Abstract
The purpose of our study was to determine the association between biomechanical outcomes of walking gait (peak vertical ground reaction force [vGRF], vGRF loading rate [vGRF-LR] and knee adduction moment [KAM]) six months following anterior cruciate ligament reconstruction (ACLR) and biochemical markers of serum type-II collagen turnover (collagen type-II cleavage product to collagen type-II C-propeptide [C2C:CPII]), plasma degenerative enzymes (matrix metalloproteinase-3 [MMP-3]), and a pro-inflammatory cytokine (interleukin-6 [IL-6]). Biochemical markers were evaluated within the first two weeks (6.5±3.8 days) following ACL injury and again six months following ACLR in eighteen participants. All peak biomechanical outcomes were extracted from the first 50% of the stance phase of walking gait during a six-month follow-up exam. Limb symmetry indices (LSI) were used to normalize the biomechanical outcomes in the ACLR limb to that of the contralateral limb (ACLR /contralateral). Bivariate correlations were used to assess associations between biomechanical and biochemical outcomes. Greater plasma MMP-3 concentrations after ACL injury and at the six-month follow-up exam were associated with lesser KAM LSI. Lesser KAM was associated with greater plasma IL-6 at the six-month follow-up exam. Similarly, lesser vGRF-LR LSI was associated with greater plasma MMP-3 concentrations at the six-month follow-up exam. Lesser peak vGRF LSI was associated with higher C2C:CPII after ACL injury, yet this association was not significant after accounting for walking speed. Therefore, lesser biomechanical loading in the ACLR limb, compared to the contralateral limb, six months following ACLR may be related to deleterious joint tissue metabolism that could influence future cartilage breakdown.
Keywords: Matrix metalloproteinase-3, Knee Adduction Moment, Collagen, Interleukin, Osteoarthritis
INTRODUCTION
Individuals who have sustained an anterior cruciate ligament (ACL) injury are at high risk of developing early onset post-traumatic osteoarthritis (PTOA).[1,2] Approximately one-third of individuals will demonstrate radiographic PTOA within the first decade following ACL injury.[3] The development of PTOA in younger individuals is associated with more years lived with disability[4] as well as worse outcomes following knee replacement compared to those with idiopathic knee osteoarthritis phenotypes.[5,6] The development of PTOA following ACL injury and ACLR is influenced by multiple factors[7,8] including changes in joint structure,[9] altered tissue metabolism[10] and aberrant mechanical joint loading.[11],[12] The severity and time to onset of PTOA following injury may be complicated by how changes in joint structure, tissue metabolism and joint loading interact following knee injury.[7] It is critical to understand the interaction between biomechanics and tissue metabolism following ACLR in order to develop comprehensive therapeutic strategies to decrease the risk of PTOA following ACL injury.
Deleterious metabolic and biomechanical alterations that contribute to PTOA onset likely begin before structural joint changes can be detected with traditional radiographs.[13,14] Detrimental alterations in the composition of the tibiofemoral articular cartilage are detectable as early as 12-months following ACLR.[13,14] Furthermore, individuals with ACLR present with greater type–II collagen turnover approximately 1.5 years following ACLR compared to uninjured controls.[15] It has been hypothesized that metabolic joint changes that occur soon after ACL injury and ACLR may influence long-term cartilage health and the onset of PTOA.[10] Matrix metalloproteinase-3 (MMP-3) concentration, one of several enzymes involved in the signaling of cartilage matrix protein degradation,[16] is increased in knee synovial fluid as early as three weeks following ACL injury compared to uninjured controls.[17,18] Pro-inflammatory cytokine concentrations are increased following ACL injury as well as in the initial days following ACLR,[19–21] and are known to stimulate MMP production.[16]
The addition of altered mechanical loading to injured joint tissues may further promote adverse changes to articular cartilage metabolism and hasten PTOA onset. Both excessive[22] and insufficient[23,24] mechanical loading can lead to cartilage breakdown or activate metabolic processes that signal cartilage breakdown. High loads are directed to the tibiofemoral surfaces during ACL rupture,[25] resulting in traumatic bone edema-like lesions that may have a negative long-term impact on the composition of the overlying cartilage matrix.[26] Following ACLR, individuals may offload the injured extremity,[27,28] which may lead to insufficient loading of the cartilage in the ACLR knee. Insufficient joint loading has been found to increase inflammation and the expression of degenerative cartilage enzymes (MMPs) in animal models,[16,23] which may result in cartilage breakdown if normalized loading is not reintroduced to the joint.[24] Similarly, lower peak vertical ground reaction forces (vGRF) during walking gait are associated with greater serum type-II collagen turnover three years following ACLR in humans.[29] Individuals with ACLR who develop PTOA in the first five years following injury demonstrate lower tibiofemoral contact forces six-months following ACLR, suggesting that joint loading is important for the maintenance of long-term cartilage health following ACLR.
Peak vGRF magnitude and loading rate (vGRF-LR) during walking gait reflect the collective vertical forces exerted upon the lower extremity, and are associated with cartilage matrix damage and turnover.[29,30] It remains unknown if peak vGRF and vGRF-LR during gait associate with biochemical markers of joint metabolism as early as six-months following ACLR, when many patients begin to return to unrestricted physical activity.[31] Knee adduction moment (KAM) has been reported to predict medial knee compartment contact forces in individuals with ACLR, [32] and greater KAM is associated with the progression of radiographic knee OA.[33,34] Finally, preliminary work has linked greater KAM to compositional articular cartilage alterations in the medial tibia and femur following ACLR, which is consistent with decreased cartilage proteoglycan density and collagen fiber orientation that may be indicative of early PTOA development.[35] The purpose of our study was to determine if biomechanical outcomes of peak vGRF, vGRF-LR and KAM symmetry during walking gait assessed six months following ACLR were associated with serum markers of type-II collagen turnover (i.e. ratio of collagen type-II cleavage product to collagen type-II C-propeptide [C2C:CPII]), as well as plasma concentrations of a degenerative enzyme MMP-3 and a pro-inflammatory cytokine (i.e. interleukin-6 [IL-6]) collected following ACL injury and 6-months following ACLR. We hypothesized that individuals with lesser biomechanical loading (vGRF, vGRF-LR and KAM) on the ACLR limb, compared to the contralateral limb, would have a greater concentrations of collagen turnover, MMP-3, and pro-inflammatory cytokines at initial presentation and at the six-month follow-up exam.
