Abstract
There is accumulating evidence for the existence of a phenotype of isolated cardiac sarcoidosis (ICS), or sarcoidosis that only involves the heart. In the absence of biopsy-confirmed cardiac sarcoidosis (CS), existing diagnostic criteria require the presence of extra-cardiac sarcoidosis as an inclusion criterion for the diagnosis of CS. Consequently, in the absence of a positive endomyocardial biopsy, ICS is not diagnosable by current guidelines. Therefore, there is uncertainty regarding the epidemiology, pathobiology, clinical characteristics, prognosis, and optimal treatment of ICS. This review will summarize the available data related to the prevalence and prognosis of ICS and will discuss challenges surrounding the diagnosis and management of this under-recognized entity.
Keywords: Cardiac sarcoidosis, cardiac PET, cardiac MRI
INTRODUCTION
Sarcoidosis is thought to be a systemic disease characterized by multi-organ infiltration by non-necrotizing granulomas. Sarcoidosis can affect any organ in the body, including the heart.1 The prevalence of cardiac sarcoidosis (CS) among patients with systemic sarcoidosis is 20–27% in the United States2–4 and has been reported to be as high as 58% in Japan.5
There is accumulating evidence for a distinct clinical phenotype characterized by isolated cardiac involvement.6–22 Established criteria for the diagnosis of CS are insensitive for ICS since they require either evidence of extra-cardiac disease or a positive endomyocardial biopsy (EMB), which itself is highly limited in its sensitivity.23,24 Therefore, alternative diagnostic approaches to diagnose ICS would facilitate further investigation into the epidemiology, pathobiology, clinical course, and optimal treatment of ICS. The need for such alternative approaches is underscored by recent data suggesting that patients with ICS may have worse long-term outcomes as compared with patients with systemic sarcoidosis and CS.20 If this is true, then early recognition of ICS and timey initiation of appropriate therapy would be of paramount importance.
This review will focus on 4 key questions related to ICS: 1. How common is it? 2. How does it present clinically? 3. What is the optimal approach to diagnosis?, and 4. What data exist to guide therapy and assess prognosis? Box 1 provides a summary of the current knowledge gaps in these areas. To address these knowledge gaps, we performed a comprehensive search of the literature using the search items “cardiac sarcoidosis” and “isolated cardiac sarcoidosis” and reviewed all relevant results.
Box 1. Summary of current knowledge about ICS.
Epidemiology
As many as 25% of patients with CS may have ICS
Patients with ICS have worse LV systolic function at presentation as compared with patients with systemic sarcoidosis and CS
Diagnosis
The diagnosis of ICS is challenging, in part because the sensitivity of EMB is limited
Current clinical criteria do not provide a means of diagnosing ICS in the absence a positive EMB, even when advanced imaging techniques such as MRI or PET are abnormal
Therapy
ICD therapy is effective at aborting ventricular dysrhythmias in patients with ICS
Prognosis
Patients with ICS have more ventricular arrhythmias compared with patients with systemic sarcoidosis and CS
Patients with ICS may have worse event-free survival compared with patients with systemic sarcoidosis and CS
How Common is ICS?
Because established diagnostic criteria for CS are insensitive for the detection of ICS, the true prevalence of ICS is not known. Reported rates of ICS among patients with CS vary widely, and range from 27 to 54%.8,20–22,25 The frequency of ICS also appears to vary significantly depending on the diagnostic method and clinical presentation. For instance, ICS occurs at a higher frequency when the presentation is with advanced heart failure (52%),22 and at a lower frequency in cases of sudden cardiac death examined post-mortem (29%).25
Tavora et al.25 reviewed all autopsies performed at the Armed Forces Institute of Pathology from 1995 to 2008 on decedents with sudden cardiac death and either a history of, or findings at autopsy consistent with, CS. Of the 46 patients included, 41 had pathologic evidence of CS. Of these, 30 (73%) had no known clinical history of extra-cardiac sarcoidosis (ECS). However, only 12 (29%) were free of ECS on autopsy. Hence, in this autopsy study of patients with sudden death and known or suspected CS, the rate of ICS was 29%.
Kandolin et al.8 studied a series of 74 consecutive patients treated for definite or suspected CS at a single center in Finland from 2000 to 2010. Using EMB, explant pathology at cardiac transplantation, or autopsy as the basis for diagnosing CS, and using clinical evaluation, chest x-ray, and liver enzymes as the basis for excluding ECS, these authors reported a rate of ICS of 33 out of 74 patients (45%). However, 15 of these 33 patients underwent whole-body positron emission tomographic (PET) imaging with F-18 fluorodeoxyglucose (FDG), which demonstrated focal extra-thoracic uptake in 7. If, based on this, it is assumed that the rate of clinically silent but radiographically evident ECS among those initially suspected to have ICS is approximately 50%, then the estimated rate of ICS in this study would fall to approximately 23%.
