeuroImage. 2007;38:261–270. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Belcheva I, Bryer JB, Starkstein SE, Honig M, Moran TH, Robinson RG. Hemispheric asymmetry in behavioral response to D1 and D2 receptor agonists in the nucleus accumbens. Brain Res. 1990;533:286–291. doi: 10.1016/0006-8993(90)91351-g. [DOI] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW., Jr A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87:1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Budilin SY, Midzyanovskaya IS, Shchegolevskii NV, Ioffe ME, Bazyan AS. Asymmetry in dopamine levels in the nucleus accumbens and motor preference in rats. Neurosci Behav Physiol. 2008;38:991–994. doi: 10.1007/s11055-008-9082-6. [DOI] [PubMed] [Google Scholar]
- Burianova H, Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention-to-memory: mapping functional connectivity in two distinct networks that underlie cued and uncued recognition memory. NeuroImage. 2012;63:1343–1352. doi: 10.1016/j.neuroimage.2012.07.057. [DOI] [PubMed] [Google Scholar]
- Cabib S, D’Amato FR, Neveu PJ, Deleplanque B, Le Moal M, Puglisi-Allegra S. Paw preference and brain dopamine asymmetries. Neuroscience. 1995;64:427–432. doi: 10.1016/0306-4522(94)00401-p. [DOI] [PubMed] [Google Scholar]
- Cai W, Cannistraci CJ, Gore JC, Leung HC. Sensorimotor-independent prefrontal activity during response inhibition. Hum Brain Mapp. 2014a;35:2119–2136. doi: 10.1002/hbm.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci. 2014b;34:14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JN, Visker KE, Keller RW, Jr, Glick SD. Left and right 6-hydroxydopamine lesions of the medial prefrontal cortex differentially alter subcortical dopamine utilization and the behavioral response to stress. Brain Res. 1996;711:1–9. doi: 10.1016/0006-8993(95)01290-7. [DOI] [PubMed] [Google Scholar]
- Chao HH, Luo X, Chang JL, Li CS. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time–an intra-subject analysis. BMC Neurosci. 2009;10:75. doi: 10.1186/1471-2202-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Michels L, Supekar K, Kochalka J, Ryali S, Menon V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory-visual attention. Eur J Neurosci. 2015;41:264–274. doi: 10.1111/ejn.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Cordes D, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Walsh V, Frith CD, Nobre AC. Distinct neural substrates for visual search amongst spatial versus temporal distractors. Brain Res Cogn Brain Res. 2003;17:368–379. doi: 10.1016/s0926-6410(03)00138-1. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014;24:49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kim EH, Chen P, Biswal BB. Lateralized resting-state functional connectivity in the task-positive and task-negative networks. Brain Connect. 2014;4:641–648. doi: 10.1089/brain.2013.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Downar J, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176–185. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and pre-supplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Woodside B, Lam E, Olmsted M, Colton P, Giacobbe P, Downar J. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. NeuroImage Clin. 2015;8:611–618. doi: 10.1016/j.nicl.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, et al. Normative development of ventral striatal resting state connectivity in humans. NeuroImage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Zhang S, Hu S, Matuskey D, Abdelghany O, Malison RT, Li CS. The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int J Neuropsychopharmacol. 2014;17:1177–1191. doi: 10.1017/S1461145714000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Conscious Cogn. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging nature reviews. Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, Polone J, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Gordon HW. Laterality of brain activation for risk factors of addiction. Curr Drug Abuse Rev. 2016;9:1–18. doi: 10.2174/1874473709666151217121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Yucel M, Harrison BJ, Pantelis C, Breakspear M. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. NeuroImage. 2015;106:144–153. doi: 10.1016/j.neuroimage.2014.11.039. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo X, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PloS one. 2010;5:e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Li CS. Anticipating conflict: neural correlates of a Bayesian belief and its motor consequence. Neuro-Image. 2015;119:286–295. doi: 10.1016/j.neuroimage.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, et al. A parietal-frontal network studied by somatosensory oddball MEG responses, and its cross-modal consistency. NeuroImage. 2005;28:99–114. doi: 10.1016/j.neuroimage.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. NeuroImage. 2011;54:455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral analysis and its applications. Holden-Day; San Francisco: 1968. [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorso-lateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[(11)C]raclopride PET study. Eur J Neurosci. 