METHODS
All participants were recruited into a prospective longitudinal cohort study (Level of Evidence II) upon initial presentation in the orthopaedic clinic following ACL injury and prior to ACLR. Biochemical outcomes were assessed from serum and plasma samples collected at initial presentation following ACL injury and at a clinical follow-up exam approximately six months after ACLR. All biomechanical loading outcomes were collected six months following ACLR. Study personnel utilized electronic mail and telephone communication to retain study participants and schedule the six-month follow-up exam. We administered the International Knee Documentation Committee Index (IKDC) and the Marx activity rating scale at the six-month follow-up exam, which are valid and reliable measures of self-reported function[36] and physical activity,[37] respectively. All self-reported function outcomes were assessed using an electronic internet-based healthcare informatics system (Socrates GP, Healthcare Ltd, Sligo, Australia) that automatically forwarded a reminder to participants to complete the questionnaire six months following ACLR using a secure link. All participants signed an informed consent form that was approved by Institutional Review Board at the University of North Carolina at Chapel Hill prior to participating in any research related procedures.
Participants
We included individuals between the ages of 18 and 35 years who had sustained an ACL injury within 14 days prior to the initial presentation at the orthopaedic clinic. Participants were excluded from the study if they were currently pregnant or planned to become pregnant in the next 12 months, had been previously diagnosed with any form of inflammatory arthritis, were in need of a multi-ligament reconstruction, or were not planning to undergo ACLR. We did not exclude patients if they had a previous history of an ACLR on the knee of interest or the contralateral knee. We also excluded participants who had a history of a cardiac pacemaker, cochlear implant, clinical hypertension, claustrophobia, hepatic disease, diabetes or seizures. We estimated that we would detect a moderate association (r = 0.59) between biomechanical and biochemical outcomes, as we have reported in a previous study in individuals with an ACLR. [29] Therefore, we would need to include 17 ACLR participants in order to detect statistical significance for a two-tailed bivariate association of this magnitude (−0.59 or 0.59) with an alpha level set at 0.05 and 80% power (G*Power, v3.1.9.2).[38]
Anterior Cruciate Ligament Reconstruction
All participants underwent an arthroscopically assisted single incision ACLR using a patellar tendon autograft performed by one of the three participating orthopaedic surgeons. The autograft was harvested from the middle third of the patellar tendon via an anterior longitudinal incision. A target point was identified on the lateral wall of the intercondylar notch of the femur and a femoral tunnel was drilled through the infra-medial arthroscopic portal with the knee in approximately 120° of flexion. Using a targeting guide, a pin was drilled and over-reamed into the ACL footprint to create a tibial tunnel. Bone-plugs of the graft were affixed to the femur and tibia with a metal interference screw. All participants were referred to a licensed physical therapist or athletic trainer for a supervised, structured rehabilitation regimen that began during the first week following surgery and progressed over the next six months.
Collection and Analyses of Serum and Plasma
Blood samples were collected at both the initial presentation and the six-month follow-up exam. Blood was immediately processed for separation of serum and plasma which were aliquoted into 1.0 mL cryovials and stored in an −80°C freezer until analysis. Serum was assessed for markers of type-II collagen breakdown (C2C) and type-II collagen synthesis (CPII) using commercially available enzyme-linked immunosorbent assays (ELISA) (IBEX Technologies, Inc., Canada). Plasma was assessed for MMP-3 and IL-6 using ELISA (R & D Systems, Minneapolis, MN). Specific assay detection sensitivities were as follows; C2C = 10 ng/ml, CPII = 50 ng/ml, MMP-3 = 0.009 ng/ml and IL-6< 0.7pg/ml. Per assay manufacture recommendations, plasma IL-6 were not diluted while plasma MMP-3 and serum CPII and C2C samples were diluted by 10× and 2×, respectively. All assays were performed in duplicate determinations for standards and unknowns, and demonstrated inter-assay and intra-assay variability of less than 10%.
Analysis of Mechanical Loading
The current study was part of a larger ongoing project that assessed three-dimensional biomechanical outcomes following ACLR. Therefore, all participants were outfitted with 25 retroreflective markers (bilateral acromioclavicular joints, anterior superior iliac spines, greater trochanters, anterior thighs, medial and lateral femoral epicondyles, anterior shanks, medial and lateral malleoli, first metatarsal heads, fifth metatarsal heads, posterior calcanei, as well as the manubrium of the sternum and L4-L5 vertebral space) and a ridged cluster of three additional markers secured over the sacrum. Marker positions were collected using a 7-camera three-dimensional motion capture system (Vicon Nexus) and post-processed with Vicon Nexus v1.4.1 motion capture software (Vicon Motion Systems). Trajectories from the anterior superior iliac spine and sacral cluster markers were used to estimate center of mass for the calculation of gait speed.[39]
Participants walked barefoot at a self-selected speed over two Bertec force plates (40×60cm, FP406010, Bertec Corporation, Columbus, Ohio, United States) embedded in a six-meter walkway such that the entire stance phase for both the right and left limbs could be collected from a single trial.[29,39] Participants were instructed to walk at a self-selected speed described as if they were “comfortably walking over a sidewalk” and to look straight ahead and maintain a constant speed through two sets of timing gates (TF100, Trac Tronix, Lenexa, Kansas, United States) centered around the force plates. Five practice trials were performed to familiarize the participants with the gait task as well as to determine the average walking speed to be used during the test trials. During data collection, participants performed five acceptable gait trials that included 1) both right and left feet individually striking and toeing off a single force plate, 2) maintaining forward eye contact and not “aiming” for the force plates, 3) maintaining a consistent gait speed within 5% of the average speed determined during the practice trials, and 4) not visibly altering gait during the trial (i.e. trip or stutter step).