In a subsequent analysis, Kandolin et al.20 studied all patients with a histologic diagnosis of CS in Finland from 1988 to 2012, a total of 110 patients. The diagnosis of CS in this analysis required either positive cardiac histology (EMB, mediastinal lymph node biopsy, explant pathology at cardiac transplantation, or autopsy) or histologic evidence of ECS plus clinical and imaging findings consistent with CS (e.g., late gadolinium enhancement on cardiac magnetic resonance imaging). The exclusion of ECS was based on clinical evaluation and chest X-ray. The authors reported a rate of ICS of 71 out of 110 patients (65%). However, 30 of these 71 were additionally studied with whole-body FDG PET, and 12 were found to have focal extra-cardiac FDG uptake. If, based on this, it is assumed that the rate of clinically silent but radiographically evident ECS among those initially diagnosed with ICS is approximately 40%, then the estimated rate of ICS in this study would fall to approximately 26%.
Tezuka et al.21 studied 83 consecutive patients with a clinical diagnosis of sarcoidosis treated at a single center in Japan from 1997 to 2013. The diagnosis of CS was based on the 2006 Japanese Ministry of Health and Welfare criteria (JMHW). The method of excluding ECS, which was only described for the 11 patients with ICS, included clinical skin examination and clinical ophthalmologic examination. Of the 83 patients studied, 41 had CS by JMHW criteria. Of these, 11 (27%) had no evidence of ECS. Hence, in this study, the rate of ICS was 27%.
Lastly, Sperry et al.22 reported 27 cases of individuals presenting with advanced heart failure and biopsy-proven CS, usually at the time of heart transplantation. Among these, only 30% met JMHW criteria for CS before cardiac biopsy, and only 48% had known extra-cardiac involvement. Table 1 summarizes the current data regarding the prevalence of ICS.
Table 1.
Summary of selected data on prevalence of ICS
| Study | Population (n) | Method of diagnosing CS (n) | Method of evaluating for ECS (n) | Prevalence of ICS (%)* |
|---|---|---|---|---|
| Kandolin et al.8 | All patients treated for definite or suspected CS at a single center in Finland from 2000–2010 (74) | EMB (17) Explant (4) Autopsy (1) Mediastinal LN biopsy (11) Clinical evaluation (22)** |
Clinical evaluation (74) Chest x-ray (74) Liver enzymes (74) Whole-body PET (21) |
Definite or suspected: 55/74 (74%) Definite: 33/74 (45%) |
| Kandolin et al.20 | All patients receiving a diagnosis of CS based on cardiac or other histology in Finland from 1988–2012 (110) | EMB (55) Explant (6) Autopsy (2) Mediastinal LN biopsy (18) CMR and/or PET (38) Other (9) |
Clinical evaluation (110) Chest x-ray (110) Whole-body PET (39) |
Clinical exclusion of ECS: 71/110 (65%) After whole-body PET exclusion of ECS: 59/100 (54%) |
| Tavora et al.25 | All patients with sudden death and CS at autopsy at AFIP*** from 1995–2008 (41) | Autopsy (41) | Autopsy (41) Clinical history (41) |
After clinical/historical exclusion of ECS: 30/41 (73%) After pathologic exclusion of ECS: 12/41 (29%) |
| Tezuka et al.21 | All patients with a clinical diagnosis of sarcoidosis who were treated at a single center in Japan from 1997–2013 (83) | 2006 JMHW Criteria (41)+ | Clinical evaluation including ophthalmologic and skin examination (11)++ | 11/41 (27%) |
| Sperry et al.22 | Heart failure patients at the Cleveland clinic with CS proven by myocardial biopsy specimen from 2002–2014 (27) | Myocardial biopsies (27) | Clinical history Diagnostic biopsy Imaging findings, including FDG PET (16) |
After clinical/historical exclusion of ECS: 30/41 (73%) |
Prevalence of ICS among patients with CS
Among these 22, 9 had LGE on CMR, 12 had focal FDG uptake on cardiac PET, and 12 had resting perfusion deficits on SPECT. While the number of patients examined with each modality is reported, the number of patients studied with multiple modalities is not. Therefore, at least 12 of these patients had diagnoses of suspected CS based on advanced imaging, but it is not clear whether all 22 were diagnosed with advanced imaging
AFIP, Armed Forces Institute of Pathology, Office of the Armed Forces Medical Examiner, and Office of the Chief Medical Examiner
Revised 2006 Japanese Ministry of Health and Welfare Criteria
Method of excluding ECS was only reported for those with ICS
How Does ICS Present Clinically?