2008;28:2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, et al. Association of ventral striatum monoamine oxidase-A binding and functional connectivity in antisocial personality disorder with high impulsivity: A positron emission tomography and functional magnetic resonance imaging study European neuropsychopharmacology : the journal of the European College of. Neuropsychopharmacology. 2016;26:777–786. doi: 10.1016/j.euroneuro.2015.12.030. [DOI] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA psychiatry. 2013;70:857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Espinoza R, Joshi SH, Vasavada M, Njau S, Woods RP, Narr KL. Desynchronization and Plasticity of Striato-frontal Connectivity in Major Depressive Disorder. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The inherent reward of choice. Psychol Sci. 2011a;22:1310–1318. doi: 10.1177/0956797611417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. Processing social and nonsocial rewards in the human brain. In: Decety J, Cacioppo J, editors. The Oxford Handbook of Social Neuroscience. Oxford University; New York: 2011b. pp. 178–194. [Google Scholar]
- Lepsien J, Nobre AC. Cognitive control of attention in the human brain: insights from orienting attention to mental representations. Brain Res. 2006;1105:20–31. doi: 10.1016/j.brainres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Li J, Daw ND. Signals in human striatum are appropriate for policy update rather than value prediction. J Neurosci. 2011;31:5504–5511. doi: 10.1523/JNEUROSCI.6316-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nat Neurosci. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. NeuroImage. 2014;97:321–332. doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Zhou Y, Du Y, Zhao Z, Qin L, Xu J, Lei H. Aberrant corticostriatal functional circuits in adolescents with Internet addiction disorder. Front Hum Neurosci. 2015;9:356. doi: 10.3389/fnhum.2015.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive–compulsive disorder: relevance of the right hemisphere. Neurosurgery. 1999;44:452–458. doi: 10.1097/00006123-199903000-00005. (discussion 458–460) [DOI] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bengson J, Huang H, Mangun GR, Ding M. Top-down Modulation of Neural Activity in Anticipatory Visual Attention: Control Mechanisms Revealed by Simultaneous EEG-fMRI. Cereb cortex. 2014 doi: 10.1093/cercor/bhu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louilot A, Le Moal M. Lateralized interdependence between limbicotemporal and ventrostriatal dopaminergic transmission. Neuroscience. 1994;59:495–500. doi: 10.1016/0306-4522(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Giragosian L, Monterosso JR. Striatal hyposensitivity to delayed rewards among cigarette smokers. Drug Alcohol Depend. 2011;116:18–23. doi: 10.1016/j.drugalcdep.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res. 2007;89:59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Zhang S, Hu S, Chao HH, Leung HC, Li CS. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. NeuroImage. 2015;107:311–322. doi: 10.1016/j.neuroimage.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W. Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front Integr Neurosci. 2013;7:2. doi: 10.3389/fnint.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molochnikov I, Cohen D. Hemispheric differences in the mesostriatal dopaminergic system. Front Syst Neurosci. 2014;8:110. doi: 10.3389/fnsys.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlert N, Lawrence AD. Brain structure correlates of emotion-based rash impulsivity. NeuroImage. 2015;115:138–146. doi: 10.1016/j.neuroimage.2015.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RL, Fryxell KJ. Sex-specific lateralization of mesocorticolimbic dopamine receptor mRNAs in adolescent mice. Paper presented at the Program No. 180.07. 2015 Neuroscience Meeting Planner, Society for Neuroscience; Chicago. 2015. Online. [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36(Suppl 2):T155–163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation. 2008;11:13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Ferguson MA, Lainhart JE, Anderson JS. An evaluation of the left-brain vs. right-brain hypothesis with resting state functional connectivity magnetic resonance imaging. PloS One. 2013;8:e71275. doi: 10.1371/journal.pone.0071275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. J Cogn Neurosci. 2004;16:363–373. doi: 10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123(Pt 11):2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Porter JN, et al. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev Cogn Neurosci. 2015;11:83–95. doi: 10.1016/j.dcn.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Somerville LH, Kelley WM, Heatherton TF. Rejection sensitivity polarizes striatal-medial prefrontal activity when anticipating social feedback. J Cogn Neurosci. 