Kinematics were sampled at 120Hz and lowpass filtered at 12 Hz (4th order recursive Butterworth), while vGRF data were sampled at 1200 Hz and lowpass filtered at 75 Hz (4th order recursive Butterworth). The stance phase was defined as the interval between heel strike (vGRF > 20N) and toe off (vGRF < 20N) and outcomes were averaged across the five gait trials. The peak vGRF and peak KAM were extracted from the first 50% of the stance phase of gait. Additionally, the instantaneous vGRF- LR was calculated as the highest loading rate from heel strike to the peak vGRF in the first 50% of the stance phase for each gait trial by determining the peak of the first derivative of the force-time curve. Peak vGRF (BW) and vGRF-LR(BW/s) were normalized to each participant’s body weight. Joint moments were calculated using an inverse dynamics and KAM was normalized to the product of body weight and height (xBW*H) and expressed as an external moment. Limb symmetry indices (LSI) was calculated for peak vGRF, vGRF-LR and KAM (ACLR limb/ contralateral limb) such that ratios < 1.00 denoted lesser loading on the ACLR limb while those > 1.00 denoted greater loading on the ACLR limb compared to the contralateral limb. Gait speed of the total body center of mass was estimated from pelvic markers by calculating the time required for the pelvis COM to translate over a 1m distance, which included 0.5m prior to and 0.5m following heel strike of the ACLR limb on the force plate.[40]
Statistical Analysis
Prior to our primary analyses, normality was assessed for the biomechanical and biochemical outcomes using a Shapiro – Wilk test. Data found to be non-normally distributed were Log-10 transformed and further assessed for outliers using stem and leaf plots. We considered any data points ≥ 2.5 standard deviations from the mean to be statistical outliers, which we subsequently removed from the data set for further analyses. For the primary analyses we used separate Pearson Product Moment correlations (r) to assess associations between each biomechanical outcome (peak vGRF LSI and vGRF-LR LSI) at the six month follow-up exam and each biochemical outcome (plasma IL-6, plasma MMP-3, and serum C2C:CPII ratio) measured using samples collected at both the initial presentation and the six-month follow-up exam. Walking speed has been demonstrated to influence peak vGRF LSI and vGRF-LR LSI during gait[41] and serum C2C concentrations in individuals with and ACLR.[39] Therefore, we further examined all using a partial correlation between the biomechanical and the biochemical variables of interest while controlling for gait speed.
We performed an exploratory secondary analysis to determine if self-reported disability (International Knee Documentation Committee Index) and physical activity (Marx scores) influenced any statistically significant associations between one of the biomechanical and biochemical markers of interest demonstrated during the primary analyses. Finally, in order to better discuss our results, we conducted a post hoc analysis that excluded participants who reported a previous history of an ACLR on either limb. Therefore, post hoc bivariate association analyses were specifically conducted for variables found to be significantly associated in the primary analyses after controlling for walking speed. Our current study was not powered to conduct further secondary partial correlations as described in the exploratory analyses part of a separate post hoc analysis. Associations were classified as negligible (0.0–0.3), low (0.31–0.5) moderate (0.51–0.7), high (0.71–0.9), and very high (0.9–1.0).[42] We considered correlations to be statistically significant if alpha levels were ≤ 0.05. Statistical Package for the Social Sciences software (SPSS, Version 19.0, IBM Corp., Somers, NY) was used to perform all analyses.
RESULTS
We recruited and retained 18 participants with biomarker data from the initial presentation and six-month follow-up exam, as well as biomechanical loading data from the six-month follow-up exam (Table 1). Two of the participants reported a previous ACLR on the contralateral limb > 2 years prior to sustaining the ACL injury of interest, while a third participant reported previous bilateral ACLR on both the limb of interest (> 3 years prior) and contralateral limb (> 2 years prior). Plasma IL-6 and MMP-3 data from initial presentation and the six-month follow-up exam, as well as vGRF-LR LSI were non-normally distributed and were Log-10 transformed prior to analysis, all other mechanical and biochemical variables were normally distributed. A single outlier was identified and removed from each of the following distributions for the final analyses: Log -10 transformed vGRF-LR LSI, KAM LSI and C2C:CPII concentrations at baseline. After removal of the outliers, the majority of the final analysis included a total of 17 or 18 participants, yet two of our bivariate associations included a total of 16 participants (Table 2). Five of the 18 IL-6 plasma samples collected at initial presentation and all of the samples collected a the six-month follow-up exam were found to be less than the lowest standard of the assay (3 pg/ml). The initial presentation exam occurred 6.5±3.8 days following injury, while ACLR occurred 32.9±15.5 days following ACL injury and the six-month follow-up exam occurred 196.2±18.4 days (6.3±0.6 months) following ACLR.
Table 1.
Means (Standard Deviation) of Participant Demographics and Outcome Measures
| Sex | 9 Males, 9 Females |
| Age | 22.1 (4.6) years |
| Height | 176.6 (11.4)cm |
| Mass | 74.0 (12.8) kg |
| IKDC at Six-month Follow-up Exam | 70.6 (11.0) % |
| Marx Activity Score | 7.7 (4.5) |
| Number of Concomitant Medial Meniscus Injuries | 2 (11.1%) |
| Number of Concomitant Lateral Meniscus Injuries | 11 (61.1%) |
| Serum C2C Concentrations | |
| Baseline | 160.51 (33.49) ng/ml |
| Six Month Follow-up | 127.18 (24.78) ng/ml |
| Serum CPII Concentrations | |
| Baseline | 1084.78 (139.13) ng/ml |
| Six Month Follow-up | 841.4 (167.75) ng/ml |
| Serum C2C:CPII Ratios | |
| Baseline | 0.15 (0.02) |
| Six Month Follow-up | 0.15 (0.03) |
| Plasma IL-6 Concentrations | |
| Baseline | 3.09 (4.21) pg/ml |
| Six Month Follow-up | 1.09 (0.9) pg/ml |
| Plasma MMP-3 Concentrations | |
| Baseline | 6.41 (5.04) ng/ml |
| Six Month Follow-up | 8.03 (6.06) ng/ml |
| Gait Speed | 1.28 (0.14) m/sec |
| Peak Vertical Ground Reaction Force | |
| ACLR Limb | 1.05 (0.06) BW |
| Contralateral Limb | 1.10 (0.09) BW |
| Limb Symmetry Index | 0.96 (0.04) |
| Peak Vertical Ground Reaction Force Loading Rate | |
| ACLR Limb | 55.03 (16.33) BW/sec |
| Contralateral Limb | 59.4 (24.32) BW/sec |
| Limb Symmetry Index | 1.06 (0.57) |
| Peak External Knee Adduction Moment | |
| ACLR Limb | 0.026 (0.006) BW* H |
| Contralateral Limb | 0.029 (0.009) BW* H |
| Limb Symmetry Index | 0.94 (0.19) |
ACLR- anterior cruciate ligament reconstruction
BW- body weight
H - Height
MMP-3 - Matrix metalloproteninase-3
C2C - collagen type-II cleavage product
CPII - collagen type-II C-propeptide
IKDC- International Knee Documentation Committee Index
IL-6 - Interleukin- 6
Table 2.
