Abstract
Among John Saunders’ many seminal contributions to developmental biology, his discovery of the limb ‘zone of polarizing activity’ (ZPA) is arguably one of the most memorable and ground-breaking. This discovery introduced the limb as a premier model for understanding developmental patterning and promoted the concept of patterning by a morphogen gradient. In the 50 years since the discovery of the ZPA, Sonic hedgehog (Shh) has been identified as the ZPA factor and the basic components of the signaling pathway and many aspects of its regulation have been elucidated. Although much has also been learned about how it regulates growth, the mechanism by which Shh patterns the limb, how it acts to instruct digit ‘identity’, nevertheless remains an enigma. This review focuses on what has been learned about Shh function in the limb and the outstanding puzzles that remain to be solved.
Keywords: ZPA, Sonic hedgehog, limb development, digit patterning
I. Introduction
The limb has served as a major and enduring model for studying developmental patterning and morphogenesis, partly owing to the seminal contributions of John Saunders that identified early signaling centers and highlighted its utility as a model amenable to experimental manipulation in the chick. The paired limbs in tetrapod vertebrates share a common basic blueprint, comprised of three major proximo-distal (PD) components: single stylopod (upper arm humerus in forelimb), paired zeugopod (radius and ulna in forearm), and multiple autopod (hand plate) elements (Fig. 1). Much attention has focused on the autopod elements, ranging from one to five digits in extant tetrapods with distinct anterior-posterior (AP) morphologies from digit 1 (thumb) to digit 5 (pinky). AP patterning of distinct digit ‘identities’, initiated by ZPA signaling, is a major target of evolutionary functional adaptations and, being a bony structure readily appreciated in the fossil record, the limb has consequently become a preeminent model for evo-devo studies (Schneider and Shubin, 2013; Zuniga, 2015). With the identification of Shh as the endogenous ZPA signaling molecule, digit patterning has also become a model system for uncovering the normal function of Hedgehog (Hh) signaling factors, which play key roles in multiple developmental processes and in adult homeostasis and disease, including neoplasia (Alman, 2015; Briscoe and Therond, 2013; Petrova and Joyner, 2014).
Fig 1.
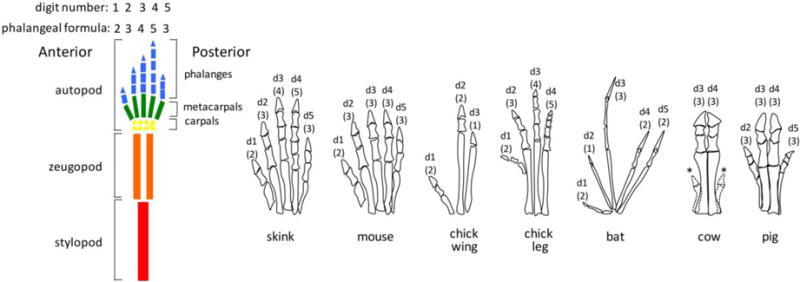
Morphologic features of basal pentadactyl limb and digit adaptations in chick, lizard and several mammalian species in which limb patterning has been examined. Diagram of basal pentadactyl limb components showing digits 1 through 5, from anterior to posterior, having phalangeal formulas of 2-3-4-5-3. Schematics of autopod morphology for the different species shown are not drawn to same scale. Numbers indicate digit type (d1–d5) and numbers in parentheses indicate phalangeal formulas. * indicates vestigial digits (dew claws).
In the 20+ years since the identification of Shh, great strides have been made in understanding the cellular mechanics of Hh transduction and transport, including the discovery of cilia (Goetz and Anderson, 2010; Huangfu and Anderson, 2006; Sasai and Briscoe, 2012) and long filopodia for secretion that may obviate the need for diffusion in long-range signaling (Sanders et al., 2013). Major advances in uncovering the regulation of ZPA-selective Shh expression by remote enhancers and the factors involved (Lettice et al., 2003; Lettice et al., 2012) have also been achieved. Yet, how Shh signaling acts to regulate digit identity is still largely a mystery. This review focuses on recent insights and remaining questions on how Shh instructs digit pattern.
II. Historical overview: from ZPA and the morphogen concept to Shh
Among John Saunders’ major contributions to the field of limb development were the discovery and characterization of the two major signaling centers that orchestrate early limb bud patterning and growth: the apical ectodermal ridge (AER), a specialized columnar ectoderm running along the distal limb bud margin and source of Fgf signals critical for PD outgrowth (reviewed by Verheyden and Sun in this special issue), and a functionally-defined region of mesoderm in the posterior-distal limb bud that became known as the ZPA (Saunders, 1948; Saunders and Gasseling, 1968). In the course of grafting experiments to investigate the basis for a posterior necrotic zone (PNZ) present in mesoderm along the posterior margin of the chick limb bud, John Saunders made the unexpected discovery that grafts of the presumptive PNZ area under the AER could induce mirror-image digit duplications in the host limb bud (Fig. 2A) with inverted AP polarity (posterior-most induced digits always nearest to the graft) (Saunders and Gasseling, 1968). Because of these attributes, Saunders named it the “Zone of Polarizing Activity” (Balcuns et al., 1970). Interestingly, Saunders also noted that the AER immediately overlying these ZPA grafts became attenuated, suggesting a negative regulatory interaction between these signaling centers; an important point that was not followed up on until much later (see section VIII below).
Fig 2.
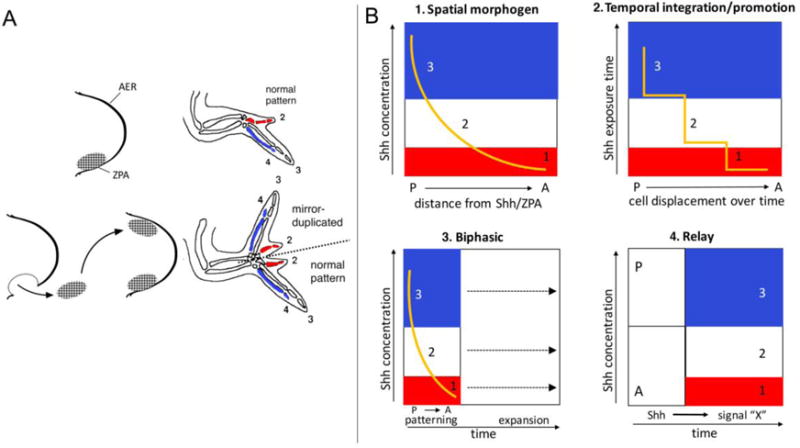
Saunders’ ZPA grafting experiment and models for Shh function in patterning. A. grafting ZPA region to the anterior margin of another limb bud results in mirror-image duplicated digits. Note that digit types in chicken wing are traditionally assigned as 2, 3, 4 (as shown in A), while recent studies attribute them to 1, 2, 3 (Towers et al., 2011) (as used in B). Color coding indicates specification of particular digit identity by exposure to either different levels or duration of Shh signals, as indicated in graphs in B. B. Graphs B1–B4 represent different models for specification of AP digit types by Shh signaling. Blue area represents the concentration or temporal range of Shh (orange line) required to specify the most posterior digit (digit 3 in these graphs), white the middle digit (digit 2), and red the most anterior digit (digit 1). B1. Spatial morphogen model: Digits are specified by different Shh signal levels that decline with increasing distance from the source (ZPA). B2. Temporal integration/promotion model: Digits are specified by different durations of Shh exposure, determined by growth and displacement away from short-range signaling near the source (ZPA). With increasing exposure duration to Shh, digit progenitors may be sequentially promoted (from digit 1 to 2 to 3 identity). B3. Biphasic model: Digit progenitors are specified early by very transient Shh exposure (early phase), possibly by a spatial Shh concentration gradient (shown), or by a relay mechanism (as in B4). Expansion of these specified progenitor ‘zones’ requires sustained Shh exposure over the entire duration of its expression to produce all digits (late phase). B4. Relay model: The relay graph shows one hypothetical example of a possible relay mechanism, in which high (P) or low (A) Shh levels in the early limb bud differentially induce secondary target relay signals (e.g. signal “X”) that specify different digit types downstream of Shh, or may act in conjunction with Shh.
Saunders’ discovery was a major milestone in developmental biology that fueled interest in digit AP patterning as a model for morphogen function and the ZPA factor as a likely candidate to act as a long-range signal in a graded distribution. Lewis Wolpert proposed that ZPA signaling provides positional information to responding cells in a concentration-dependent manner (Wolpert, 1969). Wolpert’s morphogen gradient model became known as the French flag model, with the 3 color zones representing different AP concentration-thresholds of the diffusible posterior ZPA morphogen providing positional information that patterns digit progenitors to form morphologically distinct digits along the limb AP axis (Fig. 2B-1). This model gained further support from work showing dosage-dependent effects by grafting different ZPA cell numbers (Tickle, 1981; Tickle et al., 1975). The identification of other embryonic tissues with ZPA-like activity when grafted in the limb, including the node/organizer and notochord, further suggested that a common morphogen signal might polarize the primary embryo axis, the dorsoventral neural tube, and the limb AP axis (Hornbruch and Wolpert, 1986; Saunders and Gasseling, 1983; Wagner et al., 1990), highlighting the utility of the limb as a general model for understanding organ morphogenesis.