2013;25:1887–1895. doi: 10.1162/jocn_a_00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane P, Cochran D, Hodge SM, Haselgrove C, Kennedy DN, Frazier JA. Connectivity in Autism: a review of MRI connectivity studies. Harv Rev Psychiatry. 2015;23:223–244. doi: 10.1097/HRP.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. NeuroImage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Finklestein S, Stoll AL, Yutzey DA, Denenberg VH. Neurochemical asymmetries in the albino rat’s cortex, striatum, and nucleus accumbens. Life Sci. 1984;34:1143–1148. doi: 10.1016/0024-3205(84)90085-7. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LH, Murphy RB, Coons EE. Lateralization of striatal dopamine (D2) receptors in normal rats. Neurosci Lett. 1982;33:281–284. doi: 10.1016/0304-3940(82)90385-8. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Crews TM, Vandiver PA. The tridimensional personality questionnaire: reliability and validity studies and derivation of a short form. Psychol Assess. 1995;7:195–208. [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Heller W, Miller GA. Hierarchical brain networks active in approach and avoidance goal pursuit. Front Hum Neurosci. 2013;7:284. doi: 10.3389/fnhum.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN. The fallacy of a “task-negative” network. Front Psychol. 2012;3:145. doi: 10.3389/fpsyg.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. NeuroImage. 2009;47:713–721. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Stoy M, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Strohle A, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. NeuroImage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkotter J. The nucleus accumbens: a target for deep brain stimulation in obsessive–compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Szechtman H. Asymmetrical influence of mesocortical dopamine depletion on stress ulcer development and subcortical dopamine systems in rats: implications for psychopathology. Neuroscience. 1995;65:757–766. doi: 10.1016/0306-4522(94)00531-9. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, et al. Smoking abstinence-induced changes in resting state functional connectivity with ventral striatum predict lapse during a quit attempt. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, et al. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex. 2015;73:80–105. doi: 10.1016/j.cortex.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Tamagaki C, Sedvall GC, Jonsson EG, Okugawa G, Hall H, Pauli S, Agartz I. Altered white matter/gray matter proportions in the striatum of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry. 2005;162:2315–2321. doi: 10.1176/appi.ajp.162.12.2315. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Schwarting RK. Dopaminergic lateralisation in the forebrain: relations to behavioural asymmetries and anxiety in male Wistar rats. NeuropsychoBiology. 2001;43:192–199. doi: 10.1159/000054889. (doi:54889) [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val-Laillet D, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuro-Image. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E, et al. Abnormal Social Reward Responses in Anorexia Nervosa: An fMRI Study. PloS One. 2015;10:e0133539. doi: 10.1371/journal.pone.0133539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wei D, Li W, Qiu J. Individual differences in brain structure and resting-state functional connectivity associated with type A behavior pattern. Neuroscience. 2014;272:217–228. doi: 10.1016/j.neuroscience.2014.04.045. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Reward breaks through center-surround inhibition via anterior insula. Hum Brain Mapp. 2015a doi: 10.1002/hbm.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol Med. 2015b doi: 10.1017/S0033291715001592. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Daw ND, Shohamy D. Generalization of value in reinforcement learning by humans. Eur J Neurosci. 2012;35:1092–1104. doi: 10.1111/j.1460-9568.2012.08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JE, Cao J, Dorris DM, Meitzen J. Genetic sex and the volumes of the caudate-putamen, nucleus accumbens core and shell: original data and a review. Brain Struct Funct. 2016;221:4257–4267. doi: 10.1007/s00429-015-1158-9. [DOI] [PubMed] [Google Scholar]
- Yu J, Tseng P, Hung DL, Wu SW, Juan CH. Brain stimulation improves cognitive control by modulating medial-frontal activity and preSMA-vmPFC functional connectivity. Hum Brain Mapp. 2015;36:4004–4015. doi: 10.1002/hbm.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage. 2012;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect. 2014;4:53–69. doi: 10.1089/brain.2013.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22:99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Li CR. Resting-state functional connectivity of the locus coeruleus in humans: In comparison with the ventral tegmental area/substantia nigra pars compacta and the effects of age. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv172. [DOI] [PMC free article] [PubMed] [Google Scholar]