Associations Between Peak VGRF, KAM, and VGRF Loading Rate and Biochemical Markers at Initial Presentation and Six Month Follow-Up Exams
| Peak Vertical Ground Reaction Force Limb Symmetry Index (vGRF LSI) | Vertical Ground Reaction Force Instantaneous Loading Rate Limb Symmetry Index (vGRF-LR LSI) | External Knee Adduction Moment Limb Symmetry Index (KAM LSI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Association between vGRF LSI and Biomarker r (P value) | Partial Correlation Accounting for Walking Speed Partial r (P value) | n | Association between vGRF-LR LSI and Biomarker (P value) | Partial Correlation Accounting for Walking Speed Partial r (P value) | n | Association between KAM LSI and Biomarker (P value) | Partial Correlation Accounting for Walking Speed Partial r (P value) | |
| Initial Presentation IL-6 | 18 | −0.06 (0.83) | −0.02 (0.95) | 17 | −0.31 (0.23) | −0.21 (0.44) | 17 | −0.21 (0.41) | 0.04 (0.89) |
| Six-Month IL-6 | 18 | −0.19 (0.45) | −0.13 (0.59) | 17 | −0.20 (0.45) | −0.28 (0.29) | 17 | −0.42 (0.1) | −0.6 (0.01) |
| Initial Presentation C2C:CPII | 17 | −0.5 (0.04) | −0.24 (0.36) | 16 | −0.37 (0.16) | 0.27(0.64) | 16 | 0.15 (0.57) | 0.16 (0.57) |
| Six-Month C2C:CPII | 18 | −0.15 (0.56) | −0.16 (0.53) | 17 | 0.09 (0.73) | 0.11 (0.69) | 17 | 0.26 (0.31) | 0.21 (0.45) |
| Initial Presentation MMP-3 | 18 | −0.03 (0.91) | −0.21 (0.42) | 17 | −0.26 (0.32) | −0.12(0.66) | 17 | −0.64 (0.01) | −0.54 (0.03) |
| Six-Month MMP-3 | 18 | −0.4 (0.89) | −0.17 (0.52) | 17 | −0.60 (0.01) | −0.53 (0.04) | 17 | −0.67 (0.01) | −0.59 (0.02) |
MMP-3 - Matrix metalloproteninase-3, IL-6 - Interleukin- 6, C2C - collagen type-II cleavage product; CPII - collagen type-II C-propeptide
Primary Analyses: Associations between Mechanical Loading and Biochemical Markers
Bivariate associations and partial correlations accounting for walking speed are presented in Table 2. Greater plasma MMP-3 concentrations at the initial presentation exam associated with lesser KAM LSI at the six-month follow-up exam (r=− 0.64; P= 0.01; partial r=− 0.54; P= 0.03; Table 2; Figure 1). Greater plasma MMP-3 concentrations at the six-month follow-up exam associated with lesser vGRF-LR LSI (r=− 0.6; P= 0.01 and partial r=−0.59; P=0.02 Table 2; Figure 2) and lesser KAM LSI (r=− 0.67; P= 0.01; partial r=−0.53; P=0.04 Table 2; Figure 3) at the six-month follow-up exam. Lower vGRF LSI at the six-month follow-up exam associated with higher serum concentrations of C2C:CPII ratios collected at the initial presentation exam (r=− 0.5; P= 0.04; Table 2, Figure 4), yet this association was not significant after controlling for walking speed (partial r =− 0.24; P= 0.36; Table 2). There was a significant association between greater IL-6 at the six-month follow-up exam and lesser KAM LSI (partial r =− 0.60; P= 0.02; Table 1; Figure 5) after controlling for walking speed. None of the other associations were significant (Table 2).
Figure 1. Association Between Metallopeptidease- 3 (MMP-3) at the Initial Presentation and External Knee Adduction Moment Limb Symmetry Index (KAM-LSI).
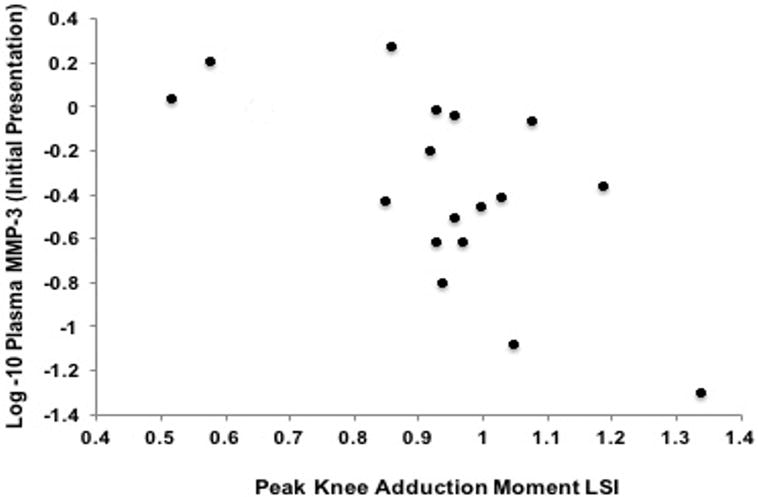
Greater plasma MMP-3 concentrations at the initial presentation exam (Y-axis) was associated with lesser KAM (X-axis) at the six-month follow-up exam (r=− 0.64; P= 0.01; partial r=− 0.54; P= 0.03).
Figure 2. Association Between Metallopeptidease- 3 (MMP-3) at the Six-Month Follow-up Exam and Vertical Ground Reaction Force Loading Rate Limb Symmetry Index (vGRF-LR LSI).