Early screens for small diffusible molecules that might act as the ZPA signal led to the discovery of Retinoic acid (RA) as a promising candidate able to induce mirror-image digit duplications in a concentration-dependent manner (Tickle et al., 1982), but was later found to act by inducing a new ZPA in host tissue (Noji et al., 1991; Wanek et al., 1991). Subsequent efforts focused on identifying homologs of Drosophila developmental control genes encoding secreted factors involved in regulating cell fate and morphogenesis. Among Hh homologs identified in several vertebrates (Chang et al., 1994; Echelard et al., 1993; Krauss et al., 1993; Riddle et al., 1993; Roelink et al., 1994), Shh was expressed in several tissues with organizer and ZPA-like activity (including posterior limb bud), and was induced by RA. Furthermore, Shh protein-loaded beads or Shh-expressing cell pellets both recapitulated digit duplication phenotypes in a dosage-dependent manner when placed in anterior limb bud (Chang et al., 1994; Riddle et al., 1993; Yang et al., 1997), and substituted for endogenous ZPA activity in place of posterior mesoderm in the chick limb (Ros et al., 2003). Molecular genetic studies in mouse have likewise confirmed the critical role of Shh in regulating digit number and pattern, and uncovered a major role for downstream target de-repression in digit formation discussed below (Chiang et al., 2001; Krauss et al., 1993; Litingtung et al., 2002; te Welscher et al., 2002b).
III. Shh pathway and the role of the Gli3 effector in the limb
A large body of work in both Drosophila and several vertebrates has illuminated the basic signal transduction components critical for Hh pathway activation (reviewed by (Briscoe and Therond, 2013; Ingham et al., 2011; Varjosalo and Taipale, 2008)). In brief, binding of Hh ligands to Patched receptors (Ptch1 is the major mouse receptor) releases the transmembrane protein Smoothened (Smo) from tonic inhibition by Ptch. Through a series of steps modulating several kinases, Smo activation blocks the proteolysis of the nuclear Hh transcriptional effectors Gli2 and Gli3, thereby preventing conversion of full length Hh target activators (Gli2A, Gli3A) to truncated transcriptional repressors (Gli2R, Gli3R). Since Shh activity blocks the production of Gli3R, Shh and Gli3R form opposing gradients across the limb bud AP axis (Wang et al., 2000). Although pathway components and regulation are remarkably conserved between Drosophila and vertebrates, in vertebrates the Shh-Ptch1 receptor interaction and downstream events including Gli2/3 processing all take place in primary cilia (reviewed by (Goetz and Anderson, 2010; Huangfu and Anderson, 2006)).
Both the mouse Shh knockout (Chiang et al., 2001; Kraus et al., 2001) and a chick Shh null mutant (Ros et al., 2003) display long-bone limb abnormalities; strikingly, autopod formation is virtually absent in the forelimb, and reduced to a single dysmorphic digit in the hindlimb, which has been interpreted as digit 1 based on marker gene expression (Chiang et al., 2001). Of the 2 nuclear Hh transducers, Gli3 has the strongest repressor activity and Gli2 is the strongest activator (Bai and Joyner, 2001; Sasaki et al., 1999). Gli1 is not regulated by proteolysis, but is itself a direct transcriptional Hh target as well as a constitutive activator of Hh-targets (Bai et al., 2002; Sasaki et al., 1999). Gli1 is largely dispensable for normal limb development, although subtle digit phenotypes occur in Gli1 null mutants in the context of other pathway mutations (Park et al., 2000). Mouse mutant analyses have demonstrated that Gli3 plays the major role in the limb, whereas Gli2 plays a minor role revealed in compound knock-outs (Bowers et al., 2012; Hui and Joyner, 1993; Mo et al., 1997). Loss of Gli3 results in polydactyly and a partial loss of distinctive AP pattern, suggesting that a Gli3R gradient may both restrain digit number and regulate digit AP polarity, corroborated by analysis of Gli3R protein levels across limb buds in chick and mouse (Wang et al., 2000). Furthermore, Gli3 is epistatic to Shh. The Shh;Gli3 compound knockout has a polydactylous limb phenotype identical to the Gli3 mutant alone, indicating that the major role of Shh in the autopod is to modulate Gli3R formation (Litingtung et al., 2002; te Welscher et al., 2002b). Whether a GliR gradient alone suffices to impart AP polarity, or graded GliA contributes as well, remains an open question. To definitively settle this issue will require the selective removal of Gli2A, Gli3A without perturbing graded GliR production. The selective removal of all GliR, using a Gli1 (activator) knock-in to replace Gli2 together with Gli3 deletion, does not restore normal AP polarity (Bowers et al., 2012).
IV. Models for Shh signal integration in limb patterning
Manipulation of Shh activity in the chick limb has produced results strongly supportive of a morphogen model of action (Fig. 2B-1). An in-depth examination varying the amount, duration, and range of Shh exposure clearly demonstrated dosage-dependence of both digit number and polarity on each of these parameters (increased digit number and more ‘posterior’ digit types induced by higher dosage, longer exposure and/or shorter distance in chick (Yang et al., 1997)). Surprisingly, however, implantation of cells expressing a membrane-tethered Shh-CD4 fusion protein also displayed long-range patterning effects, suggesting a possible relay mechanism, although several alternate explanations are plausible, for example proteolysis to produce free ligand, or long-range Shh-Ptch receptor interactions via filopodial cell extensions (Sanders et al., 2013). Another report showing that Bmps could potentiate the effects of Shh and reduce the effective Shh dosage/duration required to induce digit duplications also suggested the possibility of downstream relay signals (Drossopoulou et al., 2000). But there has been little follow-up on this finding, in part because the protean, overlapping, and context-dependent roles of multiple Bmp family members complicates analysis (Pignatti et al., 2014; Salazar et al., 2016). Furthermore, removal of Bmp2, the main Shh-induced Bmp, doesn’t alter digit patterning in mouse (Bandyopadhyay et al., 2006).
Although Shh action as a spatial morphogen signal via graded diffusion or filopodial delivery is a conceptually appealing model, several aspects of morphogen gradients are problematic. The limb bud expands and more than doubles in size during the period in which Shh is active although Shh expression does not change appreciably, raising the question of how a stable spatial gradient is maintained during substantial growth. In addition, modeling of morphogen gradients indicates that they are only transiently sustainable and tend to resolve into bi-stable switches at steady state (Lander, 2011; Nahmad and Lander, 2011).
Genetic lineage mapping experiments in mouse and pharmacologic Shh inhibition in chick have suggested one potential alternative mechanism in which temporal integration of Shh signals leads to a graded response. Lineage tracing of the ZPA using a ShhCre knock-in allele and RosaLacZ reporter to label Shh-expressing descendant cells demonstrated that ZPA cells don’t only signal to limb digit-forming cells, but give rise to all of digits 4 and 5 (Harfe et al., 2004). It was proposed that a combination of autocrine signaling combined with cell expansion (and cessation of Shh expression in cells displaced anteriorly away from the ZPA) would lead to varying temporal exposure to Shh, with the anterior Shh-dependent digits (d2,3) specified primarily by early paracrine signaling and posterior digits (d4,5) specified by sustained autocrine signaling. Additional support for the idea that signaling duration, rather than varying Shh concentration, is critical for specifying positional information to pattern digits came from genetic experiments in mouse. Deletion of a conditional Shh mutant allele using ShhCre led to a low-level of residual sustained signaling (due to the inherent delay in Shh deletion by ShhCre and homeostatic feedback regulation to generate ZPA cells), and resulted in loss of only a single anterior Shh-dependent digit (Scherz et al., 2007). Timed removal of Shh signaling in chick using pharmacologic inhibition of Smo (cyclopamine) results in a posterior-to-anterior order of digit loss, with progressively earlier Shh pathway inhibition (Scherz et al., 2007; Towers et al., 2008). In contrast, treatment with the deacetylase inhibitor trichostatin A or colchicine to inhibit proliferation without terminating Shh signaling also caused digit loss, but a remaining digit of posterior-type identity formed (Pickering and Towers, 2016; Towers et al., 2008). Based on these results, it was proposed that Shh patterning and growth functions are integrated so that digit progenitors are both expanded and ‘promoted’ to more posterior identities as the duration of Shh exposure increases (Fig. 2B-2). Curtailing both functions leads to anterior digit specification, whereas uncoupling them by selectively inhibiting expansion leads to posterior specification, again suggesting that temporal integration of signaling is required to specify more posterior identities.