Owing to the lack of consensus on diagnostic criteria for ICS, relatively little is known about its clinical features and if they differ from those of systemic sarcoidosis with CS. However, data from several studies suggest that patients with ICS may present with more advanced heart disease than those with systemic sarcoidosis and CS. In their 2011 study, Kandolin and colleagues found that 82% of patients with ICS had impaired left ventricular (LV) systolic function, defined as a an LV ejection fraction <50%.8 In their subsequent 2015 study, the same authors found that 69% of patients with ICS had impaired LV systolic function, compared with 41% of patients with systemic sarcoidosis and CS, a difference that was statistically significant.20 Similarly, in their 2015 study described above, Tezuka and colleagues found that 82% of patients with ICS had impaired LV systolic function, compared with 50% of patients with systemic sarcoidosis and CS, a difference that was not statistically significant.21 Finally, Tavora et al.25 reported that 10 out of 12 patients (83%) with ICS presented with sudden cardiac death attributable to CS. By comparison, 15 of 29 patients (52%) with systemic sarcoidosis presented with sudden cardiac death attributable to CS.
While these comparisons are mostly underpowered due to the small sizes of the sub-groups compared, these data raise the question of whether patients with ICS are at greater risk for sudden cardiac death than their counterparts who have systemic sarcoidosis with CS. It is unclear if such differences are due to delayed recognition, and/or treatment of ICS vs. systemic sarcoidosis with CS, or if patients with ICS have a distinct clinical course. Lastly, a selection/detection bias effect cannot be excluded as increasing numbers of patients with systemic sarcoidosis are being “screened” for cardiac involvement, whereas ICS will generally come to clinical attention after the onset of cardiac symptoms due complete heart block, sustained ventricular arrhythmias, and/or significant left ventricular systolic dysfunction.
What Is the Optimal Diagnostic Approach to Cases of Suspected ICS?
The diagnosis of ICS is challenging for a number of reasons. First, the diagnosis of CS itself is fraught with difficulty, as the clinical manifestations of CS can be varied and non-specific, and existing diagnostic criteria have suboptimal sensitivity.26 Second, because existing diagnostic criteria for CS require either positive EMB or evidence of ECS, the diagnosis of ICS in effect relies on EMB. However, the sensitivity of EMB for the diagnosis of CS is suboptimal at approximately 20–40%, which may in part be due to the patchy nature of the disease.8,21,23,24 Therefore, alternative methods for diagnosing ICS are needed.
Nery and colleagues reported an illustrative case of EMB guided by electro-anatomical mapping in the workup of suspected ICS.13 In this case, the patient fulfilled clinical criteria for arrhythmogenic right ventricular cardiomyopathy, but there was strong suspicion for CS based on intermittent heart block. An initial EMB was inconclusive. A repeat EMB was guided by electro-anatomical mapping, with samples taken from low-voltage regions in the right ventricular septum. This revealed the classic non-caseating granulomas of sarcoidosis. While such an approach would likely improve the diagnostic yield of EMB, it may not be appropriate for all cases of suspected ICS. First, not all patients may demonstrate low-voltage regions accessible from the right heart. Second, not all areas of low voltage may represent the active inflammatory phase of CS, and biopsies may be inconclusive in cases of non-specific fibrosis.
While mediastinal lymph node involvement would not be consistent with a strict definition of ICS, it is instructive to consider the diagnostic yield of lymph node (LN) biopsy in sarcoidosis that is limited to the mediastinum. Otsuka and colleagues reported the results of chest computed tomographic (CT) imaging in a series of 8 patients who underwent left ventriculoplasty for idiopathic dilated cardiomyopathy and were found to have evidence of CS on pathologic inspection of the resected myocardium.27 On retrospective review of the CT imaging, all 8 patients had enlarged mediastinal LN. In their 2011 study, Kandolin et al.8 reported the results of mediastinal LN biopsies in 12 patients with suspected CS. Only-FDG positive nodes were sampled, which is salient in that lymph node enlargement may be due either to granulomatous infiltration or to heart failure physiology itself. Among the 12 biopsies, 11 had positive histology for sarcoidosis. Notably, 10 of these patients had negative EMB and 1 did not have EMB performed.
Despite potential strategies for improving the diagnostic yield of biopsies in the evaluation of suspected ICS, these procedures are invasive and the risk to benefit ratio remains undefined. On the other hand, advanced cardiac imaging, including cardiac magnetic resonance imaging (CMR) and cardiac PET, may provide non-invasive and sensitive techniques for the evaluation of suspected ICS.