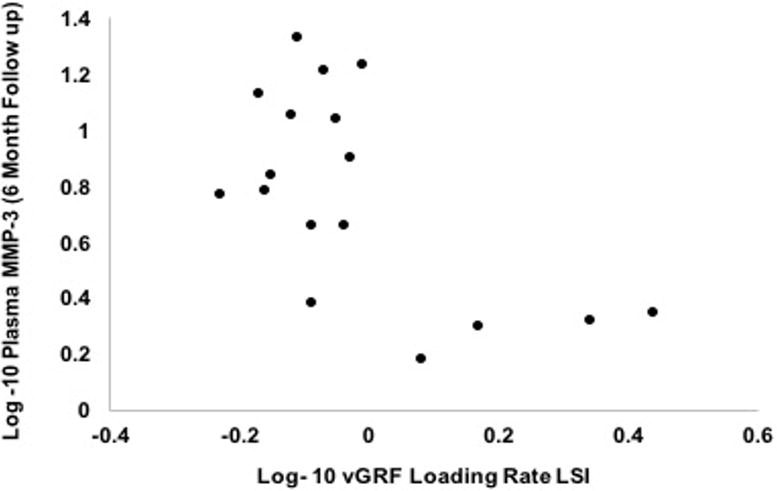
Higher log-10 transformed plasma MMP-3 concentrations (Y-axis) associated with lower log-10 transformed vGRF-LR LSI (X-axis) at the six-month follow-up exam (r=− 0.6; P= 0.01).
Figure 3. Association Between Metallopeptidease- 3 (MMP-3) at the Six-Month Follow-up Exam and External Knee Adduction Moment Limb Symmetry Index (KAM-LSI).
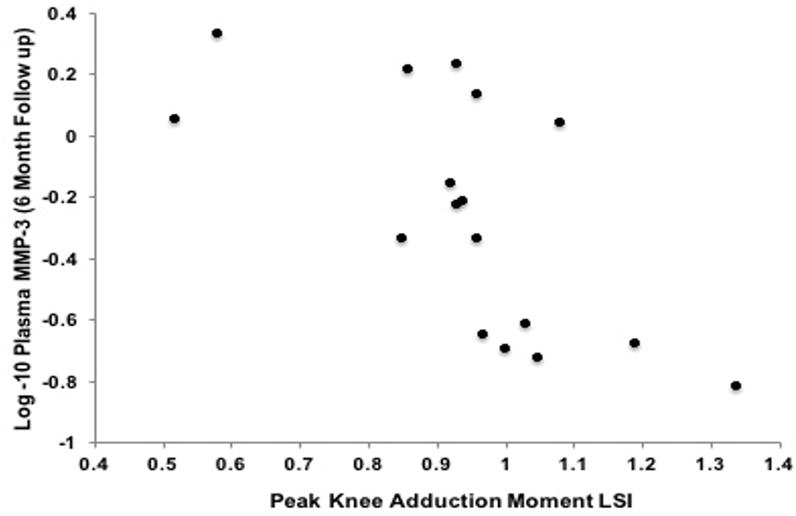
Greater plasma MMP-3 concentrations (Y-axis) at the six-month follow-up exam was associated lesser KAM LSI (X-axis) at the six-month follow-up exam (r=− 0.67; P= 0.01; partial r=−0.53; P=0.04).
Figure 4. Association Between Serum Collagen Type-II Cleavage Product to Collagen Type-II C-Propeptide [C2C:CPII] Ratios at the Six-Month Follow-up Exam and External Knee Adduction Moment Limb Symmetry Index (KAM-LSI).
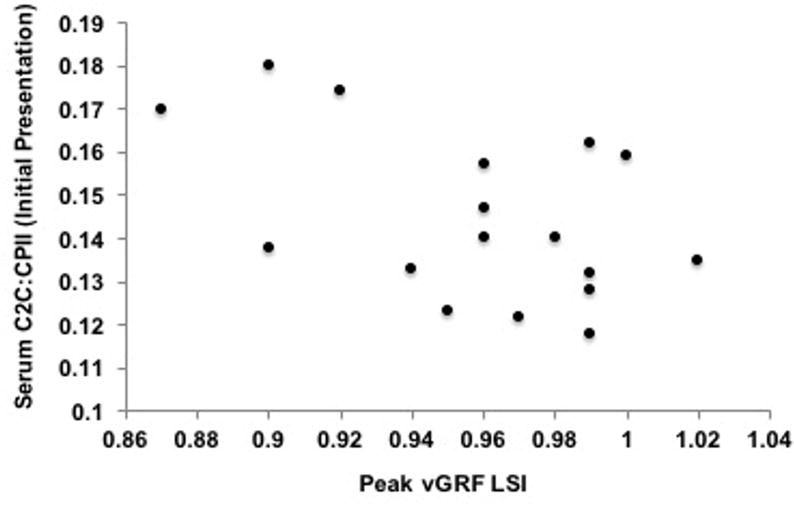
Lower vGRF LSI (X-axis) at the six-month follow-up exam associated with higher serum concentrations of C2C:CPII ratios (Y-axis) collected at the initial presentation exam (r=− 0.5; P= 0.04) yet this association was not significant after controlling for walking speed (partial r =− 0.24; P= 0.36).
Figure 5. Association Between Interleukin-6 (IL-6) at the Six-Month Follow-up Exam and External Knee Adduction Moment Limb Symmetry Index (KAM-LSI).
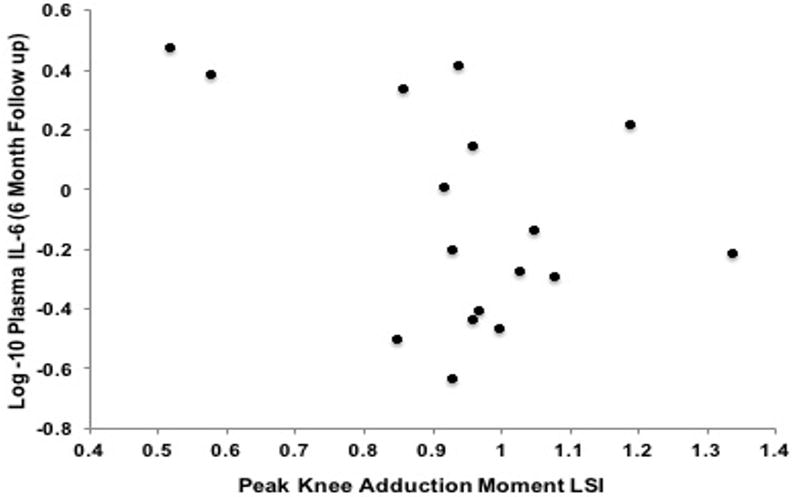
There was a significant association between greater IL-6 (Y-axis) at the six-month follow-up exam and lesser KAM LSI(X-axis) after controlling for walking speed (partial r =− 0.60; P= 0.02).