However, genetic analyses of Shh response and the temporal requirements for Shh function in mouse have yielded results incompatible with a temporal integration/promotion model. Evaluation of Shh-response at different times in mouse limb buds using a tamoxifen-inducible Gli1CreER and RosaLacZ to pulse-label responding cells and their descendants, revealed that the posterior digit progenitors become refractory to Shh signaling during the “specification” period (Ahn and Joyner, 2004). Since Gli1 is a direct target of Shh activation (GliA), this data is inconsistent with the concept that posterior digit progenitors are specified by the longest duration of Shh exposure. Such a block to autocrine signaling is similarly seen in Hh producing cells in Drosophila wing disc, which are refractory to Hh pathway response due to a lack of Ci (Gli) expression (Ramirez-Weber et al., 2000), and in floorplate cells of vertebrate neural tube, which are only transiently Shh responsive due to down-regulation of Gli2 (Ribes et al., 2010), and may be a general characteristic of Hh-producing cells. An analogous situation may exist in the vertebrate limb bud; both Gli2/3 expression are repressed in the ZPA region (Mo et al., 1997). Indirect evidence in chick and mouse also supports a Shh-refractory state in Hh producing cells; ZPA cells and their descendants don’t upregulate the mesodermal Shh target Grem1 in response to Hh signaling (Nissim et al., 2006; Scherz et al., 2004).
Direct analysis of the temporal requirement for Shh signaling in mouse limb buds by examining genetic removal of a Shh conditional allele, using a tamoxifen-inducible Cre expressed prior to and independent of Shh, likewise showed that the most posterior digits were not the most sensitive to Shh removal after expression had initiated (Zhu et al., 2008). Although progressively more digits were lost as Shh was removed at earlier times, the order of digit loss reflected the normal order in which their progenitor condensations form, rather than their positional identity. These results were more consistent with a failure of AP expansion when Shh expression was truncated, because the latest forming condensations were the first lost, and correlated with both G1 cell cycle arrest and apoptosis. In particular, the posterior most digit (digit 5) was not lost first, and marker analyses suggested that digit 4 required only the shortest Shh exposure to be specified (Zhu and Mackem, 2011; Zhu et al., 2008). An alternate biphasic model was proposed, in which early transient Shh exposure (~6 hours) suffices to provide positional information and specify digit progenitors, while sustained exposure is required to expand the cell population to form the normal complement of 5 digits (Fig. 2B-3). Additionally, comparison of mutants with altered Shh levels in which either posterior or anterior digits were selectively lost revealed that posterior digit specification required high transient Shh levels when expression first initiates, whereas anterior digits were preferentially lost when Shh levels declined after this transient period (Zhu and Mackem, 2011).
Although in principle compatible with a spatial morphogen gradient operating over a very short time window, the biphasic model raises the possibility of relay signaling as a mechanism for determining positional differences at early stages (e.g. Fig. 2B-4). As mentioned above, such a relay has been suggested by certain experiments in chick demonstrating potentiation of Shh by Bmp signaling (Drossopoulou et al., 2000). In addition, work in both chick and mouse has provided clear evidence for ongoing late regulation of digit identity that is non-autonomous but occurs well after normal Shh expression has ceased (Dahn and Fallon, 2000; Huang et al., 2016; Suzuki et al., 2008), indicating the likelihood of relay mechanisms involving a signal cascade, but the nature of the factors involved remains contentious.
A recent report examining the surprising result that late removal of Shh function in chick can produce a post-axial (posterior digit) polydactyly, highlights negative feedback interactions between AER/Fgfs and Shh/ZPA that restrain expansion to form more digits (discussed in section VIII below), and proposes a different explanation to reconcile mouse and chick results: that positional signaling is blunted in mouse by an apparent lack of Shh-responsiveness, allowing expansion (of multiple digits with digit 2 identity) in the absence of positional promotion (Pickering and Towers, 2016). This model assumes that late read-outs of digit specification reflect the effects of Shh, rather than other late-acting regulators – discussed below.
V. The problem of digit identity as a readout for ‘positional information’ provided by Shh
To someone outside the field of limb development, it may seem surprising that the mechanism by which Shh regulates digit identity is still a matter of debate. The problem that has impeded resolving which models accurately portray how Shh signaling directs AP patterning is the nature of the reporter used as a definitive readout for digit identity. Importantly, in the limb, in contrast to paraxial mesoderm and neural tube patterning, the final read-out of signaling is a morphologic ‘identity’ of complex structures (digits) having similar tissue constituents (cartilage, tendons, joints), rather than simply changes in cell fate per se. This distinction has complicated analysis and interpretation of ZPA/Shh function despite its attractiveness as a model. The readout, of necessity, is a late, very delayed assay of how Shh may function to provide positional information that cannot be assessed until well after Shh signaling has ceased (several days later in both mouse and chick), and after late-stage regulatory signals have intervened to regulate final identity (Dahn and Fallon, 2000; Huang et al., 2016; Sanz-Ezquerro and Tickle, 2003; Suzuki et al., 2008). Any early reporter would require the use of marker gene expression, since relying strictly on the position of early condensations ignores potential transformations in identity. However, there are currently no definitive markers for digit identity; those used may correlate with digit identity in the wildtype, but are not actually required to specify a particular identity, and may be uninformative or even misleading in a mutant scenario. For example, Hoxd13 is expressed exclusively in digit 1 progenitors in the absence of other 5′Hoxd expression in the wild type; however, Hoxd12 expression can substitute in the absence of Hoxd13 (Kmita et al., 2002). It can also be argued that because the morphologic identity of digits being assayed is based on late emergent differences (number of phalanges, joints) in structures that are all composed of the same tissue types, it is unlikely that such early digit-specific marker genes even exist.
Further complicating the use of morphologic identity as a reporter for early AP patterning, the reliance on such a late readout reflects post-Shh regulation that may also include the differential regulatory effects of evolutionary adaptive modifications in final digit morphologies. Both of the mainstay embryologic and genetic models for limb development (chick and mouse) have derived limbs that deviate substantially from the ancestral pentadactyl “ground state” (see Fig. 1). The mouse, although pentadactyl, has been criticized for a lack of easily identified differences in digit morphologies since the non-digit 1 digits are all triphalangeal (carpal/tarsal elements at the wrist/ankle are distinct). Although the chick hindlimb approximates the ancestral digit formula, digit number is reduced to four and moreover, much of the chick work on AP patterning has focused on the forelimb (wing), which has both a reduced digit number and highly derived, truncated digits with curtailed phalanx formation.
A complete understanding of the hierarchy of targets acting downstream of Shh signaling could provide a framework for unraveling those that might respond to integration of signaling over time, or act as tiers in signal relays. Defining their exact roles is also complicated by the normally tight coupling between Shh roles in regulating mesodermal patterning and growth. Some insights on Shh targets have been gained, reviewed below, from genetic analyses modifying either signaling levels or response, and genome-wide analysis of Gli3 targets, as well as studies of evolutionary adaptation. But more such studies aimed at parsing out the hierarchical cascade of targets are needed.
VI. Shh pathway targets in the limb – patterning, expansion, and feedback circuits
Shh pathway targets in the limb have been identified both from genome-wide analysis of direct Gli3 binding sites in mouse limb buds by ChIP-Chip with a Gli3-flag tagged transgene (Vokes et al., 2008) and from several gene expression analyses following Shh pathway activation and inhibition (Lewandowski et al., 2015; Lopez-Rios et al., 2012; McGlinn et al., 2005; Probst et al., 2011; Vokes et al., 2008). The identification of in vivo limb Gli binding sites revealed over 200 target genes with strong Gli Binding Sequence (GBS) domains as well as a similarly large target set lacking GBS, that remain less well characterized (Vokes et al., 2008). Additional undiscovered target genes may derive from binding regions too far removed (>100 kbp) from neighboring genes. The majority of the GBS+ target genes that are expressed in a posterior Shh-responsive domain are regulated mainly by de-repression (release from Gli3R). This characteristic of Shh function in the limb adds an additional level of complexity in analyzing the role of different target classes. The expression patterns of different derepressed targets are dictated in part by the particular transcription factors (TFs) that mediate their positive regulation, and these appear to differ among targets; consequently, their regulation may occur by divergent routes (examples shown in Fig. 3). This feature of derepressed targets may also be relevant to patterning by graded Gli3R activity.
Fig 3.
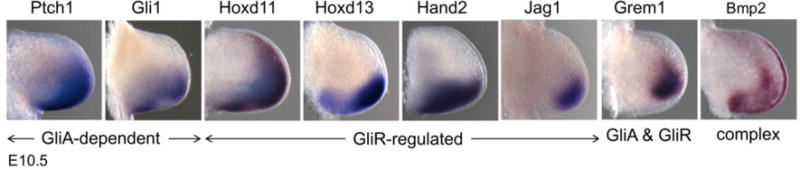
Shh activity range: derepression (GliR) vs activation (GliA) targets compared. Examples of Shh target gene RNAs expressed in E10.5 mouse forelimbs (anterior at top and distal at right of each panel). Bmp2 expression is designated as ‘complex’ because the overall expression domain is determined by both Shh-dependent and independent inputs (Litingtung et al., 2002; Vokes et al., 2008) and expression is also present in the AER. Images were provided courtesy of J. Lewandoski and S.A. Vokes and are adapted from Lewandowski et al, 2015, Dev. Biol., 406, 92–103.