Late gadolinium enhancement (LGE) on CMR has been well described as an imaging biomarker for myocardial fibrosis. In the 2011 study by Kandolin and colleagues described above, 16 of the 32 patients with ICS underwent CMR. Using EMB, explant pathology at cardiac transplantation, or autopsy as the reference standard, the presence of LGE in 15 of these patients corresponded to a sensitivity of approximately 94% for the diagnosis of ICS.8 In the subsequent 2015 study by Kandolin et al.,20 38 of the 71 patients with ICS underwent CMR. The presence of LGE in 36 of these patients corresponded to a sensitivity of approximately 95% for the diagnosis of ICS. However, given the dynamic nature of CS, with both active inflammatory and chronic fibrotic phases, LGE may be less sensitive for the detection of early inflammatory disease.
Cardiac PET, on the other hand, may be more useful in the detection of the active inflammatory phase of CS. The combination of both perfusion PET with either rubidium-82 or N-13-ammonia and FDG PET allows differentiation of the spectrum of CS. Either perfusion or FDG PET findings, or both, may be present in the active inflammatory phase of CS and include patterns of focal, focal-on-diffuse, or diffuse FDG uptake, with or without perfusion defects.28–31 In the 2011 study by Kandolin and co-workers, 25 of 32 patients with ICS underwent cardiac PET imaging. The presence of focal FDG uptake with perfusion defects in 20 of these patients corresponded to a sensitivity of approximately 80% for the diagnosis of ICS.8 In their subsequent 2015 study, 46 of 71 patients with ICS underwent cardiac PET. The presence of focal FDG uptake in 34 of these patients corresponded to a sensitivity of approximately 74% for the diagnosis of ICS.20
The combined use of CMR and PET in the evaluation of suspected ICS offers complementary data31–33 and may be particularly attractive given the fact that each imaging technique has different strengths and limitations.30 When technically feasible, such an approach enables imaging of perfusion, inflammation, and fibrosis, while also allowing for the identification of extra-cardiac disease. As a result, combining CMR and PET imaging likely provides the highest diagnostic accuracy for detecting ICS.
Finally, the optimal method of excluding ECS in cases of suspected ICS has not been studied. An approach based solely on clinical history, physical examination, and laboratory testing will miss a significant amount of clinically silent but radiographically evident ECS. As described above, in both the 2011 and 2015 studies by Kandolin and co-workers, at least 40% of patients with suspected ICS undergoing whole-body PET were found to have clinically silent but radiographically evident ECS.8,20 Hence, an approach involving whole-body imaging is likely to provide the most reliable exclusion of ECS in the evaluation of suspected ICS. Importantly, ECS may develop after initial cardiac involvement in cases of apparent ICS. Adamson and co-workers reported a series of 18 patients treated for CS at a single center in New Zealand from 2005 to 2013. Among 3 patients initially thought to have ICS, 2 subsequently developed evidence of ECS. The time interval from initial negative assessment to the development of evidence of ECS was not reported.15 Table 2 summarizes the current evidence regarding the diagnosis of ICS.
Table 2.
Summary of selected data on the diagnosis of ICS
| Study | Population | Reference Standard | Sensitivity |
|---|---|---|---|
| Endomyocardial biopsy | |||
| Kandolin et al.8 | 33 patients with histologically proven CS and no clinical evidence of ECS; 31 underwent EMB | Subsequent biopsy, explant pathology, or autopsy | 11/31 or 34% |
| Tezuka et al.21 | 11 patients with a clinical diagnosis of ICS; 5 underwent EMB | Clinical suspicion for and imaging features consistent with CS | 2/5 or 40% |
| LGE on CMR | |||
| Kandolin et al.8 | 33 patients with histologically proven CS and no clinical evidence of ECS; 16 underwent CMR | EMB, explant pathology, or autopsy | 15/16 or 94% |
| Kandolin et al.20 | 71 patients with a clinical diagnosis of ICS; 38 underwent CMR | EMB, explant pathology, or autopsy | 36/38 or 95% |
| Focal FDG uptake on cardiac PET | |||
| Kandolin et al.8 | 33 patients with histologically proven CS and no clinical evidence of ECS; 25 underwent cardiac PET | EMB, explant pathology, or autopsy | 20/25 or 80% |
| Kandolin et al.20 | 71 patients with a clinical diagnosis of ICS; 46 underwent cardiac PET | EMB, explant pathology, or autopsy | 34/46 or 74% |
| Mediastinal lymphadenopathy on chest CT | |||
| Ostuka 2007 | 8 patients who underwent cardiac transplant for DCM with evidence of CS on explant pathology; all 8 underwent chest CT | Explant pathology | 7/8 or 88% |
What Data Exist to Help Assess Prognosis and Guide Therapy in ICS?