Secondary Analysis: Partial Correlations Accounting for International Knee Documentation Committee Index and Marx Score
After accounting for International Knee Documentation Committee Index, (partial r =− 0.62; P= 0.02), and Marx activity score (partial r =− 0.66; P= 0.008), the association between higher plasma MMP-3 concentrations and lower vGRF-LR LSI at the six-month follow-up exam remained significant. Similarly, the association between lower KAM LSI and higher IL-6 remained significant after accounting for International Knee Documentation Committee Index, (partial r =− 0.57; P= 0.02), and Marx activity score (partial r =− 0.62; P= 0.01). The associations between KAM LSI and MMP-3 at initial presentation and the six-month follow-up exam were not significant after accounting for International Knee Documentation committee index (partial r =− 0.44; P= 0.09 and partial r =− 0.47; P= 0.07), and Marx activity score (partial r =− 0.48; P= 0.06 and partial r =− 0.47; P= 0.07).
Post Hoc Analysis
We performed post hoc analyses removing the three participants who reported a previous history of an ACLR for associations that were found to be significant in our primary analyses. Higher plasma MMP-3 concentrations were associated with lower vGRF-LR LSI at the six-month follow-up exam in participants who reported no previous history of ACL injury other than the ACLR of interest (n= 14, r=−0.53; P= 0.05). Similarly, lower KAM LSI was associated with higher MMP-3 at initial presentation (n= 15, r=−0.64; P= 0.01) and at the 6-month follow-up exam (n= 15, r=−0.68; P= 0.006) in those without a history of an ACLR. There was a notable but non-significant association between higher KAM LSI and lower IL-6 at the 6-month follow-up exam in those without a previous ACLR (n= 15, r=−0.41; P= 0.13, Table 2).
DISCUSSION
Our study provides evidence that individuals with lesser biomechanical loading on the ACLR limb at the six-month follow-up exam, compared to the contralateral limb, demonstrate greater concentrations of plasma MMP-3 and IL-6, as well as serum type-II collagen turnover (Table 2). Specifically, those with lesser KAM LSI at the six-month follow-up exam demonstrated greater concentrations of degenerative MMP-3 enzymes at initial presentation, as well as greater concentrations of MMP-3 and IL-6 at the six-month follow up exam. Similarly, those with lesser vGRF-LR demonstrated greater concentrations of MMP-3 at the six-month follow up exam. We found limited evidence that any of the biomechanical outcomes that we studied were directly associated with type-II collagen breakdown; the moderate association between lesser peak vGRF LSI and greater serum concentrations of type-II collagen turnover originally observed at initial presentation was not significant after accounting for walking speed. Overall the associations between lesser loading on the ACLR limb (KAM LSI and vGRF-LR LSI) and increased concentrations of IL-6 and MMP-3 may be indicative of early interactions between mechanical joint loading and metabolic processes that may influence the future breakdown of cartilage.
Our study is the first to evaluate associations between mechanical outcomes of peak vGRF LSI, vGRF-LR LSI and KAM LSI and biochemical markers of plasma IL-6 and MMP-3, as well as serum C2C:CPII concentrations at both initial presentation and at the six-month follow-up exam following ACLR. We previously demonstrated that lower peak vGRF magnitude, but not linear vGRF-LR, associated with greater serum type-II collagen turnover (C2C:CPII) in individuals with ACLR (38.0± 29.3 months following ACLR).[29] The current study evaluated the peak instantaneous vGRF-LR rather than the linear vGRF-LR, potentially explaining the discrepancies between the results of the current study and those of our previous study.[29] Peak instantaneous vGRF-LR occurs immediately after initial ground contact and may be a better marker of impulsive loading linked to development of knee osteoarthritis compared to linear vGRF-LR, which is averaged over the entire loading phase of gait.[43] Our results may indicate that instantaneous vGRF loading rate is associated with early changes in joint tissue metabolism following ACLR, while peak vGRF is related to cartilage turnover years after injury.[29] Longitudinal studies are needed to evaluate how associations between biomechanical variables and metabolism change over time following ACLR. While both the magnitude of loading and the rate of loading, as well as loading of the medial tibiofemoral compartment can influence acute cartilage injury and altered cartilage metabolism,[35,44] it remains unknown if one of these biomechanical variables is better at predicting PTOA risk.
External KAM is a biomechanical marker that can be linked to contact forces in the medial tibiofemoral compartment [32] and those with medial compartment knee OA have demonstrated higher KAM than healthy control participants.[45] The influence of KAM on the pathogenesis of PTOA following ACLR is unclear. KAM has been reported to be higher following ACLR [46] compared to control participants while others have reported no difference in KAM compared to uninjured participants.[47] Conversely, individuals with an ACLR who developed PTOA five years following surgery demonstrated lesser peak KAM during walking gait assessments that occurred six months following ACLR.[48] Similarly, we found that individuals with higher levels of degenerative MMP-3 enzymes collected in the first 6.5±3.8 days following ACL injury walked with lesser medial compartment loading at the six-month follow-up exam. Greater concentrations of MMP-3 at the initial presentation exam may be due to increased cartilage metabolism following greater tissue damage after ACL injury. While we are unable to determine the causal nature of the relationship at this time, it is possible that greater MMP-3 concentrations immediately after ACL injury may influence how people load the medial knee compartment in the first six months after ACLR. Interestingly, lesser medial compartment loading associated with greater concentrations of plasma MMP-3 and IL-6 at the six-month follow-up exam. It is also possible that consistently walking with lesser KAM over the six months following ACLR perpetuated metabolic changes resulting in greater concentrations of plasma IL-6 and MMP-3. The magnitude of the associations between KAM LSI and MMP-3 were lower after accounting for IKDC and Marx score. The partial correlations were exploratory and the current study was not originally powered to determine how patient-reported outcomes may affect associations between biomechanical and biochemical outcomes. However, these data suggest that self-reported disability and physical activity influence the association between KAM LSI and MMP-3. Future research should seek to better understand interactions between patient-reported outcomes, biomechanics and tissue metabolism.