Shh targets include three major functional classes: 1) targets involved in ‘patterning’ that directly provide positional information ultimately leading to specific digit identities and/or relays of positional cues (eg. AP zones) to other downstream signals; and 2) targets with roles in limb bud expansion – which includes both direct targets acting in the mesoderm and relays to the ectoderm to modulate AER/Fgf function. Considerably more has been learned about the second than the first group. Yet a third and sizable group are direct targets involved in the transduction or regulation of the Hh pathway itself (Fig. 4A–B), contributing to extensive, interconnected feedback circuits and again, adding to the complexity of pathway functional analysis in the limb. The elaborate cross- and feedback regulation between Shh itself, Gli3 expression, and ‘downstream’ targets has made it difficult to neatly separate the functional contributions of different inputs (Fig. 4B). These targets include multiple regulators of Shh expression such as Prdm1 (Robertson et al., 2007), Ets, Etv4,5 (Lettice et al., 2012), Alx4 (te Welscher et al., 2002b), Gata4,6 (Kozhemyakina et al., 2014); regulators of Gli3 such as Irx3,5 (Li et al., 2014a), Sall4 (Akiyama et al., 2015) and Tbx3 (Emechebe et al., 2016; Osterwalder et al., 2014); and regulators of both Shh expression and Gli3R expression/function, such as Hand2, Hoxd/Hoxa (Chen et al., 2004; Galli et al., 2010; Osterwalder et al., 2014; te Welscher et al., 2002a; Zakany et al., 2004) and also Gata6 (Hayashi et al., 2016); as well as the Hh pathway components and modulators Ptch, Boc, Cdo, Gas1, Smo, Hhip (Fig. 4A) (Allen et al., 2011; Holtz et al., 2013; Izzi et al., 2011; Tenzen et al., 2006; Vokes et al., 2008).
Fig 4.
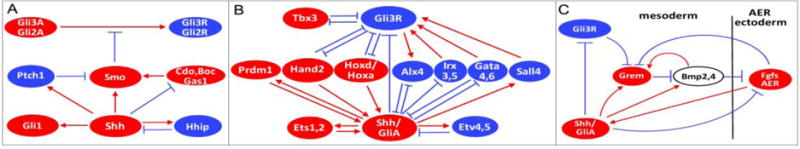
Shh pathway targets and regulatory networks. Interactions are based on both genetic data and on genome-wide transcriptional and chromatin binding analyses. Blue indicates pathway inhibitory interactions, red indicates activating interactions, non-colored circle represents genes with complex activities; lines do not necessarily denote direct interactions and may occur at the transcriptional or protein level; circle sizes have no significance. A. Shh pathway feedback regulation involving signal transduction components. B. Shh pathway feedback regulation by target TFs. For simplicity, only interactions with Shh/GliA and Gli3R are shown and cross-regulation among targets are not indicated (for e.g. Hand2 also regulates Irx3,5 and Tbx3). C. Ectodermal-mesodermal feedback loops operating in Shh pathway to regulate AP patterning and expansion. As noted in the text, expression levels are highly dynamic and some of the regulatory interactions shown occur at different times (e.g. initiation, or termination of feedback loops).
VII. Shh targets involved in patterning limb mesoderm
Of targets potentially regulating digit identity, the 5′Hoxd and Hoxa genes have been the most highly implicated based on the digit phenotypes of single and compound mutants (Davis and Capecchi, 1996; Davis et al., 1995; FromentalRamain et al., 1996; Zakany and Duboule, 1996; Zakany et al., 1997). However, there is no ‘Hox code’; their targets are as yet poorly understood and the mechanisms by which they impart early positional cues remain unclear. The biphasic model, if correct, would introduce an added constraint; the apparent transient Shh requirement for digit patterning in mouse predicts that the target class important for early positional information should become rapidly Shh-independent (or be required only very transiently). Based on Shh pathway inhibition studies in mouse limb buds cultured with cyclopamine, very few targets have yet been identified that require only transient Shh exposure for stable expression (Lewandowski et al., 2015; Panman et al., 2006), and the 5′Hoxd genes are not among them, instead requiring sustained Shh signaling. However, a recent analysis of 5′Hoxd function using genetic manipulation in mouse indicates that the 5′Hoxd role in patterning may occur relatively late, well after Shh signaling has ceased, during a Shh-independent Hoxd expression phase (Huang et al., 2016). This suggests that 5′Hoxd genes may act indirectly to pattern digits downstream of a signal relay, even though they are also direct Shh targets.
Bmps have been proposed as synergistic relay signals in patterning based on manipulations in chick embryos showing that Shh exposure times required to alter digit pattern could be shortened if followed by Bmp2 exposure (Drossopoulou et al., 2000), and have also been implicated in interdigit signaling that regulates final digit identity at late stages (Dahn and Fallon, 2000; Suzuki et al., 2008). Interestingly, the Bmp pathway is also highly enriched in GBS+ direct targets identified by ChIP-Chip (Vokes et al., 2008). However, the role of Bmps remains controversial, partly because of functional redundancy and context-dependence, as well as confounding effects on both AER induction and maintenance (discussed below), which both impact cell survival (Pizette et al., 2001; Pizette and Niswander, 1999; Soshnikova et al., 2003). Simultaneous genetic removal of several major Bmp ligands in mouse has failed to alter digit identity, although in one study, affects on chondrogenesis complicate interpretation of digit phenotypes (Bandyopadhyay et al., 2006), and in another study the timing of Bmp deletion may be too late (Kaltcheva et al., 2016). On the other hand, genetic manipulation of Bmp pathway at late stages in mouse suggest that the proper balance of Bmp activity may play a role in regulating aspects of digit identity; albeit at a late stage more consistent with action as an indirect relay target (Huang et al., 2016).
VIII. Shh signaling effects on the AER and limb expansion
There is greater agreement from chick and mouse studies that limb bud expansion in response to Shh, not surprisingly, requires sustained signaling over time. Cell cycle analyses in both models have shown that Shh inhibition causes a G1 arrest and reduced proliferation (Towers et al., 2008; Zhu et al., 2008), and conversely Gli3 removal has the opposite effect, because of the predominant role of Gli3R (Lopez-Rios et al., 2012). Gli3 target analyses have identified Cdk6 and MycN as direct, and the cyclin Ccnd1 as indirect mesodermal Shh targets (Lopez-Rios et al., 2012; Vokes et al., 2008). Sustained Shh signaling also plays an important role in regulating cell survival, particularly via effects on the AER.
Fgf signaling from the AER plays a critical role is sustaining limb mesoderm outgrowth and the interplay between Shh/ZPA and AER/Fgf in regulating cell survival and expansion has been the focus of much study dating back to Saunders’ original work characterizing both signaling centers (Saunders, 1948; Saunders and Gasseling, 1968). Shh modulates AER function both indirectly via target relay signals to AER, and directly (Fig. 4C). Experiments in chick manipulating the AER in conjunction with exposure to exogenous Shh or Fgf4 established that the AER is necessary to induce Shh expression and can be substituted for by Fgf4 (Laufer et al., 1994; Niswander et al., 1994). Shh, in turn, induces Fgf4 expression in posterior AER, forming a positive feedback loop between ZPA and AER. Deletion of both Fgf4;Fgf8 in mouse definitively showed that AER/Fgfs are essential for initiation of Shh expression in the limb (Sun et al., 2002), and in fact Ets family TFs, nuclear effectors of Fgf signals, are key regulators of the Shh limb enhancer (Lettice et al., 2012). Genetic studies in mouse revealed that the Shh mesodermal target Gremlin (Grem1), a secreted BMP antagonist initially characterized through the limb deformity mutation that disrupts the function of a long-range Grem1 limb enhancer (Zuniga et al., 2004), relays the Shh signal to the AER by modulating the negative effects of Bmps on AER maintenance (Zuniga et al., 1999). Grem1 enhancer characterization has uncovered complex regulation by the Shh pathway, involving a potential role for GliA in addition to a major effect of Gli3R derepression, as well as induction by Shh targets Bmp2,4 (Benazet et al., 2009; Li et al., 2014b; Nissim et al., 2006; Vokes et al., 2008; Zuniga et al., 2012). The balance between Gli3R, Bmps and Grem1 governs AER activity to constrain normal digit number. Genetic manipulation of Fgf activity levels in mouse indicates that high-level Fgf signaling at late stages also acts itself as a negative feedback input on AER maintenance, by repressing Grem1 expression in the underlying mesoderm (Verheyden and Sun, 2008). It is important to note that many of these regulatory effects are highly dynamic, dependent on expression levels and the context of other inputs, both of which change over developmental time. For example, in the early limb bud, mesodermal Bmps induce Grem1 expression prior to Shh activation. At the protein level, accumulating Grem1 antagonizes Bmp function, thereby promoting AER/Fgf maintenance and Shh activation as effective Bmp activity declines, and initiating a mesodermal-ectodermal Grem1-Fgf-Shh feedback loop (Benazet et al., 2009). At late stages, the accumulation of high Fgf levels act to terminate the feedback loop by repressing Grem1, and thereby extinguish AER and ZPA function.