The complications of ICS are similar to those of systemic sarcoidosis with CS and include ventricular arrhythmias, heart failure, and conduction system disease.8
Patients with ICS have a risk of ventricular tachycardia (VT) that is at least as high as, and possibly greater than, the risk of VT among patients with systemic sarcoidosis and CS. In their 2011 study, Kandolin and co-workers reported that 36% of patients with ICS presented with VT and 6% presented with ventricular fibrillation.8 In their subsequent 2015 study, these authors reported that 38% of patients with ICS presented with VT, compared with 23% of patients with systemic sarcoidosis and CS; the statistical significance of this difference was not reported.20
Among patients with ICS, the benefit of device therapy with implantable cardioverter defibrillators (ICD) for primary prevention of VT appears to be greater than that among patients with systemic sarcoidosis with CS. Kron and co-workers studied 235 patients with CS who had undergone placement of an ICD.32 Among these, 15 had suspected or definite ICS. Over 4 years of follow-up, 9 of these 15 patients (69%) received appropriate device therapy (anti-tachycardia pacing or defibrillation), compared with 75 of 222 patients (34%) with systemic sarcoidosis and CS, a difference that was statistically significant. This again suggests that VT may occur more commonly in patients with ICS as compared with systemic sarcoidosis and CS, and that device therapy may be appropriate and effective in patients with ICS at risk for ventricular dysrhythmias.
The optimal approach to the medical management of heart failure associated with ICS has not been studied. Patients with systemic sarcoidosis and CS who are treated with prednisone may experience improvements in LV systolic function, especially those with mild to moderate dysfunction (e.g., LV ejection fraction of 30–55%).33,34 While changes in LV systolic function in patients with ICS receiving immunosuppressive therapy have not been separately reported, it would be reasonable to expect a similar benefit in such individuals.
In end-stage heart failure associated with ICS, cardiac transplantation may be a reasonable option. Chang and co-workers reviewed 411 cardiac transplantations at a single center in Taiwan from 1988 to 2011.9 Explanted hearts from 5 patients demonstrated pathology consistent with CS. None of these 5 patients had historical or clinical evidence of ECS; hence at least some may have had ICS. During up to 8 years of follow-up, none of these 5 patients demonstrated recurrent CS, clinically or on routine surveillance EMB, and none died. Given the small number of patients studied, these data should be interpreted with caution, but they suggest that when indicated, cardiac transplantation for end-stage heart failure associated with ICS may be appropriate. This observation is notwithstanding the limitation that in clinical practice the diagnosis of ICS in such cases may only be recognized following explantation. Moreover, it is noteworthy that recurrence of CS has rarely been reported following heart transplantation.35-38
Data regarding overall prognosis and long-term survival of ICS vs. systemic sarcoidosis with CS are summarized in Table 3.
Table 3.
Summary of selected data on prognosis in ICS
| Study | Population (n) | Follow-up | Outcomes | ICS vs systemic sarcoidosis with CS |
|---|---|---|---|---|
| Kandolin et al.20 | All patients receiving a histologic diagnosis of CS in Finland from 1988–2012 (110) | Median of 79 months, range of 12–303 months | Survival free of cardiac transplantation or aborted sudden death | 1/71 in the ICS group vs 4/39 in the systemic sarcoidosis and CS group (log rank P = 0.005) |
| Kron et al.34 | Patients with CS and ICD implantation at multiple centers (time period not specified) (235) | Questionnaire mailed at a single time point | Appropriate device therapies (ATP or defibrillation) | 9/13 in the ICS group vs 75/222 in the systemic sarcoidosis and CS group (P = 0.015) |
| Tezuka et al.21 | All patients with a clinical diagnosis of sarcoidosis who were treated at a single center in Japan from 1997 to 2013 (83) | Mean of 18 months forICS and 14 months for SCS | Survival | Reported as no difference; specific values not reported |
DISCUSSION
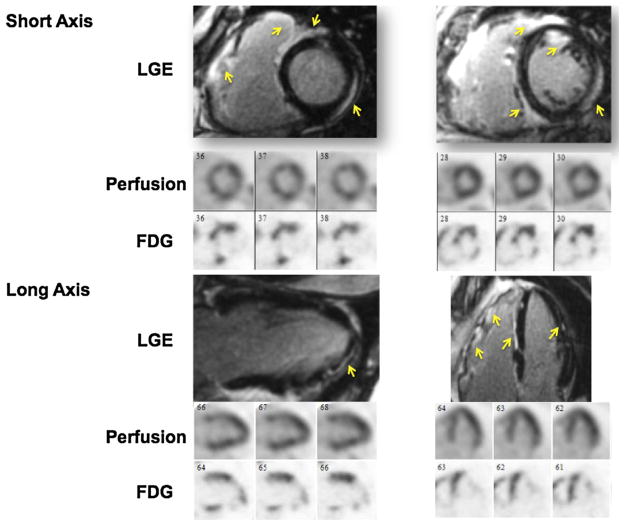
Based on our review of the available data, the rate of ICS among patients with CS appears to be approximately 25%. In addition, patients with ICS appear to have worse LV systolic function at presentation and a higher rate of incident ventricular tachycardia as compared with patients with systemic sarcoidosis with CS. Because of limitations in current diagnostic criteria, there is an important role for advanced cardiac imaging techniques in the evaluation of patients with suspected ICS. A combined approach using FDG PET and CMR may provide complementary data on both inflammation and scar or fibrosis, respectively (Figure 1). In addition to improved detection of earlier stages of ICS, a combined approach may provide enhanced diagnostic certainty and improved accuracy.39–41 Finally, by utilizing whole-body imaging, FDG PET may be a useful technique for screening for extra-cardiac involvement in cases of suspected ICS.