Previous studies have demonstrated higher MMP concentrations in osteoarthritic articular cartilage.[49,50] MMP-3 is involved in the breakdown of collagen and extracellular matrix proteins including proteoglycans and may also activate other MMPs, which impact further proteoglycan breakdown and later collagen degradation.[16] Increased MMP-3 expression can be triggered by acute mechanical injury to articular cartilage,[51] and synovial fluid concentrations of MMP-3 are increased within the first week of ACL injury.[18] IL-6 is increased following cartilage damage and acts to regulate the activation other interleukins that can increase the expression of MMPs in the cartilage or synovial fluid,[52] which may explain the concurrent associations between lesser KAM and greater MMP-3 and IL-6 in our study (Table 2). Greater loads, that can damage cartilage, increase production and release of MMP-3.[53,54] Conversely, it has also been demonstrated that lesser mechanical loading also increases MMP-3 expression.[23,24] Both excessive and diminished mechanical loading may stimulate expression of MMPs and influence the metabolic processes that are associated with joint tissue breakdown and the eventual development of PTOA.
Mechanical loading is necessary for maintaining proper cartilage health, and diminished mechanical loading may increase catabolic activity.[23] Recent meta-analyses have demonstrated decreased knee joint loading in the injured limb compared to the contralateral limb in individuals with an ACLR.[55,56] Conversely, it can be hypothesized that a decrease in joint loading following ACLR is a biomechanical adaptation that is initiated in response to the underlying catabolic process occurring in knee,[39] which may be due to the extent of the tissue that was injured at the time of ACL injury. Bone contusions, which occur frequently during ACL injury,[57] may cause alterations in the overlying articular cartilage, which may persist months after the bone contusion has healed.[14] The association between greater MMP-3 plasma concentration and lesser vGRF-LR and KAM may be influenced by the extent of tissue damage that occurred during ACL injury and a longer, more robust healing process may be necessary in joints that have sustained greater injury in order to regain optimal joint function.
While the current study improves our understanding of the association between cartilage metabolism and mechanical loading following ACLR, there are limitations that should be addressed. The sample size of the current study is relatively small, which precludes us from performing adequately powered sub-analyses to determine how concomitant injuries (i.e. meniscal injury, bone marrow edema-like lesions) or concomitant procedures may have influenced the associations between loading and the included biochemical markers. However, we performed post hoc analyses and determined that magnitudes of the associations found between biomechanical loading outcomes and biochemical markers in the entire cohort (N= 18) were similar to those found in only the individuals without a history of previous ACLR (n=14 or 15 depending on analysis). A future study with a larger sample size may be able to make more definitive conclusions regarding the associations between biomechanical loading and biochemical outcomes in those with and without a history of multiple ACL injuries. Additionally, the collection of biochemical markers that we analyzed is far from comprehensive, and future studies may seek to evaluate associations between loading and other biochemical markers of inflammation, proteinases, collagen metabolism, as well as proteoglycan metabolism,[10] which we did not measure in this analysis. We assessed plasma IL-6 and MMP-3 as well as serum C2C:CPII concentrations; therefore it is impossible to discern if associations between increased MMP-3 and decreased vGRF-LR are due to metabolism specifically related to the ACLR knee, the contralateral knee or other structures throughout the body.
In conclusion, individuals with a lesser vGRF-LR LSI in the first 50% of the stance phase of gait demonstrated greater plasma MMP-3 concentrations six months following ACLR. Similarly lesser KAM LSI was associated with greater plasma MMP-3 at initial presentation, as well as greater plasma MMP-3 and IL-6 concentrations six months following ACLR. There was a significant association between lesser peak vGRF-LSI and greater type-II collagen breakdown, yet this association was not significant after accounting for walking speed. The results of the current study support the hypothesis that biomechanical outcomes are associated with biochemical markers related to joint tissue metabolism following ACLR. The association between lesser biomechanical loading in the ACLR limb with greater concentrations of a degenerative enzyme and a pro-inflammatory cytokine early after ACL injury and during the early postoperative period may be related to future cartilage breakdown, which impact future PTOA onset.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (1R03AR066840-01A1), North Carolina Translational and Clinical Sciences (TraCS) Institute and National Athletic Trainers Association Research and Education Foundation (#14NewInv001). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, TraCS Institute or the National Athletic Trainers Association Research and Education Foundation.
Footnotes
Author Contributions
All authors made contributions to: 1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, 2) drafting the article or revising it critically for important intellectual content and 3) final approval of the version to be submitted.
References
- 1.Claes S, Hermie L, Verdonk R, et al. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21:1967–1976. doi: 10.1007/s00167-012-2251-8. [DOI] [PubMed] [Google Scholar]
- 2.Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42:2242–2252. doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 3.Luc B, Gribble P, Pietrosimone B. Osteoarthritis Prevalence Following Anterior Cruciate Ligament Reconstruction: A Systematic Review and Numbers Needed to Treat Analysis. J Athl Train. 2014;49:806–819. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerman IN, Bucknill A, Page RS, et al. The substantial personal burden experienced by younger people with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2015;23:1276–1284. doi: 10.1016/j.joca.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Lonner J, Pedlow F, Siliski J. Total knee arthroplasty for post-traumatic arthrosis. J Arthroplasty. 1999;14:969–975. doi: 10.1016/s0883-5403(99)90012-8. [DOI] [PubMed] [Google Scholar]
- 6.Weiss N, Parvizi J, Hanssen A, et al. Total knee arthroplasty in post-traumatic arthrosis of the knee. J Arthroplasty. 2003;18:23–26. doi: 10.1054/arth.2003.50068. [DOI] [PubMed] [Google Scholar]
- 7.Andriacchi TP, Favre J, Erhart-Hledik JC, Chu CR. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann Biomed Eng. 2015;43:376–387. doi: 10.1007/s10439-014-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: A systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015;33:939–947. doi: 10.1002/jor.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter DJ, Lohmander LS, Makovey J, et al. The effect of anterior cruciate ligament injury on bone curvature: exploratory analysis in the KANON trial. Osteoarthritis Cartilage. 2014;22:959–968. doi: 10.1016/j.joca.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage. 2015;23:1–12. doi: 10.1016/j.joca.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech (Bristol, Avon) 25:277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 13.Theologis AA, Haughom B, Liang F, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22:298–307. doi: 10.1007/s00167-013-2397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1rho and T2–initial experience with 1-year follow-up. Radiology. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tourville TW, Johnson RJ, Slauterbeck JR, et al. Relationship between markers of type II collagen metabolism and tibiofemoral joint space width changes after ACL injury and reconstruction. Am J Sports Med. 2013;41:779–787. doi: 10.1177/0363546513476481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlberg L, Friden T, Roos H, et al. A longitudinal study of cartilage matrix metabolism in patients with cruciate ligament rupture–synovial fluid concentrations of aggrecan fragments, stromelysin-1 and tissue inhibitor of metalloproteinase-1. Br J Rheumatol. 1994;33:1107–1111. doi: 10.1093/rheumatology/33.12.1107. [DOI] [PubMed] [Google Scholar]