In addition to indirect effects on AER/Fgf activity via Grem1-Bmp modulation, the ZPA/Shh also signals directly to the overlying ectoderm in a negative feedback circuit to regulate the posterior extent of the functional AER and thereby modulate ZPA activity (Bouldin et al., 2010). Selective genetic removal of Shh response in the AER (by conditional inactivation of Smo) resulted in increased AER/Fgf8 expansion along the posterior limb bud margin. In contrast, exogenous Shh application in chick limb inhibited AER/Fgf8 expression and reduced AER extent. Notably, a similar observation was made by John Saunders in the context of ZPA grafting experiments; finding that a thickened AER was not maintained immediately overlying ZPA grafts (Saunders and Gasseling, 1968). Recently, pharmacologic inhibition of Shh response in chick with cyclopamine treatment at different times confirmed this negative feedback circuit (Pickering and Towers, 2016). The time course of inhibition revealed that, in contrast to digit loss caused by early Shh pathway inhibition, late inhibition of Shh signaling resulted in postaxial polydactyly associated with extended Fgf8 expression and AER along the posterior marginal ectoderm to overlie the ZPA region (Pickering and Towers, 2016). In the chick wing, lineage tracing has shown that the ZPA normally does not give rise to any digits and digit 4,5 progenitors regress, due to extensive apoptosis (Towers et al., 2011). Late Shh inhibition and AER expansion enables these progenitors to persist, but they adopt digit 2 positional values owing to the interrupted Shh exposure. In mouse, the ZPA underlies the AER and gives rise to posterior digits, leading the authors to speculate that mouse digit progenitors become refractory to Shh patterning function once they have acquired a positional value of digit 2, but proliferate to produce several digit 2 copies via a Turing mechanism later on. This interpretation assumes that phalanx number can be used as an indicator of earlier Shh effect on positional values across species, despite adaptive morphologic changes (see section V above). Nevertheless, these studies indicate that ZPA-AER interactions also act to restrain posterior digit number. In fact, recent work has provided evidence for the regulation of digit number by a Turing type self-organizing mechanism in which antagonistic Bmp—Wnt interactions specify periodic Sox9 (digit progenitor) expression in a Substrate (Sox9)—Depletion model (Raspopovic et al., 2014). Intriguingly, Gli3R levels have been shown to modulate the periodicity with which digit condensations arise, and thereby total digit number (Sheth et al., 2012), possibly acting to regulate Bmp expression and set the net Bmp activity level (Lopez-Rios et al., 2012). However, since Sox9 expression and digit condensation appearance commence at a time when Shh expression has already ceased, the extent to which Shh signaling regulates digit number by impacting the Gli3R level at this self-organizing stage is unclear.
Shh activity also plays a supporting role in P-D progression of limb outgrowth, which is governed by antagonistic interactions between RA proximally and AER/Fgfs distally (Cooper et al., 2011; Mercader et al., 2000; Rosello-Diez et al., 2011). Both genome-wide expression analysis in Shh pathway mutants and GBS-site analysis suggest that Shh activity may down-regulate levels of the RA synthetic enzyme Aldh1a2 as well as the proximalizing RA target gene Meis1 (Lewandowski et al., 2015; Probst et al., 2011; Vokes et al., 2008). Induction of the degradative enzyme Cyp26a1, which is initially regulated by Fgfs independent of Shh, also becomes reduced in the distal domain of Shh mutant limb buds due to the indirect effects of reduced AER/Fgf activity. In this manner, Shh also helps to promote the distal progression of limb development.
IX. Requirement for “polarized’ Shh activity
Although Shh pathway activation is frequently linked to polydactyly in mutants in several species, precocious pathway activation in the early limb bud by genetic removal of Ptch1 prior to establishment of polarized gene expression (AER and ZPA centers) results in a severe outgrowth defect with fewer and symmetrical, truncated digits (Butterfield et al., 2009). An extensive analysis of several mutants with different levels of precocious pathway activation (Ptch1, Kif7, SuFu; together with Gli3R removal) revealed that the level of GliA relative to Gli3R is a critical factor in producing this phenotype (Zhulyn et al., 2014). With Gli3 loss alone (primarily GliR), which results in polydactyly, polarized Shh expression is still maintained. In contrast, in single and compound mutants with high levels of GliA (primarily Gli2 activation), Shh expression becomes apical, diffuse and symmetric, and failure to establish normal AER/Fgf8 and ZPA formation ensues. Precocious pathway activation by the Hh agonist SAG in chick produces similar effects. Although the mechanism by which nonpolarized and precocious GliA inhibits normal AER and ZPA formation is as yet not entirely clear, reduced Shh ligand levels (either by a Shh +/− allele, or the normally lower endogenous Shh level in forelimb compared to hindlimb) mitigates the phenotype. This suggests the possibility that early mislocalized, apical Shh may inhibit normal AER formation by direct signaling to the ectoderm, and consequently cause failure of distal limb expansion. In less severe cases of precocious and nonpolarized pathway activation (with lower GliA level) in which AER/Fgf signaling is still initiated (eg. Ptch1 mutant), another possibility is that too high an initial level of Fgf expression may prematurely inhibit the Grem1 feedback loop and terminate AER maintenance prematurely (Verheyden and Sun, 2008).
These results indicate a requirement to induce Shh in a polarized manner, which is ensured by high anterior Gli3 expression (Gli3R level) and mutual cross-repression with Hand2 expressed in the posterior limb bud (Osterwalder et al., 2014; te Welscher et al., 2002a; Vokes et al., 2008). Recent analysis of Irx3,5 gene function in mouse identified an essential role in specifying anterior limb elements that normally form independent of Shh activity (eg. femur, tibia, digit 1 in hindlimb) (Li et al., 2014a). Irx3,5 are expressed in the anterior limb bud and act genetically upstream to regulate Gli3 expression prior to Shh induction. Establishment of an anterior zone is essential for formation of anterior elements and for the timely induction of polarized Shh/ZPA in the posterior limb bud, after the AER/Fgf8 signaling center, to initiate specification of posterior elements and digits, and limb bud expansion.
X. Insights from evolutionary adaptation
Modifications of both digit number and identity across different vertebrate taxa, and even among closely related species, are frequent in the course of evolutionary adaptation. Early tetrapod ancestors (eg. acanthostega) were polydactylous, having up to 8 digits with little AP distinction (Schneider and Shubin, 2013). These were probably more akin to the bony rays of fish fins, which are homologous structures that employ regulatory networks highly conserved across fish and tetrapods in their development (Gehrke and Shubin, 2016; Nakamura et al., 2016). In the course of tetrapod evolution, it has been proposed that digit number became restrained to what is referred to as the “pentadactyl ground state” (see Fig. 1) (Laurin, 1998). The acquisition of a bona fide autopod and constrained digit number has been partly attributed to the evolutionary expansion of late phase 5′Hox expression into the distal fin/limb bud, producing fewer and larger, more distinctive digits (Freitas et al., 2012; Sheth et al., 2012; Woltering et al., 2014; Zakany et al., 1997). The appearance of polarized Gli3-Shh may also have played a role; the ancestral digit ‘rays’ in tetrapod forebears, such as acanthostega, are reminiscent of the Gli3 null mouse phenotype. Consistent with this view, the fin rays of cartilaginous fish, such as the catshark, lack robust Shh expression and an anteriorized Gli3R gradient (Onimaru et al., 2015; Tanaka, 2016). The requirement to express Shh in a polarized manner also indicates that modulation of both Shh expression timing and domains can act to constrain digit number.
Although experimental manipulation of Shh levels in chick at later stages (Dahn and Fallon, 2000; Sanz-Ezquerro and Tickle, 2003) can produce apparent changes in digit identity (hyperphalangy and digit lengthening), the normal late stage re-expression of Shh that occurs in bat wing interdigits is associated with lengthened phalanges but without increases in phalangeal number and may be linked to a role in extending Fgf8 duration and sustaining cell survival of the extensive interdigital webbing of the bat wing (Hockman et al., 2008; Weatherbee et al., 2006). In taxa that do display hyperphalangy (such as marine mammals), the molecular mechanisms have not been explored (Fedak and Hall, 2004; Richardson et al., 2004), although a recent dolphin Shh enhancer analysis in transgenic mice suggests a potential Shh role (Kvon et al., 2016).
Most adaptations in modern extant species, for specialized functions such as running or burrowing, usually trend toward digit loss from the pentadactyl state (Fig. 1). Two major mechanisms that accomplish this are enhanced apoptosis to reduce autopod extent and changes in the timing of Shh expression – either duration or onset (Cooper et al., 2014; Lopez-Rios et al., 2014). Digit loss in skink species has been shown to correlate directly with reduced duration in Shh expression (Shapiro et al., 2003). Although the digit pattern is very different, the observed reduction in digit number without substantial changes in the morphology of remaining digits that occurs in skink species is analogous to the phenotypes observed in mice when Shh expression is curtailed by genetic removal. The biphasic model provides a framework for uncoupling digit identity from number and preserving relatively normal digit morphologies while reducing number (Zhu et al., 2008).