Figure 1.
Example of the complementary value of CMR and cardiac PET imaging in detecting cardiac sarcoidosis. A 61-year-old male presented with systolic heart failure of unknown etiology. CMR showed late gadolinium enhancement in the basal and mid anterior, anteroseptal, and inferoseptal segments that extended into the right ventricle. Myocardial perfusion imaging showed mild perfusion defects in the mid inferoseptal and the mid anterior segments. There was FDG uptake involving the basal to mid anterior and anteroseptal, basal inferior and apical septal segments. In this case, the combined information provided by PET and CMR can enhance the diagnostic certainty for diagnosing cardiac sarcoidosis, as subsequently was also confirmed via biopsy.
Challenges Related to Management of ICS
A challenging clinical scenario occurs when a patient has a clinical presentation and cardiac imaging consistent with CS, but no evidence of ECS, and the results of EMB—if performed—are negative or inconclusive. In such cases, it is helpful to establish the likelihood of CS by integrating all clinical and imaging data. If the likelihood is >50% and significant inflammation by cardiac PET is present, a trial of immunosuppressive therapies may be reasonable. Importantly, myocardial inflammation should be conclusively demonstrated in such cases, as opposed to cases where non-specific FDG uptake or failure to suppress physiologic FDG uptake from the normal myocardium is present.42
In keeping with the 2014 expert consensus statement on cardiac sarcoidosis from the Heart Rhythm Society, reduction in FDG uptake upon treatment could serve as a potential criterion to support the diagnosis of ICS.43 Nevertheless, it is important to acknowledge that FDG uptake may be due to other conditions, including failure to suppress physiologic glucose uptake by normal myocardium, inflammatory myopathies associated with rheumatologic disorders and some subtypes of myocarditis.
Efforts to enhance clinical detection of ICS and to aid in the management of suspected ICS may benefit from guidelines for categorizing the likelihood of CS as probable (>50%) or highly probable (>90%). This approach is exemplified by the World Association of Sarcoidosis and Other Granulomatous Disorders Organ Assessment Instrument,44 which establishes categories to assess the probability of individual organ involvement in patients with sarcoidosis.
Areas for Future Work
An important step towards further investigation in the area of ICS will be the development of improved diagnostic methods for both the detection of CS and the exclusion of ECS. Along with the important role of CMR and cardiac PET in the detection of ICS, radio-tracers targeting inflammatory cells such as F-18 fluorothymidine (FLT)45 and Ga-68 DOTATOC46 may provide additional and complementary information. Improved detection and diagnosis may facilitate a deeper understanding of the pathobiology, epidemiology, clinical characteristics, and prognosis of ICS. Given that ICS patients appear to present with more advanced left ventricular systolic dysfunction and may be at higher risk for the development of ventricular tachycardia, another important area of inquiry will be the role of early anti-inflammatory therapy and primary prevention device therapy in ICS. Box 2 lists selected questions that may represent opportunities for future research in ICS.
Box 2. Summary of key questions relating to ICS.
Epidemiology
Do ICS and systemic sarcoidosis with CS occur with similar frequencies in male and female patients, in different age groups, in different racial and ethnic groups, and in different geographic locations?
Diagnosis
What is the optimal strategy for utilizing advanced cardiac imaging techniques in the workup of suspected ICS?
What is the optimal method of excluding extra-cardiac sarcoidosis?
Therapy
Which ICS patients should receive steroid therapy; when is the optimal time to initiate steroid therapy; and what is the best strategy for monitoring response to steroid therapy?
Which ICS patients should undergo device therapy with ICD placement?
Prognosis
What are the differences in long-term outcomes between ICS and systemic sarcoidosis with CS, and can medical and device therapy improve event-free survival?