- 18.Lohmander LS, Roos H, Dahlberg L, et al. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Ortho Res. 1994;12:21–28. doi: 10.1002/jor.1100120104. [DOI] [PubMed] [Google Scholar]
- 19.Hayward AL, Deehan DJ, Aspden RM, Sutherland AG. Analysis of sequential cytokine release after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:1709–1715. doi: 10.1007/s00167-011-1486-0. [DOI] [PubMed] [Google Scholar]
- 20.Mendias CL, Lynch EB, Davis ME, et al. Changes in circulating biomarkers of muscle atrophy, inflammation, and cartilage turnover in patients undergoing anterior cruciate ligament reconstruction and rehabilitation. Am J Sports Med. 2013;41:1819–1826. doi: 10.1177/0363546513490651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron ML, Fu FH, Paessler HH, et al. Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc. 1994;2:38–44. doi: 10.1007/BF01552652. [DOI] [PubMed] [Google Scholar]
- 22.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–521. [PubMed] [Google Scholar]
- 23.Leong DJ, Li YH, Gu XI, et al. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25:182–191. doi: 10.1096/fj.10-164277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun HB, Zhao L, Tanaka S, Yokota H. Moderate joint loading reduces degenerative actions of matrix metalloproteinases in the articular cartilage of mouse ulnae. Connect Tissue Res. 2012;53:180–186. doi: 10.3109/03008207.2011.628765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SY, Spritzer CE, Utturkar GM, et al. Knee Kinematics During Noncontact Anterior Cruciate Ligament Injury as Determined From Bone Bruise Location. Am J Sports Med. 2015;43:2515–2521. doi: 10.1177/0363546515594446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theologis A, Kuo D, Cheng J, et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament-injured and -reconstructed knees using quantitative t(1ρ) magnetic resonance imaging: 1-year cohort study. Arthroscopy. 2011;27:65–76. doi: 10.1016/j.arthro.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterno MV, Ford KR, Myer GD, et al. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17:258–262. doi: 10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt LC, Paterno MV, Ford KR, et al. Strength Asymmetry and Landing Mechanics at Return to Sport after Anterior Cruciate Ligament Reconstruction. Med Sci Sports Exerc. 2015;47:1426–1434. doi: 10.1249/MSS.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrosimone B, Blackburn JT, Harkey MS, et al. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44:425–432. doi: 10.1177/0363546515618380. [DOI] [PubMed] [Google Scholar]
- 30.Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Ortho Res. 2001;19:779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 31.Beynnon BD, JR, Naud S, Fleming BC, Abate JA, Brattbakk B, Nichols CE. Accelerated versus nonaccelerated rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. Am J Sport Med. 2011;39:2536–2548. doi: 10.1177/0363546511422349. [DOI] [PubMed] [Google Scholar]
- 32.Wellsandt E, Khandha A, Manal K, et al. Predictors of knee joint loading after anterior cruciate ligament reconstruction. J Ortho Res. 2016 doi: 10.1002/jor.23408. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma L, Hurwitz DE, Thonar EJ, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki T, Wada M, Kawahara H, et al. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar D, Kothari A, Souza R, et al. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: A pilot study. Knee. 2014;21:881–885. doi: 10.1016/j.knee.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins LD, Taylor MK, Park D, et al. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007;74:594–599. doi: 10.1016/j.jbspin.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 37.Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83-a:1459–1469. doi: 10.2106/00004623-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 39.Pietrosimone B, Blackburn JT, Harkey MS, et al. Walking speed as a potential indicator of cartilage breakdown following anterior cruciate ligament reconstruction. Arthritis Care Res. 2015;66:793–800. doi: 10.1002/acr.22773. [DOI] [PubMed] [Google Scholar]
- 40.Dempster WT, Gabel WC, Felts WJ. The anthropometry of the manual work space for the seated subject. Am J Phys Anthropol. 1959;17:289–317. doi: 10.1002/ajpa.1330170405. [DOI] [PubMed] [Google Scholar]
- 41.Zeni JA, Jr, Higginson JS. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: a result of altered walking speed? Clin Biomech (Bristol, Avon) 2009;24:372–378. doi: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukaka M. A guide to appropriate use of Correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 43.Pamukoff DN, Lewek MD, Blackburn JT. Greater vertical loading rate in obese compared to normal weight young adults. Clin Biomech (Bristol, Avon) 2016;33:61–65. doi: 10.1016/j.clinbiomech.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech. 2005;38:493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Baliunas A, Hurwitz D, Ryals A, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 46.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43:366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 47.Patterson MR, Delahunt E, Caulfield B. Peak knee adduction moment during gait in anterior cruciate ligament reconstructed females. Clin Biomech (Bristol, Avon) 2014;29:138–142. doi: 10.1016/j.clinbiomech.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Wellsandt E, Gardinier ES, Manal K, et al. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am J Sports Med. 2016;44:143–151. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bau B, Gebhard PM, Haag J, et al. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 50.Swingler TE, Waters JG, Davidson RK, et al. Degradome expression profiling in human articular cartilage. Arthritis Res Ther. 2009;11:R96. doi: 10.1186/ar2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watt FE, Paterson E, Freidin A, et al. Acute molecular changes in synovial fluid following human knee injury are associated with early clinical outcomes. Arthritis Rheumatol (Hoboken, NJ) 2016;68(9):2129–40. doi: 10.1002/art.39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusano K, Miyaura C, Inada M, et al. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139:1338–1345. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 53.Stevens AL, Wishnok JS, White FM, et al. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics. 2009 doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bevill SL, Briant PL, Levenston ME, Andriacchi TP. Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarthritis Cartilage. 2009;17:980–987. doi: 10.1016/j.joca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016;50:597–612. doi: 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 56.Kaur M, Ribeiro DC, Theis JC, et al. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sports Med (Auckland, NZ) 2016;46(12):1869–1895. doi: 10.1007/s40279-016-0510-4. [DOI] [PubMed] [Google Scholar]
- 57.Yoon KH, Yoo JH, Kim KI. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. T J Bone Joint Surg Am. 2011;93:1510–1518. doi: 10.2106/JBJS.J.01320. [DOI] [PubMed] [Google Scholar]