In other examples of adaptive evolution, particularly those highly specialized for cursorial locomotion, substantial change in morphologies occur coincident with reduction in the number of both digits and phalanges. The requirement to express Shh in a polarized manner indicates that inappropriate pathway activation across the limb, paradoxically, can lead to digit reduction phenotypes by interfering with normal ZPA and AER formation or maintenance. In fact, this mechanism may be fine-tuned to reduce digit number and generate the small, symmetrical digits adapted for land locomotion/running in certain ungulates (hooved mammals). In cows, it was shown that reduced Ptch1 expression is associated with depolarized Shh pathway activity and broader spread of Shh protein across the distal limb bud margin (Lopez-Rios et al., 2014). Since Ptch1 negatively regulates Shh signaling, both by virtue of tonically inhibiting Smo activation and by sequestering Shh ligand, reduced Ptch1 causes expansion of Shh activity, leading to loss of limb asymmetry and premature AER regression and digit reduction (e.g. Fig. 1) that phenocopies the genetic removal of Ptch1 in mouse limb buds. Functional transgenic analysis of the bovine compared to murine Ptch1 limb-regulatory module revealed a loss of responsiveness to induction by Shh, implicating reduced Ptch1 as the primary basis for digit loss in cows.
In snakes with the most extreme adaptation for trunk-based locomotion (extensive to complete loss of limb elements), recent comparative genomic analyses, transgenic reporter analyses and gene editing to test snake sequence alterations in mouse, have revealed that the limb-specific loss of Shh function is the most frequent cause of limb reduction phenotypes in different snake species (Kvon et al., 2016; Leal and Cohn, 2016). This occurs via alterations leading to the progressive loss of Hoxd and/or Ets1 transcription factor binding sites, resulting in loss of Shh limb enhancer function in modern snakes. However, particularly in advanced snake species, other evolutionary changes must come into play as well, since complete limblessness is more severe than the phenotype due to complete absence of Shh function in limbed vertebrates (Chiang et al., 2001; Ros et al., 2003). Altogether, these studies reveal that both gain and loss of function changes in the Shh pathway can lead to evolutionary digit reduction and adaptive modifications.
XI. Questions and future directions
With his discovery of the ZPA, Saunders opened up a vibrant and stimulating area of research that has made the developing limb an enduring model for studying patterning and morphogenesis, ultimately leading to the identification of key Shh roles in limb patterning and growth, the discovery of filopodia as a physical mechanism for extending spatial signaling range, and the elucidation of a robust and complex regulatory network centered on maintaining and restraining ZPA activity, among many other discoveries. At the same time, it is a model for morphogenesis still filled with many puzzles for future work.
Because the morphogenesis of the appendicular skeleton involves a number of processes and steps to realize final digit identities downstream of Shh signaling, how Shh regulates positional information remains a matter of debate. Complete elucidation of targets together with their temporal order of activation (or derepression), and of potential relay steps will be required to organize targets into a hierarchical network that may provide insights on the validity of different models which are testable using molecular genetic strategies.
Shh signaling to both mesoderm and directly and indirectly to ectoderm to regulate limb AP expansion as well as patterning also obscures/complicates dissecting out different roles. The indirect effects of Shh certainly promote AER maintenance at early stages. To what extent this also impacts the later progenitor pool for forming phalanges in digit tips is unclear (Suzuki et al., 2008). However, the size of the pool and duration of late AER/Fgf function likely affect final phalanx number and digit identity (Sanz-Ezquerro and Tickle, 2003). An intriguing possibility, yet to be examined, is whether polarized Shh may lead to AP polarized AER function that contributes to patterning as well as growth by modulating the formation of late digit tip progenitor pools.
Genetic studies and manipulation of Bmp-Wnt levels in cultured mouse limb buds support a Turing type reaction-diffusion mechanism in the formation of digits (Raspopovic et al., 2014; Sheth et al., 2012), and recent analysis of formation of cartilaginous fish fin rays raises the possibility that such a mechanism played an ancestral role preceding the regulation of AP polarity (Onimaru et al., 2015; Onimaru et al., 2016). If digit formation does proceed via a Turing mechanism, how is this integrated with early Shh-regulated positional information to produce distinct digit types?
The ZPA has been the foundation for a large body of work that has increased our understanding of how morphogenesis is orchestrated to produce a complex structure, but there are still many important fundamental questions to tackle in deciphering how Shh patterns the limb.
Highlights.
John W. Saunders’ pioneering work established the limb as a key model for developmental patterning
Saunders’ discovery of the Zone of Polarizing Activity (ZPA) identified a candidate “morphogen”
Sonic hedgehog (Shh) is the ZPA signal regulating digit identity and number
The problem of digit identity and why the mechanism(s) by which Shh instructs digit identity remains unresolved
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Akiyama R, Kawakami H, Wong J, Oishi I, Nishinakamura R, Kawakami Y. Sall4-Gli3 system in early limb progenitors is essential for the development of limb skeletal elements. Proc Natl Acad Sci U S A. 2015;112:5075–5080. doi: 10.1073/pnas.1421949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 2011;20:775–787. doi: 10.1016/j.devcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alman BA. The role of hedgehog signalling in skeletal health and disease. Nature Reviews Rheumatology. 2015;11:552–560. doi: 10.1038/nrrheum.2015.84. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai CYB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Balcuns A, Gasseling MT, Saunders JW. Spatio Temporal Distribution of a Zone That Controls Antero-Posterior Polarity in Limb Bud of Chick and Other Bird Embryos. Am Zool. 1970;10:323. [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazet JD, Bischofberger M, Tiecke E, Goncalves A, Martin JF, Zuniga A, Naef F, Zeller R. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science. 2009;323:1050–1053. doi: 10.1126/science.1168755. [DOI] [PubMed] [Google Scholar]
- Bouldin CM, Gritli-Linde A, Ahn S, Harfe BD. Shh pathway activation is present and required within the vertebrate limb bud apical ectodermal ridge for normal autopod patterning. Proc Natl Acad Sci U S A. 2010;107:5489–5494. doi: 10.1073/pnas.0912818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M, Eng LE, Lao ZM, Turnbull RK, Bao XZ, Riedel E, Mackem S, Joyner AL. Limb anterior-posterior polarity integrates activator and repressor functions of GLI2 as well as GLI3. Developmental Biology. 2012;370:110–124. doi: 10.1016/j.ydbio.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nature Reviews Molecular Cell Biology. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Butterfield NC, Metzis V, McGlinn E, Bruce SJ, Wainwright BJ, Wicking C. Patched 1 is a crucial determinant of asymmetry and digit number in the vertebrate limb. Development. 2009;136:3515–3524. doi: 10.1242/dev.037507. [DOI] [PubMed] [Google Scholar]
- Chang DT, Lopez A, Vonkessler DP, Chiang C, Simandl BK, Zhao RB, Seldin MF, Fallon JF, Beachy PA. Products, Genetic-Linkage and Limb Patterning Activity of a Murine Hedgehog Gene. Development. 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knezevic V, Ervin V, Hutson R, Ward Y, Mackem S. Direct interaction with Hoxd proteins reverses Gli3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–2347. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Hu JK, ten Berge D, Fernandez-Teran M, Ros MA, Tabin CJ. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332:1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, Sears KE, Uygur A, Maier J, Baczkowski KS, Brosnahan M, Antczak D, Skidmore JA, Tabin CJ. Patterning and post-patterning modes of evolutionary digit loss in mammals. Nature. 2014;511:41–45. doi: 10.1038/nature13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahn RD, Fallon JF. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science. 2000;289:438–441. doi: 10.1126/science.289.5478.438. [DOI] [PubMed] [Google Scholar]
- Davis AP, Capecchi MR. A mutational analysis of the 5′ HoxD genes: dissection of genetic interactions during limb development in the mouse. Development. 1996;122:1175–1185. doi: 10.1242/dev.122.4.1175. [DOI] [PubMed] [Google Scholar]
- Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375:791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C, Tickle C. A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signalling and Bmp signalling. Development. 2000;127:1337–1348. doi: 10.1242/dev.127.7.1337. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Emechebe U, Kumar PP, Rozenberg JM, Moore B, Firment A, Mirshahi T, Moon AM. T-box3 is a ciliary protein and regulates stability of the Gli3 transcription factor to control digit number. Elife. 2016;5:e07897. doi: 10.7554/eLife.07897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedak TJ, Hall BK. Perspectives on hyperphalangy: patterns and processes. Journal of Anatomy. 2004;204:151–163. doi: 10.1111/j.0021-8782.2004.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas R, Gomez-Marin C, Wilson JM, Casares F, Gomez-Skarmeta JL. Hoxd13 Contribution to the Evolution of Vertebrate Appendages. Dev Cell. 2012;23:1219–1229. doi: 10.1016/j.devcel.2012.10.015. [DOI] [PubMed] [Google Scholar]
- FromentalRamain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- Galli A, Robay D, Osterwalder M, Bao XZ, Benazet JD, Tariq M, Paro R, Mackem S, Zeller R. Distinct Roles of Hand2 in Initiating Polarity and Posterior Shh Expression during the Onset of Mouse Limb Bud Development. Plos Genetics. 2010;6:e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke AR, Shubin NH. Cis-regulatory programs in the development and evolution of vertebrate paired appendages. Seminars in Cell & Developmental Biology. 2016;57:31–39. doi: 10.1016/j.semcdb.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Akiyama R, Wong J, Tahara N, Kawakami H, Kawakami Y. Gata6-Dependent GLI3 Repressor Function is Essential in Anterior Limb Progenitor Cells for Proper Limb Development. PLoS Genet. 2016;12:e1006138. doi: 10.1371/journal.pgen.1006138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockman D, Cretekos CJ, Mason MK, Behringer RR, Jacobs DS, Illing N. A second wave of Sonic hedgehog expression during the development of the bat limb. Proc Natl Acad Sci U S A. 2008;105:16982–16987. doi: 10.1073/pnas.0805308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz AM, Peterson KA, Nishi Y, Morin S, Song JY, Charron F, McMahon AP, Allen BL. Essential role for ligand-dependent feedback antagonism of vertebrate hedgehog signaling by PTCH1, PTCH2 and HHIP1 during neural patterning. Development. 2013;140:3423–3434. doi: 10.1242/dev.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbruch A, Wolpert L. Positional Signaling by Hensens Node When Grafted to the Chick Limb Bud. J Embryol Exp Morph. 1986;94:257–265. [PubMed] [Google Scholar]
- Huang BL, Trofka A, Furusawa A, Norrie JL, Rabinowitz AH, Vokes SA, Mark Taketo M, Zakany J, Mackem S. An interdigit signalling centre instructs coordinate phalanx-joint formation governed by 5′Hoxd-Gli3 antagonism. Nat Commun. 2016;7:12903. doi: 10.1038/ncomms12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A Mouse Model of Greig Cephalopolysyndactyly Syndrome - the Extra-Toes(J) Mutation Contains an Intragenic Deletion of the Gli3 Gene. Nature Genetics. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. [Google Scholar]
- Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 Each Form Distinct Shh Receptor Complexes with Ptch1 and Are Required for Shh-Mediated Cell Proliferation. Dev Cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltcheva MM, Anderson MJ, Harfe BD, Lewandoski M. BMPs are direct triggers of interdigital programmed cell death. Dev Biol. 2016;411:266–276. doi: 10.1016/j.ydbio.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmita M, Fraudeau N, Herault Y, Duboule D. Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature. 2002;420:145–150. doi: 10.1038/nature01189. [DOI] [PubMed] [Google Scholar]
- Kozhemyakina E, Ionescu A, Lassar AB. GATA6 is a crucial regulator of Shh in the limb bud. PLoS Genet. 2014;10:e1004072. doi: 10.1371/journal.pgen.1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A Functionally Conserved Homology of the Drosophila Segment Polarity Gene-Hh Is Expressed in Tissues with Polarizing Activity in Zebrafish Embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Kvon EZ, Kamneva OK, Melo US, Barozzi I, Osterwalder M, Mannion BJ, Tissieres V, Pickle CS, Plajzer-Frick I, Lee EA, Kato M, Garvin TH, Akiyama JA, Afzal V, Lopez-Rios J, Rubin EM, Dickel DE, Pennacchio LA, Visel A. Progressive Loss of Function in a Limb Enhancer during Snake Evolution. Cell. 2016;167:633–642. doi: 10.1016/j.cell.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. Pattern, Growth, and Control. Cell. 2011;144:955–969. doi: 10.1016/j.cell.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Laurin M. A Reevaluation of the Origin of Pentadactyly. Evolution. 1998;52:1476–1482. doi: 10.1111/j.1558-5646.1998.tb02028.x. [DOI] [PubMed] [Google Scholar]
- Leal F, Cohn MJ. Loss and Re-emergence of Legs in Snakes by Modular Evolution of Sonic hedgehog and HOXD Enhancers. Curr Biol. 2016;26:2966–2973. doi: 10.1016/j.cub.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Williamson I, Wiltshire JH, Peluso S, Devenney PS, Hill AE, Essafi A, Hagman J, Mort R, Grimes G, DeAngelis CL, Hill RE. Opposing functions of the ETS factor family define Shh spatial expression in limb buds and underlie polydactyly. Dev Cell. 2012;22:459–467. doi: 10.1016/j.devcel.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski JP, Du F, Zhang S, Powell MB, Falkenstein KN, Ji H, Vokes SA. Spatiotemporal regulation of GLI target genes in the mammalian limb bud. Dev Biol. 2015;406:92–103. doi: 10.1016/j.ydbio.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Sakuma R, Vakili NA, Mo R, Puviindran V, Deimling S, Zhang XY, Hopyan S, Hui CC. Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling. Dev Cell. 2014a;29:233–240. doi: 10.1016/j.devcel.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Li Q, Lewandowski JP, Powell MB, Norrie JL, Cho SH, Vokes SA. A Gli silencer is required for robust repression of gremlin in the vertebrate limb bud. Development. 2014b;141:1906–1914. doi: 10.1242/dev.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J, Duchesne A, Speziale D, Andrey G, Peterson KA, Germann P, Uuml;nal E, Liu J, Floriot S, Barbey S, Gallard Y, Muller-Gerbl M, Courtney AD, Klopp C, Rodriguez S, Ivanek R, Beisel C, Wicking C, Iber D, Robert B, McMahon AP, Duboule D, Zeller R. Attenuated sensing of SHH by Ptch1 underlies evolution of bovine limbs. Nature. 2014;511:46–51. doi: 10.1038/nature13289. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J, Speziale D, Robay D, Scotti M, Osterwalder M, Nusspaumer G, Galli A, Hollander GA, Kmita M, Zeller R. GLI3 constrains digit number by controlling both progenitor proliferation and BMP-dependent exit to chondrogenesis. Dev Cell. 2012;22:837–848. doi: 10.1016/j.devcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinn E, van Bueren KL, Fiorenza S, Mo R, Poh AM, Forrest A, Soares MB, Bonaldo Mde F, Grimmond S, Hui CC, Wainwright B, Wicking C. Pax9 and Jagged1 act downstream of Gli3 in vertebrate limb development. Mech Dev. 2005;122:1218–1233. doi: 10.1016/j.mod.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martinez AC, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HHQ, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui CC. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Nahmad M, Lander AD. Spatiotemporal mechanisms of morphogen gradient interpretation. Curr Opin Genet Dev. 2011;21:726–731. doi: 10.1016/j.gde.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Gehrke AR, Lemberg J, Szymaszek J, Shubin NH. Digits and fin rays share common developmental histories. Nature. 2016;537:225–228. doi: 10.1038/nature19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim S, Hasso SM, Fallon JF, Tabin CJ. Regulation of Gremlin expression in the posterior limb bud. Dev Biol. 2006;299:12–21. doi: 10.1016/j.ydbio.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- Noji S, Nohno T, Koyama E, Muto K, Ohyama K, Aoki Y, Tamura K, Ohsugi K, Ide H, Taniguchi S, Saito T. Retinoic Acid Induces Polarizing Activity but Is Unlikely to Be a Morphogen in the Chick Limb Bud. Nature. 1991;350:83–86. doi: 10.1038/350083a0. [DOI] [PubMed] [Google Scholar]
- Onimaru K, Kuraku S, Takagi W, Hyodo S, Sharpe J, Tanaka M. A shift in anterior-posterior positional information underlies the fin-to-limb evolution. Elife. 2015;4:e07048. doi: 10.7554/eLife.07048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru K, Marcon L, Musy M, Tanaka M, Sharpe J. The fin-to-limb transition as the re-organization of a Turing pattern. Nat Commun. 2016;7:11582. doi: 10.1038/ncomms11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder M, Speziale D, Shoukry M, Mohan R, Ivanek R, Kohler M, Beisel C, Wen XH, Scales SJ, Christoffels VM, Visel A, Lopez-Rios J, Zeller R. HAND2 Targets Define a Network of Transcriptional Regulators that Compartmentalize the Early Limb Bud Mesenchyme. Dev Cell. 2014;31:345–357. doi: 10.1016/j.devcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panman L, Galli A, Lagarde N, Michos O, Soete G, Zuniga A, Zeller R. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development. 2006;133:3419–3428. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445–3457. doi: 10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering J, Towers M. Inhibition of Shh signalling in the chick wing gives insights into digit patterning and evolution. Development. 2016;143:3514–3521. doi: 10.1242/dev.137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti E, Zeller R, Zuniga A. To BMP or not to BMP during vertebrate limb bud development. Seminars in Cell & Developmental Biology. 2014;32:119–127. doi: 10.1016/j.semcdb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Pizette S, Abate-Shen C, Niswander L. BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development. 2001;128:4463–4474. doi: 10.1242/dev.128.22.4463. [DOI] [PubMed] [Google Scholar]