Supplementary Material
Abbreviations
- CS
Cardiac sarcoidosis
- ICS
Isolated cardiac sarcoidosis
- ECS
Extra-cardiac sarcoidosis
- EMB
Endomyocardial biopsy
- FDG
Fluorodeoxyglucose
- LGE
Late gadolinium enhancement
- PET
Positron emission tomography
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12350-016-0658-1) contains supplementary material, which is available to authorized users.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on http://SpringerLink.com.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine. 1952;31:1–132. [PubMed] [Google Scholar]
- 3.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 4.Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest. 1993;103:253–8. doi: 10.1378/chest.103.1.253. [DOI] [PubMed] [Google Scholar]
- 5.Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, Homma AH, Mikami R, Hongo O, Hiraga Y, Yamamoto M. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–69. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown ML, Reeder G, Unni KK, Mullany C. Intraoperative diagnosis of isolated cardiac sarcoid. Heart Lung Circ. 2007;16:315–7. doi: 10.1016/j.hlc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 7.White J, Sutton T, Kerr A. Isolated primary cardiac sarcoidosis: MRI diagnosis and monitoring of treatment response with cardiac enzymes. Circ Heart Fail. 2010;3:e28–9. doi: 10.1161/CIRCHEARTFAILURE.110.939686. [DOI] [PubMed] [Google Scholar]
- 8.Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivisto SM, Kupari M. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–8. doi: 10.1111/j.1365-2796.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang TI, Chi NH, Chou NK, Tsao CI, Yu HY, Chen YS, Wang SS. Isolated cardiac sarcoidosis in heart transplantation. Transpl Proc. 2012;44:903–6. doi: 10.1016/j.transproceed.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 10.Tsai JH, Chou NK, Wang SS, Shun CT. Isolated cardiac sarcoidosis: case experience in heart transplantation. J Formos Med Assoc. 2013;112:499–500. doi: 10.1016/j.jfma.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Chinitz JS, Inra LA, Stein KM, Weinsaft JW. Isolated cardiac sarcoid in a patient with unexplained syncope. J Cardiovasc Electrophysiol. 2010;21:333. doi: 10.1111/j.1540-8167.2009.01630.x. [DOI] [PubMed] [Google Scholar]
- 12.Mc Ardle BA, Leung E, Ohira H, Cocker MS, deKemp RA, DaSilva J, Birnie D, Beanlands RS, Nery PB. The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:297–306. doi: 10.1007/s12350-012-9668-9. [DOI] [PubMed] [Google Scholar]
- 13.Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated cardiac sarcoidosis: Establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol. 1015;29:e1–3. doi: 10.1016/j.cjca.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Sugizaki Y, Tanaka H, Imanishi J, Konishi A, Yamashita T, Shinke T, Ishida T, Kawai H, Hirata K. Isolated primary cardiac sarcoidosis presenting as acute heart failure. Intern Med. 2013;52:71–4. doi: 10.2169/internalmedicine.52.8470. [DOI] [PubMed] [Google Scholar]
- 15.Adamson P, Melton I, O’Donnell J, MacDonald S, Crozier I. Cardiac sarcoidosis: The Christchurch experience. Intern Med J. 2014;44:70–6. doi: 10.1111/imj.12314. [DOI] [PubMed] [Google Scholar]
- 16.Nery PB, Mc Ardle BA, Redpath CJ, Leung E, Lemery R, Dekemp R, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014;37:364–74. doi: 10.1111/pace.12277. [DOI] [PubMed] [Google Scholar]
- 17.Galati G, Leone O, Rapezzi C. The difficult diagnosis of isolated cardiac sarcoidosis: Usefulness of an integrated MRI and PET approach. Heart. 2014;100:89–90. doi: 10.1136/heartjnl-2013-304237. [DOI] [PubMed] [Google Scholar]
- 18.Vakil K, Minami E, Fishbein DP. Right ventricular sarcoidosis: Is it time for updated diagnostic criteria? Tex Heart Inst J. 2014;41:203–7. doi: 10.14503/THIJ-12-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehl NF, Maeder MT, Joerg L. Isolated cardiac sarcoidosis: Critical role of cardiac MRI for diagnosis and management. Eur Heart J. 2015;36:3434. doi: 10.1093/eurheartj/ehu513. [DOI] [PubMed] [Google Scholar]
- 20.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietila-Effati P, Utrianen S, Kupari M. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–32. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 21.Tezuka D, Terashima M, Kato Y, Toriihara A, Hirasawa K, Sasaoka T, Yoshikawa S, Maejima Y, Ashikaga T, Suzuki J, Hirao K, Isobe M. Clinical characteristics of definite or suspected isolated cardiac sarcoidosis: Application of cardiac magnetic resonance imaging and 18F-Fluoro-2-deoxyglucose positron-emission tomography/computerized tomography. J Card Fail. 2015;21:313–22. doi: 10.1016/j.cardfail.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Sperry BW, Oldan J, Hachamovitch R, Tamarappoo BK. Insights into biopsy-proven cardiac sarcoidosis in patients with heart failure. J Heart Lung Transpl. 2016;35:392–3. doi: 10.1016/j.healun.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R American Heart A, American College of C and European Society of C. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 24.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 25.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104:571–7. doi: 10.1016/j.amjcard.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 26.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsuka K, Terasaki F, Eishi Y, Shimomura H, Ogura Y, Horii T, Isomura T, Suma H, Kitaura Y. Cardiac sarcoidosis underlies idiopathic dilated cardiomyopathy: Importance of mediastinal lymphadenopathy in differential diagnosis. Circ J. 2007;71:1937–41. doi: 10.1253/circj.71.1937. [DOI] [PubMed] [Google Scholar]
- 28.Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, Ito N, Ohira H, Ikeda D, Tamaki N, Nishimura M. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–43. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 29.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867. doi: 10.1161/CIRCIMAGING.113.000867. [DOI] [PubMed] [Google Scholar]
- 31.Soussan M, Brillet PY, Nunes H, Pop G, Ouvrier MJ, Naggara N, Valeyre D, Weinmann P. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:120–7. doi: 10.1007/s12350-012-9653-3. [DOI] [PubMed] [Google Scholar]
- 32.Orii M, Hirata K, Tanimoto T, Ota S, Shiono Y, Yamano T, Matsuo Y, Ino Y, Yamaguchi T, Kubo T, Tanaka A, Akasaka T. Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complet heart block. Heart Rhythm. 2015;12:2477–85. doi: 10.1016/j.hrthm.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Ohira H, Birnie DH, Pena E, Bernick J, Mc Ardle B, Leung E, et al. Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2016;43:259–69. doi: 10.1007/s00259-015-3181-8. [DOI] [PubMed] [Google Scholar]
- 34.Kron J, Sauer W, Mueller G, Schuller J, Bogun F, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, Greenspon AJ, Ortman M, Delurgio DB, Valadri R, Narasimhan C, Swapna N, Singh JP, Danik S, Markowitz SM, Almquist AK, Krahn AD, Wolfe LG, Feinstein S, Ellenbogen KA, Crawford T. Outcomes of patients with definite and suspected isolated cardiac sarcoidosis treated with an implantable cardiac defibrillator. J Interv Card Electrophysiol. 2015;43:55–64. doi: 10.1007/s10840-015-9978-3. [DOI] [PubMed] [Google Scholar]
- 35.Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–6. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 36.Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–74. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 37.Oni AA, Hershberger RE, Norman DJ, Ray J, Hovaguimian H, Cobanoglu AM, Hosenpud JD. Recurrence of sarcoidosis in a cardiac allograft: Control with augmented corticosteroids. J Heart Lung Transpl. 1992;11:367–9. [PubMed] [Google Scholar]
- 38.Yager JE, Hernandez AF, Steenbergen C, Persing B, Russell SD, Milano C, Felker GM. Recurrence of cardiac sarcoidosis in a heart transplant recipient. J Heart Lung Transpl. 2005;24:1988–90. doi: 10.1016/j.healun.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Luk A, Lee A, Ahn E, Soor GS, Ross HJ, Butany J. Cardiac sarcoidosis: Recurrent disease in a heart transplant patient following pulmonary tuberculosis infection. Can J Cardiol. 2010;26:e273–5. doi: 10.1016/s0828-282x(10)70424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osborne M, Kolli S, Padera RF, Naya M, Lewis E, Dorbala S, Di Carli MF, Blankstein R. Use of multimodality imaging to diagnose cardiac sarcoidosis as well as identify recurrence following heart transplantation. J Nucl Cardiol. 2013;20:310–2. doi: 10.1007/s12350-013-9677-3. [DOI] [PubMed] [Google Scholar]
- 41.Tung R, Bauer B, Schelbert H, Lynch JP, 3rd, Auerbach M, Gupta P, Schiepers C, Chan S, Ferris J, Barrio M, Ajijola O, Bradfield J, Shivkumar K. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–98. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osborne MT, Hulten EA, Murthy VL, Skali H, Taqueti VR, Dorbala S, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2016 doi: 10.1007/s12350-016-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 44.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, Sweiss N, Crouser E, Morgenthau AS, Lower EE, Azuma A, Ishihara M, Morimoto S, Tetsuo Yamaguchi T, Shijubo N, Grutters JC, Rosenbach M, Li HP, Rottoli P, Inoue Y, Prasse A, Baughman RP Organ Assessment Instrument Investigators TW. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 45.Norikane T, Yamamoto Y, Maeda Y, Noma T, Nishiyama Y. 18F-FLT PET imaging in a patient with sarcoidosis with cardiac involvement. Clin Nucl Med. 2015;40:433–4. doi: 10.1097/RLU.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 46.Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J. 2015;36:2404. doi: 10.1093/eurheartj/ehv278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.