- Pizette S, Niswander L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development. 1999;126:883–894. doi: 10.1242/dev.126.5.883. [DOI] [PubMed] [Google Scholar]
- Probst S, Kraemer C, Demougin P, Sheth R, Martin GR, Shiratori H, Hamada H, Iber D, Zeller R, Zuniga A. SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling. Development. 2011;138:1913–1923. doi: 10.1242/dev.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Casso DJ, Aza-Blanc P, Tabata T, Kornberg TB. Hedgehog signal transduction in the posterior compartment of the Drosophila wing imaginal disc. Mol Cell. 2000;6:479–485. doi: 10.1016/s1097-2765(00)00046-0. [DOI] [PubMed] [Google Scholar]
- Raspopovic J, Marcon L, Russo L, Sharpe J. Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science. 2014;345:566–570. doi: 10.1126/science.1252960. [DOI] [PubMed] [Google Scholar]
- Ribes V, Balaskas N, Sasai N, Cruz C, Dessaud E, Cayuso J, Tozer S, Yang LL, Novitch B, Marti E, Briscoe J. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010;24:1186–1200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MK, Jeffery JE, Tabin CJ. Proximodistal patterning of the limb: insights from evolutionary morphology. Evolution & Development. 2004;6:1–5. doi: 10.1111/j.1525-142x.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic-Hedgehog Mediates the Polarizing Activity of the Zpa. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Charatsi I, Joyner CJ, Koonce CH, Morgan M, Islam A, Paterson C, Lejsek E, Arnold SJ, Kallies A, Nutt SL, Bikoff EK. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development. 2007;134:4335–4345. doi: 10.1242/dev.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Altaba ARI, Tanabe Y, Placzek M, Edlund T, Jessell TM, Dodd J. Floor Plate and Motor-Neuron Induction by Vhh-1, a Vertebrate Homolog of Hedgehog Expressed by the Notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Ros MA, Dahn RD, Fernandez-Teran M, Rashka K, Caruccio NC, Hasso SM, Bitgood JJ, Lancman JJ, Fallon JF. The chick oligozeugodactyly (ozd) mutant lacks sonic hedgehog function in the limb. Development. 2003;130:527–537. doi: 10.1242/dev.00245. [DOI] [PubMed] [Google Scholar]
- Rosello-Diez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nature Reviews Endocrinology. 2016;12:203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Tickle C. Fgf signaling controls the number of phalanges and tip formation in developing digits. Curr Biol. 2003;13:1830–1836. doi: 10.1016/j.cub.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Sasai N, Briscoe J. Primary cilia and graded Sonic Hedgehog signaling. Wiley Interdiscip Rev Dev Biol. 2012;1:753–772. doi: 10.1002/wdev.43. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Saunders JW. The Proximo-Distal Sequence of Origin of the Parts of the Chick Wing and the Role of the Ectoderm. Journal of Experimental Zoology. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Saunders JW, Gasseling M. New insights into the problem of pattern regulation in the limb bud of the chick embryo. In: Fallon JF, Caplan AI, editors. Limb Development and Regulation, Part A. Alan R. Liss; New York: 1983. pp. 67–76. [PubMed] [Google Scholar]
- Saunders JW, Gasseling MT. Ectodermal-mesenchymal interactions in the origin of limb symmetry. In: Fleischmajer R, Billingham RE, editors. Epithelial-Mesenchymal Interactions. Williams & Wilkins; Baltimore: 1968. pp. 78–97. [Google Scholar]
- Scherz PJ, Harfe BD, McMahon AP, Tabin CJ. The limb bud Shh-Fgf feedback loop is terminated by expansion of former ZPA cells. Science. 2004;305:396–399. doi: 10.1126/science.1096966. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I, Shubin NH. The origin of the tetrapod limb: from expeditions to enhancers. Trends in Genetics. 2013;29:419–426. doi: 10.1016/j.tig.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Shapiro MD, Hanken J, Rosenthal N. Developmental basis of evolutionary digit loss in the Australian lizard Hemiergis. J Exp Zool B Mol Dev Evol. 2003;297:48–56. doi: 10.1002/jez.b.19. [DOI] [PubMed] [Google Scholar]
- Sheth R, Marcon L, Bastida MF, Junco M, Quintana L, Dahn R, Kmita M, Sharpe J, Ros MA. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science. 2012;338:1476–1480. doi: 10.1126/science.1226804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes & Development. 2003;17:1963–1968. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hasso SM, Fallon JF. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci U S A. 2008;105:4185–4190. doi: 10.1073/pnas.0707899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. Fins into limbs: Autopod acquisition and anterior elements reduction by modifying gene networks involving 5′Hox, Gli3, and Shh. Developmental Biology. 2016;413:1–7. doi: 10.1016/j.ydbio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002a;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, Zeller R. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002b;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10:647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Tickle C. The Number of Polarizing Region Cells Required to Specify Additional Digits in the Developing Chick Wing. Nature. 1981;289:295–298. doi: 10.1038/289295a0. [DOI] [PubMed] [Google Scholar]
- Tickle C, Alberts B, Wolpert L, Lee J. Local Application of Retinoic Acid to the Limb Bud Mimics the Action of the Polarizing Region. Nature. 1982;296:564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Tickle C, Summerbell D, Wolpert L. Positional Signaling and Specification of Digits in Chick Limb Morphogenesis. Nature. 1975;254:199–202. doi: 10.1038/254199a0. [DOI] [PubMed] [Google Scholar]
- Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- Towers M, Signolet J, Sherman A, Sang H, Tickle C. Insights into bird wing evolution and digit specification from polarizing region fate maps. Nature Communications. 2011;2:426. doi: 10.1038/ncomms1437. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Verheyden JM, Sun X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 2008;454:638–641. doi: 10.1038/nature07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22:2651–2663. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Thaller C, Jessell T, Eichele G. Polarizing Activity and Retinoid Synthesis in the Floor Plate of the Neural-Tube. Nature. 1990;345:819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Wanek N, Gardiner DM, Muneoka K, Bryant SV. Conversion by Retinoic Acid of Anterior Cells into Zpa Cells in the Chick Wing Bud. Nature. 1991;350:81–83. doi: 10.1038/350081a0. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Behringer RR, Rasweiler JJT, Niswander LA. Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc Natl Acad Sci U S A. 2006;103:15103–15107. doi: 10.1073/pnas.0604934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. Positional Information and Spatial Pattern of Cellular Differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Noordermeer D, Leleu M, Duboule D. Conservation and Divergence of Regulatory Strategies at Hox Loci and the Origin of Tetrapod Digits. Plos Biology. 2014;12:e1001773. doi: 10.1371/journal.pbio.1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, Bumcrot D, Vargesson N, Clarke J, Niswander L, McMahon A, Tickle C. Relationship between dose, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124:4393–4404. doi: 10.1242/dev.124.21.4393. [DOI] [PubMed] [Google Scholar]
- Zakany J, Duboule D. Synpolydactyly in mice with a targeted deficiency in the HoxD complex. Nature. 1996;384:69–71. doi: 10.1038/384069a0. [DOI] [PubMed] [Google Scholar]
- Zakany J, FromentalRamain C, Warot X, Duboule D. Regulation of number and size of digits by posterior Hox genes: A dose-dependent mechanism with potential evolutionary implications. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mackem S. Analysis of mutants with altered shh activity and posterior digit loss supports a biphasic model for shh function as a morphogen and mitogen. Dev Dyn. 2011;240:1303–1310. doi: 10.1002/dvdy.22637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhulyn O, Li D, Deimling S, Vakili NA, Mo R, Puviindran V, Chen MH, Chuang PT, Hopyan S, Hui CC. A switch from low to high Shh activity regulates establishment of limb progenitors and signaling centers. Dev Cell. 2014;29:241–249. doi: 10.1016/j.devcel.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Zuniga A. Next generation limb development and evolution: old questions, new perspectives. Development. 2015;142:3810–3820. doi: 10.1242/dev.125757. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Laurent F, Lopez-Rios J, Klasen C, Matt N, Zeller R. Conserved cis-regulatory regions in a large genomic landscape control SHH and BMP-regulated Gremlin1 expression in mouse limb buds. Bmc Developmental Biology. 2012;12:23. doi: 10.1186/1471-213X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga A, Michos O, Spitz F, Haramis AP, Panman L, Galli A, Vintersten K, Klasen C, Mansfield W, Kuc S, Duboule D, Dono R, Zeller R. Mouse limb deformity mutations disrupt a global control region within the large regulatory landscape required for Gremlin expression. Genes Dev. 2004;18:1553–1564. doi: 10.1101/gad.299904. [DOI] [PMC free article] [PubMed] [Google Scholar]


